
Treatment strategies for borderline resectable pancreatic neuroendocrine tumors: a narrative review
Introduction
Pancreatic neuroendocrine tumors (pNETs) are a group of rare, heterogeneous tumors that originate in the endocrine tissue of the pancreas and account for 1–2% of all pancreatic neoplasms (1-5). According to the World Health Organization classification, pancreatic neuroendocrine neoplasms are categorized based on cytological/histological morphology as either poorly-differentiated neuroendocrine carcinomas or well-differentiated pNET, which will be the subject of this review (6). Depending on the biological behavior assessed by mitotic count and Ki-67 index, well-differentiated pNETs can be further classified as low-grade (G1) with a Ki-67 index of ≤2%, intermediate-grade (G2) with a Ki-67 index of 3–20%, or high-grade (G3) with a Ki-67 index >20% (7).
These pNETs are also categorized as functional or non-functional based on their ability to secrete biologically active hormones. The majority of pNETs are non-functional and typically follow a more indolent course. As such, many patients are diagnosed incidentally on cross-sectional imaging performed for another indication. While active surveillance is increasingly utilized for small, asymptomatic, non-functional pNETs, surgical resection is the primary management for most early stage localized tumors and is associated with an excellent long-term prognosis (1,8).
In contrast to small asymptomatic pNETs diagnosed incidentally, some patients will present with signs or symptoms secondary to more advanced disease (9,10). Indeed, some pNETs will present with large primary tumors with extrapancreatic organ or vascular involvement. Others will have evidence of metastatic disease. For these patients with “borderline resectable” tumors, surgical resection may be more difficult to achieve, the potential for incomplete resection exists, and risk of disease recurrence is high (11-13). Yet, complete surgical resection even for those patients with large primary tumors or low volume metastatic disease is associated with improved overall survival rates and remains the only potentially curative intent treatment among patients with advanced pNETs (14-18).
In recent years, consensus criteria have been developed to standardize the localized staging of pancreatic ductal adenocarcinoma (PDAC). Largely based on the extent of vascular involvement, patients’ tumors are described as potentially resectable, borderline resectable (BR), or locally advanced. This standardized terminology has been critical for clarifying the optimal treatment strategies for PDAC, namely advancing the role of neoadjuvant therapy and optimizing the use of vascular techniques at the time of surgery. Unlike PDAC, criteria for defining BR-pNETs and the optimal multidisciplinary treatment approach for these patients have not been established. Therefore, in this review, we introduce the concept of BR-pNETs and outline multidisciplinary treatment strategies that may optimize outcomes for this growing patient population. We present this article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-145/rc).
Methods
A literature search was conducted using PubMed, Google Scholar, and ClinicalTrials.gov for relevant articles up to October 28th, 2023. The following keywords were used: “pancreatic neuroendocrine tumor” OR “pNET” AND “locally advanced” OR “advanced” OR “borderline resectable” AND “neoadjuvant” OR “preoperative” AND “adjuvant” OR “postoperative” AND “therapy” OR “chemotherapy” OR “radiation” OR “systemic therapy”. Original articles and case reports were included. Only publications in English were considered (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | October 28th, 2023 |
| Databases and other sources searched | PubMed, Google Scholar, and ClinicalTrials.gov |
| Search terms used | Pancreatic neuroendocrine tumor, pNET, locally advanced, advanced, borderline resectable, neoadjuvant, preoperative, adjuvant, postoperative, therapy, chemotherapy, radiation, systemic therapy |
| Timeframe | Up to October 28th, 2023 |
| Inclusion criteria | All included studies were available in English with full text |
| Selection process | All authors contributed to the search and reviewed the selected literature |
pNET, pancreatic neuroendocrine tumor.
Definition of BR pNET
Determining the resectability of pNETs requires a multidisciplinary approach to thoroughly evaluate factors regarding the tumor and the patient. For PDAC, borderline resectability has been defined across anatomic (BR-A), biologic (BR-B), or condition (BR-C) domains. The pancreas has a close relationship with several critical vascular structures. As such, the anatomic relationship between the tumor and neighboring structures determines whether the tumor can be safely resected and if vascular reconstruction is necessary (14). Currently, the National Comprehensive Cancer Network (NCCN) defines BR-A criteria for PDAC as tumor contact with the inferior vena cava, tumor contact ≤180° with the superior mesenteric vein (SMV) or portal vein (PV) with contour irregularity or thrombosis, tumor contact >180° of the SMV or PV, tumor contact ≤180° with the superior mesenteric artery (SMA), head/uncinate tumor contact with common hepatic artery without extension to the celiac axis or hepatic artery bifurcation, or a body/tail tumor with ≤180° contact with the celiac axis (19,20). BR-B reflects whether resection of the tumor, regardless of technical feasibility, is clinically appropriate given the overall oncologic circumstances. This determination may be based on tumor markers, presence of indeterminate radiographic lesions, or other oncologic factors.
However, the difference in underlying tumor biology should be considered when comparing guidelines for PDAC and pNET. PDAC is an aggressive cancer with a 5-year survival of about 10–12%, while pNETs are slow growing and have a more indolent course with excellent long-term survival achieved by most (21). As such, it may be reasonable to consider more aggressive surgery in patients with pNETs and advanced disease. In addition, PDAC and pNET behave differently at the local level as well. While PDAC is often infiltrative, invading nearby structures with microscopic disease extending well beyond what is visible grossly, well-differentiated pNETs are usually well encapsulated and tend to behave as “pushers” rather than “invaders”. Still, achieving macroscopic complete resection can be challenging for locally advanced PNETs. Given the importance of margin-negative resection on long-term outcomes of patients with pNETs, strategies to facilitate surgical resection are critical.
The role of surgery for metastatic pNET remains debatable, but a large body of evidence suggests that complete surgical resection of neuroendocrine liver metastases, for example, is associated with improved long-term survival outcomes (22-25). Multiple societies recommend surgery for locally advanced and/or metastatic pNETs when disease is completely resectable, as it offers significant survival benefits (26-31). The European Neuroendocrine Tumor Society (ENETS) guideline describes the minimal requirements for resection with “curative intent” as resectable grade 1–2 liver disease with acceptable morbidity and <5% mortality, absence of right heart insufficiency, absence of unresectable lymph node and extra-abdominal metastases, and absence of diffuse peritoneal carcinomatosis (27). If clinically appropriate and technical feasible, complete resection should be considered for all advanced pNETs. Moreover, even when hepatic metastases cannot be fully removed, there is some evidence that aggressive surgical debulking can be beneficial (5).
It is crucial to carefully evaluate the recurrence rate following resection. Multiple risk factors are associated with recurrence, including pathologic factors (grade, Ki-67, size, perineural invasion, lymph node, metastasis), clinical factors (symptomatic tumors, gender, chromogranin A level, type of surgery), and molecular factors (high vimentin expression and loss of E-cadherin) (32,33). One study investigated the impact of tumor grade, lymph node status, and perineural invasion on the recurrence rate in 179 patients following curative pancreatic resection of grade 1–2 pNET >2 cm without metastasis (34). The study revealed a significant difference in 5-year recurrence-free survival (RFS), with 89.8% in low-risk group compared to 50.6% in high-risk patients.
These factors highlight possible criteria for defining BR-pNET disease: specifically, those in which the local extent of the primary tumor calls into question the feasibility of resection or the presence of metastatic disease calls into question the clinical utility of surgery. Conceptualizing these definitions would be important for standardizing the indications for multimodality treatment and aggressive surgical approaches in patients with pNETs.
Surgical resection
Vascular reconstruction
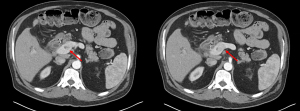
For locally advanced pNETs, a growing body of research suggests that aggressive vascular reconstruction should not be viewed as a contraindication to surgery (11,35-39). Studies demonstrate that vascular reconstruction can be done safely and confer a survival benefit in patients with both locally advanced and metastatic pNETs. In 2020, Titan et al. evaluated 99 patients who underwent resection for locally advanced T3/T4 pNET without distant metastasis (10). The entire study population demonstrated a 5-year disease-free survival (DFS) of 61% and 5-year overall survival (OS) of 91%. About 17% of these patients required vascular reconstruction, with most requiring resection of the SMV/PV confluence. On multivariable Cox regression, vascular reconstruction was not associated with increased risk of recurrence. Additionally, other studies have suggested that vascular involvement does not increase the risk of recurrence in pNETs (10,40). Norton et al. evaluated their population of 273 patients with pNETs and found that 46 (17%) had vascular involvement on preoperative imaging (37). At the time of surgery, only 9 (21%) of these patients required vascular reconstruction. This shows that vascular involvement on preoperative imaging does not always require vascular reconstruction in the operating room. Additionally, this study demonstrated that the DFS was not affected by the extent of vascular involvement or the need for vascular reconstruction. Other studies have reported similar findings that preoperative imaging may overestimate vascular involvement (10,37,38,41). Notably, pNETs can cause venous tumor thrombus in adjacent vascular structures, particularly the PV (Figure 1) (42). This characteristic resembles the tumor thrombus found in hepatocellular carcinoma (HCC) or renal cell carcinoma (RCC). Venous tumor thrombectomy, concomitant with pancreatectomy, has been shown to be a safe and effective method to resect the tumor without the need for segmental vein resection and reconstruction (43).
Multi-visceral resection
Some locally advanced pNETs can invade adjacent structures, for example the stomach, kidney, adrenal gland, and/or small bowel (24). Currently, the ENETS guidelines state that for localized pNETs >2 cm, aggressive surgery can include resection of nearby organs (stomach, colon, kidney, adrenal gland) and/or major vessel reconstruction (44-46). For pNETs that have invaded adjacent organs, concurrent resection of the primary and adjacent organ can be safely performed with low perioperative morbidity and mortality (47,48). In one study, 16 patients underwent en bloc resection of the primary pNET and adjacent organs requiring a gastrectomy, nephrectomy, adrenalectomy, and/or small bowel resection (11). There was no difference in 5-year OS between these patients and those who underwent resection of just the primary tumor without adjacent organ involvement. Hellman et al. evaluated 31 patients who underwent surgery for large pNETs (41). Of these patients, five had tumors infiltrating adjacent organs. Of these five patients, three required a subtotal or total gastrectomy and two required a colectomy. Patients had an acceptable 30-day morbidity and no 30-day mortality. Norton et al. evaluated 20 patients with advanced NETs of the pancreas and duodenum who underwent aggressive resection (36). Adjacent organ resection included nephrectomy, colectomy, and partial gastrectomy among these patients. Among the 20 patients, there were no post-operative deaths and six patients had post-operative complications. The mean hospital stay was 11.5 days, and 5-year OS was 80%. Based on these small studies, aggressive resection of the primary with adjacent organs can be safe and result in a survival benefit with 5-year OS rate around 80% when compared to unresected patients, who have a 5-year OS rate of approximately 45% (11,12,36).
Resection of liver metastases
Metastatic disease in patients with pNETs most commonly occurs in the liver and is generally associated with a worse prognosis (3,24). The goal of curative intent surgery is to treat all sites of disease. The NCCN guidelines recommend resection of both the primary tumor and metastases in appropriately selected patients with pNETs (31). Although resection of all disease is preferable, it is only feasible in about 10–25% of patients with pNETs metastatic to the liver (3). With the introduction of 68Ga-DOTATE positron emission tomography (PET) scans, assessment of metastatic disease in patients with pNETs has improved.
ENETS advocates surgical resection with curative intent as the gold standard for treating any neuroendocrine neoplasm with liver metastasis (27). The 5-year survival rate for surgical resection reaches 60–80%, compared to 30% if unresected, with low mortality (0–5%) and acceptable morbidity (~30%). Additionally, the European-African Hepato-Biliary Association (E-AHPBA) suggests that liver resection should be considered as the first choice for completely resectable grade 1 or 2 neuroendocrine liver metastasis, with or without resectable extrahepatic disease (28). The North American Neuroendocrine Tumor Society (NANETS) guideline recommends that hepatic resection should be considered for patients with NET if all hepatic metastases appear resectable (26). According to the Chinese Study Group for Neuroendocrine Tumors (CSNET), curative surgery is recommended for grade 1 or 2 pNET with single liver metastasis regardless of size (30). Curative surgery can also be performed in select patients with multifocal metastatic pNET as long as the hepatic metastases are confined to one lobe. The recurrence rate following fully resected pNET was found to be 26.5% at 3 years and 39.6% at 5 years, respectively (49).
There is some evidence that suggests liver debulking surgery in patients with unresectable metastasis can lead to long-term benefits. The NCCN guideline states that non-curative debulking surgery might be considered in select patients (31). Nevertheless, this remains an area of controversy and a consensus has not been reached (5,29,30). Previously, debulking of 90% of the tumor burden was thought to be the necessary threshold to see a survival benefit in patients with metastatic pNETs (50). More recently, Morgan et al. evaluated 42 patients with metastatic pNET and demonstrated that this threshold could be lowered to 70%. There was no significant difference in progression-free survival (PFS) or 5-year OS between patients who underwent debulking of 70%, 90%, or 100% of the existing tumor burden (51,52). Though precise indications have not been definitively established, debulking should be considered for patients with well-differentiated, grade 1 or 2, metastatic pNETs with <50% hepatic replacement, surgically amenable disease, adequate liver function, and no evidence of carcinoid heart disease or other major comorbidities (24).
The role of orthotopic liver transplantation for pNETs with unresectable liver metastases remains controversial. Following liver transplantation for pNET with liver metastases, the 5-year OS ranges between 44–53% (53). However, even after liver transplantation, the recurrence rate is around 31–57% (23,54). Additional research on liver transplantation is needed to determine the optimal selection criteria and long-term outcomes.
Intraoperative ablation of liver metastases
In pNETs with multifocal disease, resection can be combined with ablative procedures to extend the benefits of resection to more patients and/or to avoid extended hepatic resections (22). In one retrospective study, 30% of 669 patients undergoing liver resection for neuroendocrine liver metastases had concomitant intraoperative ablation (55). The study found that intraoperative ablation was not associated with higher 30-day morbidity in multivariate analysis. Mayo et al. further demonstrated the safety of intraoperative ablation for neuroendocrine liver metastases (56). Combination of ablation and resection was not associated with an increased risk of recurrence when compared to patients who had surgery alone.
Therapeutic options for advanced pNETs
A keen understanding of the systemic and locoregional treatment options is necessary for optimizing the multimodality therapeutic approaches to patients with BR-pNETs.
Somatostatin analogs (SSAs)
SSAs, including octreotide and lanreotide, are often considered the first-line treatment for metastatic pNETs (57). They inhibit hypersecretion in NETs that express somatostatin receptors (SSTRs) and help to control tumor growth and disease progression (58). The phase III CLARINET trial randomized 204 patients with well to moderately-differentiated, non-functioning gastroenteropancreatic NETs (45% were pNETs) to receive lanreotide or placebo (59). Patients included in this trial had not received chemotherapy in the previous 6 months, SSA at any time, or had major surgery for their NET within the previous 3 months. The authors found that lanreotide extended survival compared to placebo, with only mild side effects. At 24 months, the estimated PFS was 65.1% in the lanreotide group compared to 33.0% in the placebo group. There was no significant difference in overall survival. However, the analysis was complicated by crossover from the placebo group to lanreotide group and uncertainty in treatment after progression.
Cytotoxic chemotherapy in metastatic pNETs
In patients who have disease progression on SSA, cytotoxic chemotherapy can be considered. Historically, streptozotocin-based regimens were used showing objective response rates ranging from 20% to 45% in G1/G2 pNETs (3). For high-grade tumors, platinum-based regimens can be employed (23). In a study of advanced pancreatic neuroendocrine neoplasms with Ki-67 >20%, treatment with cisplatin or carboplatin plus etoposide resulted in an objective response rate was 30%, a median PFS of 5 months, and a median OS of 15 months (60). Patients with Ki-67 >55% had a higher objective response rate compared to those with Ki-67 <55% (42% versus 15%, respectively). Recently, capecitabine and temozolomide (CAPTEM) has demonstrated improved objective response rate (70%) and median PFS (18 months) in patients with G1/G2 pNETs (57). Currently, the NCCN guidelines recommend CAPTEM as one of the preferred systemic therapy options for disease progression (31).
Targeted therapy in metastatic pNETs
Currently, there are two approved targeted therapies for metastatic pNETs that can be considered for second-line therapy: sunitinib and everolimus (23). Sunitinib is a tyrosine kinase inhibitor (TKI) and everolimus is an mTOR inhibitor (24). A phase III trial randomized 171 patients with well-differentiated, advanced or metastatic pNET who were not surgical candidates to receive sunitinib or a placebo (61). They demonstrated an improved outcome in the sunitinib cohort (sunitinib: PFS 11.4 months versus placebo: 5.5 months). The trial was terminated early due to the risk of disease progression and death in the placebo cohort. The RADIANT-3 trial randomly assigned 410 patients with low or intermediate grade pNETs to receive everolimus or a placebo (62). They found that everolimus led to improved PFS (11 months) compared to the placebo cohort (4.6 months). In a phase II trial, bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, combined with everolimus was compared to everolimus alone in 150 patients with pNETs (63). The results showed that the combination of bevacizumab and everolimus compared to everolimus alone had an improved response rate (31% versus 12%) and PFS (16.7 versus 14.0 months) but no difference in overall survival. Although the combination therapy also increased the rate of treatment toxicities, the study highlighted the role of different molecular pathways in pNETs. Recently, the CABINET trial investigated the role of cabozantinib, a multi-kinase inhibitor, in locally advanced or metastatic pNET (64). Among 93 patients, the cabozantinib arm had a median PFS of 13.7 months, compared to 3.0 months for those who received a placebo.
Peptide receptor radioligand therapy (PRRT)
PRRT delivers radionuclides such as Yttrium-90 (90Y) or Lutetium-177 (177Lu) to tumors expressing SSTRs (65). It is generally well tolerated, but there is a risk of developing long-term toxicity, including severe renal toxicity in less than 5% and leukemia/myelodysplastic syndrome in approximately 2–3% of patients at a median of 2 years after therapy (66,67). In a phase II trial, 295 patients with non-functioning metastatic pNET received 90Y-DOTATOC (68). Within this cohort, 49.2% experienced a measurable decrease of tumor volume on imaging, 13.6% had decrease in tumor markers that demonstrated pre-therapeutic progression, and 28.8% reported symptomatic improvement.
In 2018, the U.S. Food and Drug Administration approved 177Lu-DOTATATE for the treatment of SSTR positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), primarily based on the success of the NETTER-1 trial. This study randomized 229 patients with well-differentiated, metastatic midgut NET to receive either 177Lu-DOTATATE or long-acting octreotide (69,70). They found that at 20 months, the estimated rate of PFS for 177Lu-DOTATATE group was 65.2%, compared to 10.8% in the octreotide group. The 177Lu-DOTATATE group also exhibited a higher objective response rate (18% versus 3% in the octreotide cohort) and preliminary evidence of improved OS. In a phase II trial, 84 patients with advanced pNET were randomized to receive PRRT or sunitinib. The PRRT arm showed an improved 12-month PFS rate of 80.5% compared to 41.9% in the sunitinib arm (71). The median PFS was 20.7 months versus 11 months for the PRRT versus sunitinib cohorts, respectively. A meta-analysis of 27 articles comparing 177Lu-DOTATATE with everolimus in advanced pNETs found evidence in favor of 177Lu-DOTATATE with an improved response rate (47% versus 12%, respectively), disease control rate (DCR) (81% versus 73%, respectively), and longer PFS (25.7 versus 14.7 months, respectively) (72). Currently, there are ongoing randomized clinical trials evaluating the efficacy of PRRT in GEP-NETs. The COMPETE trial is comparing PRRT to everolimus (NCT03049189) and the Alliance A022001 trial is comparing PRRT to CAPTEM (NCT05247905) (73).
Trans-arterial therapies
Hepatic arterial embolization is an effective therapy for patients with multifocal, bilobar liver metastases from pNETs (74). This approach takes advantage of the fact that hepatic metastases primarily derive their blood supply from the hepatic artery, while the liver parenchyma is predominantly supplied by the PV (75). Various embolization techniques are employed, including bland trans-arterial embolization (TAE), as well as in combination with other modalities such as chemotherapy in trans-arterial chemoembolization (TACE) and Yttrium-90 in trans-arterial radioembolization (TARE) (22). One retrosepective study compared the outcomes between TACE or TARE among 248 patients with unresectable GEP-NET in two institutions (76). The DCR on first post-treatment imaging [Response Evaluation Criteria in Solid Tumors (RECIST) partial/complete response or stable disease] was greater for TACE at 96%, compared to 83% in TARE. However, TACE or TARE showed comparable results in terms of long-term outcomes, including OS and PFS. Of note, TACE is typically performed with an overnight hospitalization, while TARE is an outpatient treatment but requires an additional mapping procedure. TACE can be further categorized: chemotherapy and lipidol (cTACE) or utilizing chemotherapy drug-eluting beads (DEB)-TACE (77). More recently, bland TAE has been used for patients with metastatic neuroendocrine tumors given the decrease in side effects, but likely equivalent long-term outcomes (78-80).
Mayo et al. evaluated 753 patients who underwent either surgery or TAE therapy for neuroendocrine liver metastases (81). The study found that while surgery resulted in a survival benefit over embolization for patients with low-volume (<25%) or symptomatic disease, there was no difference in long-term outcomes for asymptomatic patients with a large liver burden (>25%). After embolization, up to 90% of patients can experience postembolization syndrome, characterized by nausea, vomiting, abdominal pain, and fever (82). Serious complications, including biliary necrosis or liver abscess, can also occur (83). The RETNET trial (NCT02724540) is currently underway to compare TAE, cTACE, and DEB-TACE in order to determine the optimal trans-arterial strategy, with a primary endpoint of hepatic PFS (84). Notably, the DEB-TACE arm was terminated early due to severe complications (85).
Neoadjuvant therapy for pNETs
While neoadjuvant therapy is the standard of care for BR-PDAC, its role in the multidisciplinary management of pNET has not been extensively investigated (86). Nevertheless, there are some theoretical advantages to neoadjuvant therapy. First, it can downstage patients with unresectable or BR disease and facilitate subsequent resection. Second, certain patients with advanced pNETs are at high-risk for recurrence. Prioritizing early systemic therapy may decrease the risk of recurrence following subsequent surgery although data to support this hypothesis is lacking. In contrast, common justifications for the use of neoadjuvant therapy in PDAC do not necessarily apply to the management of pNET. For example, since all patients with PDAC benefit from systemic chemotherapy and yet many are unable to receive it following pancreatic surgery due to complications or prolonged recovery, neoadjuvant therapy ensures the receipt of systemic therapy. However, the use of adjuvant therapy has not been proven to be efficacious in patients with pNET. In addition, for patients with PDAC, neoadjuvant therapy affords a “test of time” allowing for aggressive disease biology to manifest prior to undergoing a high-risk operation. However, well-differentiated pNETs have different underlying biology than PDAC and rapid progression before or after surgery is uncommon.
Currently, there are no published randomized control trials on the use of neoadjuvant therapy for pNET, but there are studies that suggest a subset of patients with pNETs may benefit from neoadjuvant therapy (Table 2). A retrospective study of 67 patients with pNET and liver metastases who underwent R0/R1 resection at a single institution compared those who received preoperative 5-fluorouracil, doxorubicin, and streptozocin (FAS, n=27) and those who had upfront surgery (n=40) (88). The neoadjuvant therapy cohort was associated with higher rate of synchronous disease, lymph node metastases, and larger tumor size. There was no difference in OS or RFS between the two cohorts. However, on a sub-analysis of patients with synchronous liver metastases (n=46), those who received neoadjuvant therapy (n=26) had improved median OS and RFS compared to those who did not (OS 97.3 versus 65 months, RFS 24.8 versus 12.1 months, respectively). On pathologic review, there was a response seen in patients who had neoadjuvant therapy. This suggests that there may be a benefit of neoadjuvant therapy for high-risk patients with metastatic pNET. In the retrospective study by Ambe et al., six of their patients had BR-pNET (defined using the NCCN guidelines for PDAC) and received neoadjuvant CAPTEM (14). All patients had a radiographic response, and four of the six patients had a negative margin resection. At follow up (between 3–4.3 years), five of the six patients were still disease free. Squires et al. conducted a larger study which evaluated 30 patients with locally advanced or metastatic pNETs (89). The study demonstrated the effectiveness of neoadjuvant CAPTEM, revealing a partial radiologic response rate of 43%. Following CAPTEM treatment, 26 patients underwent surgical resections (87%), resulting in a median PFS of 28.2 months and a 5-year OS of 63%. Ostwal et al. studied patients with locally advanced or metastatic NET (47% had pNET) and evaluated the effectiveness of neoadjuvant CAPTEM or CAPTEM-PRRT (95). Both regimens demonstrated radiographic response, suggesting that neoadjuvant therapy may be able to downstage patients initially thought to be unresectable.
Table 2
| Author, reference | Year | Neoadjuvant therapy | Sample size | Surgery following neoadjuvant therapy |
|---|---|---|---|---|
| Prakash, (87) | 2017 | FAS | 29 | 14 |
| Cloyd, (88) | 2018 | FAS | 67 | 27 |
| Ambe, (14) | 2017 | CAPTEM | 112 | 6† |
| Squires, (89) | 2020 | CAPTEM | 30 | 26 |
| Murase, (90) | 2021 | Sunitinib | 106 | 31 |
| van Vliet, (91) | 2015 | PRRT | 29 | 9 |
| Partelli, (92) | 2018 | PRRT | 46 | 23 |
| Minczeles, (93) | 2022 | PRRT | 49 | 26 |
| Partelli, (94) (NCT04385992) | 2023 | PRRT | 31 | 29 |
†, 6 out of 23 patients who underwent resection following neoadjuvant therapy met the inclusion criteria for the study (National Comprehensive Cancer Network definition of borderline resectable pancreatic ductal adenocarcinoma and without metastasis). FAS, fluorouracil, doxorubicin, and streptozocin; CAPTEM, capecitabine and temozolomide; PRRT, peptide receptor radionuclide therapy.
Murase et al. evaluated the use of sunitinib as neoadjuvant therapy for patients with locally advanced or metastatic pNETs (90). They found that 29.2% of the patients were able to undergo an operation. Patients who underwent surgery following sunitinib showed improved 5-year OS compared to sunitinib alone (88.9% versus 14.1%, respectively). Additionally, undergoing surgical resection was associated with improved survival on multivariable regression analysis.
Given evidence that PRRT leads to both radiographic and histopathologic response, several studies have evaluated the role of neoadjuvant PRRT prior to surgery (91-94,96). Indeed, a recent literature review analyzed the outcomes of 11 studies on neoadjuvant PRRT, involving a total of 148 patients (65). This review found that studies show consistent macroscopic and microscopic changes in locally advanced pNETs following PRRT. Almost half of the patients (48.6%) were downstaged and eligible for surgery as a result of neoadjuvant PRRT. Partelli et al. studied 31 patients with G1/G2 BR pNETs (defined as tumors >4 cm with adjacent organ or vascular involvement and/or potentially resectable liver metastases) (92). This study found that those treated with neoadjuvant PRRT had increased PFS compared to those who had surgery alone (median PFS not reached versus 36 months, respectively). Minczeles et al. evaluated 49 patients with pNET that had arterial abutment, venous involvement, and/or metastatic disease (≤3 liver metastases) (93). The study found that following PRRT 53% were able to undergo pancreatic surgery with curative intent. Additionally, 28% of patients had improvement in the tumor-vessel interface. In the PRRT induction group, the median OS was 14.7 years and median PFS was 5.3 years compared to 5.5 and 3.0 years, respectively, in the PRRT alone group. A recent meta-analysis of nine studies comparing different neoadjuvant therapies [chemotherapy, PRRT/peptide receptor chemoradionuclide therapy (PRCRT), sunitinib] found no statistically significant advantage of one therapy over another (9). Further research is required to identify the optimal neoadjuvant therapy and appropriate subset of patients that will yield the best outcomes.
Postoperative management and adjuvant therapy
Given the high recurrence for patients with pNETs, routine surveillance is recommended after curative-intent resection for pNETs. Studies have found that the 5-year RFS following resection ranges from less than 40% to greater than 90% depending on identified risk factors (34,97). Risk factors for recurrence can include a positive resection margin, higher tumor grade, positive lymph nodes, lymphovascular invasion, perineural invasion, and larger tumor size as well as clinical and molecular features (32,33,98-100). For instance, studies have shown that significant elevation in the pre-operative chromogranin A level, a commonly used tumor marker for neuroendocrine tumors, was a predictive factor for tumor recurrence (97,101). Pulvirenti et al. developed an externally validated nomogram (c-index of 0.84) to estimate the probability of RFS at 5 years for grade 1 or 2 pNET using four variables: number of positive nodes, tumor diameter, Ki-67 score, and vascular/perineural invasion (102).
Currently, there is no indication for adjuvant therapy following resection of all disease in patients with well-differentiated pNETs based on NCCN guidelines (31,103,104). In one retrospective study, the role of adjuvant radiotherapy (RT) was investigated in patients with pNET who had positive or close (<1 mm) margin following resection (105). Local recurrence rates were similar for patients with low-risk tumor features and no adjuvant therapy to those with high-risk features who underwent adjuvant therapy. The finding suggests that adjuvant RT can enhance local control for high-risk tumors. In a retrospective study of 52 patients with well-differentiated metastatic GEP-NET who underwent surgery, adjuvant chemotherapy (streptozotocin and 5-fluorouracil) was given to patients with pNET, >10 liver metastases, or patients <50 years old (106). Adjuvant chemotherapy did not improve RFS. The ongoing SWOG S2104 trial (NCT05040360) aims to compare RFS in patients with high-risk pNETs who either receive adjuvant CAPTEM or undergo observation after resection (107). Eligible patients underwent resection of well-differentiated grade 2 or 3 pNETs with a Zaidi score of ≥3. The Zaidi score, a predictive tool for recurrence developed by the U.S. Neuroendocrine Tumor Study Group, incorporates factors such as symptomatic tumor, tumor size, positive lymph nodes, and Ki-67.
Conclusions
Well-differentiated pNETs are relatively rare neoplasms with heterogeneous presentations. While many are small, indolent, and incidentally discovered, other pNETs can present as large bulky tumors or with limited oligometastatic disease. While formal definitions of BR-pNET are lacking, we suggest that certain criteria for defining BR-pNET disease are adopted: specifically, those in which the local extent of the primary tumor calls into question the feasibility of resection or the presence of metastatic disease calls into question the clinical utility of surgery. Formalizing these definitions through multidisciplinary collaborative research will be important for standardizing the indications for multimodality treatment and aggressive surgical approaches for patients with pNETs.
Particularly in experienced institutions, resections involving vascular reconstruction, adjacent organ resection, and/or liver metastasis should be considered as viable treatment options for patients with pNETs (108,109). Retrospective studies have consistently demonstrated that the benefits of surgical resection are extended to those with more advanced disease despite more complex operations. Given the lack of strong evidence supporting the use of neoadjuvant therapy for BR-pNETs, upfront surgery should generally be recommended when feasible. The use of adjuvant therapies has not been proven for high-risk pNETs and thus require further investigation. Given the complex surgical approaches and access to multimodality treatments, the management of patients with BR-pNETs may be best approached at high-volume multidisciplinary centers of excellence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-145/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-145/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-145/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. There were no clinical procedures performed for this narrative review; any images obtained are de-identified and do not require consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol 2015;21:9512-25. [Crossref] [PubMed]
- Cloyd JM, Poultsides GA. The Landmark Series: Pancreatic Neuroendocrine Tumors. Ann Surg Oncol 2021;28:1039-49. [Crossref] [PubMed]
- Nigri G, Petrucciani N, Debs T, et al. Treatment options for PNET liver metastases: a systematic review. World J Surg Oncol 2018;16:142. [Crossref] [PubMed]
- Stensbøl AB, Krogh J, Holmager P, et al. Incidence, Clinical Presentation and Trends in Indication for Diagnostic Work-Up of Small Intestinal and Pancreatic Neuroendocrine Tumors. Diagnostics (Basel) 2021;11:2030. [Crossref] [PubMed]
- Howe JR, Merchant NB, Conrad C, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:1-33. [Crossref] [PubMed]
- Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 2018;31:1770-86. [Crossref] [PubMed]
- Muttillo EM, Mazzarella G, Picardi B, et al. Treatment strategies for neuroendocrine liver metastases: a systematic review. HPB (Oxford) 2022;24:1832-43. [Crossref] [PubMed]
- Hill JS, McPhee JT, McDade TP, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer 2009;115:741-51. [Crossref] [PubMed]
- Li Y, Fan Z, Zhang F, et al. Neoadjuvant therapy in pancreatic neuroendocrine neoplasms: A systematic review and meta-analysis. Front Oncol 2022;12:981575. [Crossref] [PubMed]
- Titan AL, Norton JA, Fisher AT, et al. Evaluation of Outcomes Following Surgery for Locally Advanced Pancreatic Neuroendocrine Tumors. JAMA Netw Open 2020;3:e2024318. [Crossref] [PubMed]
- Birnbaum DJ, Turrini O, Vigano L, et al. Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Ann Surg Oncol 2015;22:1000-7. [Crossref] [PubMed]
- Solorzano CC, Lee JE, Pisters PW, et al. Nonfunctioning islet cell carcinoma of the pancreas: survival results in a contemporary series of 163 patients. Surgery 2001;130:1078-85. [Crossref] [PubMed]
- Dumlu EG, Karakoç D, Özdemir A. Nonfunctional Pancreatic Neuroendocrine Tumors: Advances in Diagnosis, Management, and Controversies. Int Surg 2015;100:1089-97. [Crossref] [PubMed]
- Ambe CM, Nguyen P, Centeno BA, et al. Multimodality Management of “Borderline Resectable” Pancreatic Neuroendocrine Tumors: Report of a Single-Institution Experience. Cancer Control 2017;24:1073274817729076. [Crossref] [PubMed]
- Kulkarni R, Kabir I, Hodson J, et al. Impact of the extent of resection of neuroendocrine tumor liver metastases on survival: A systematic review and meta-analysis. Ann Hepatobiliary Pancreat Surg 2022;26:31-9. [Crossref] [PubMed]
- Frilling A, Li J, Malamutmann E, et al. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg 2009;96:175-84. [Crossref] [PubMed]
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37. [Crossref] [PubMed]
- Schurr PG, Strate T, Rese K, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg 2007;245:273-81. [Crossref] [PubMed]
- Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol 2014;20:10740-51. [Crossref] [PubMed]
- Malafa MP. Defining borderline resectable pancreatic cancer: emerging consensus for an old challenge. J Natl Compr Canc Netw 2015;13:501-4. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Cloyd JM, Wiseman JT, Pawlik TM. Surgical management of pancreatic neuroendocrine liver metastases. J Gastrointest Oncol 2020;11:590-600. [Crossref] [PubMed]
- Cloyd JM, Ejaz A, Konda B, et al. Neuroendocrine liver metastases: a contemporary review of treatment strategies. Hepatobiliary Surg Nutr 2020;9:440-51. [Crossref] [PubMed]
- Scott AT, Howe JR. Evaluation and Management of Neuroendocrine Tumors of the Pancreas. Surg Clin North Am 2019;99:793-814. [Crossref] [PubMed]
- Russo A, Gangi A. The Evolving Landscape of Neuroendocrine Tumors. Surg Oncol Clin N Am 2023;32:185-98. [Crossref] [PubMed]
- Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013;42:557-77. [Crossref] [PubMed]
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76. [Crossref] [PubMed]
- Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8-21. [Crossref] [PubMed]
- Singh S, Dey C, Kennecke H, et al. Consensus Recommendations for the Diagnosis and Management of Pancreatic Neuroendocrine Tumors: Guidelines from a Canadian National Expert Group. Ann Surg Oncol 2015;22:2685-99. [Crossref] [PubMed]
- Jin K, Xu J, Chen J, et al. Surgical management for non-functional pancreatic neuroendocrine neoplasms with synchronous liver metastasis: A consensus from the Chinese Study Group for Neuroendocrine Tumors (CSNET). Int J Oncol 2016;49:1991-2000. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Neuroendorine and Adrenal Tumors (Version 1.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Kos-Kudła B, Castaño JP, Denecke T, et al. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J Neuroendocrinol 2023;35:e13343. [Crossref] [PubMed]
- Zhou B, Xiang J, Jin M, et al. High vimentin expression with E-cadherin expression loss predicts a poor prognosis after resection of grade 1 and 2 pancreatic neuroendocrine tumors. BMC Cancer 2021;21:334. [Crossref] [PubMed]
- Heidsma CM, van Roessel S, van Dieren S, et al. International Validation of a Nomogram to Predict Recurrence after Resection of Grade 1 and 2 Nonfunctioning Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2022;112:571-9. [Crossref] [PubMed]
- Deguelte S, de Mestier L, Hentic O, et al. Sporadic pancreatic neuroendocrine tumor: Surgery of the primary tumor. J Visc Surg 2018;155:483-92. [Crossref] [PubMed]
- Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg 2003;138:859-66. [Crossref] [PubMed]
- Norton JA, Harris EJ, Chen Y, et al. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg 2011;146:724-32. [Crossref] [PubMed]
- Li AY, Visser BC, Dua MM. Surgical Indications and Outcomes of Resection for Pancreatic Neuroendocrine Tumors with Vascular Involvement. Cancers (Basel) 2022;14:2312. [Crossref] [PubMed]
- Gudmundsdottir H, Tomlinson JL, Graham RP, et al. Outcomes of pancreatectomy with portomesenteric venous resection and reconstruction for locally advanced pancreatic neuroendocrine neoplasms. HPB (Oxford) 2022;24:1186-93. [Crossref] [PubMed]
- Dong DH, Zhang XF, Lopez-Aguiar AG, et al. Tumor burden score predicts tumor recurrence of non-functional pancreatic neuroendocrine tumors after curative resection. HPB (Oxford) 2020;22:1149-57. [Crossref] [PubMed]
- Hellman P, Andersson M, Rastad J, et al. Surgical strategy for large or malignant endocrine pancreatic tumors. World J Surg 2000;24:1353-60. [Crossref] [PubMed]
- Balachandran A, Tamm EP, Bhosale PR, et al. Venous tumor thrombus in nonfunctional pancreatic neuroendocrine tumors. AJR Am J Roentgenol 2012;199:602-8. [Crossref] [PubMed]
- Prakash L, Lee JE, Yao J, et al. Role and Operative Technique of Portal Venous Tumor Thrombectomy in Patients with Pancreatic Neuroendocrine Tumors. J Gastrointest Surg 2015;19:2011-8. [Crossref] [PubMed]
- Falconi M, Plockinger U, Kwekkeboom DJ, et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology 2006;84:196-211. [Crossref] [PubMed]
- Gundara JS, Alvarado-Bachmann R, Williams N, et al. Multivisceral resection of pancreatic neuroendocrine tumours: a report of two cases. World J Surg Oncol 2011;9:93. [Crossref] [PubMed]
- Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016;103:153-71. [Crossref] [PubMed]
- Teh SH, Deveney C, Sheppard BC. Aggressive pancreatic resection for primary pancreatic neuroendocrine tumor: is it justifiable? Am J Surg 2007;193:610-3; discussion 613. [Crossref] [PubMed]
- Abu Hilal M, McPhail MJ, Zeidan BA, et al. Aggressive multi-visceral pancreatic resections for locally advanced neuroendocrine tumours. Is it worth it? JOP 2009;10:276-9. [PubMed]
- Singh S, Chan DL, Moody L, et al. Recurrence in Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol 2018;4:583-5. [Crossref] [PubMed]
- Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin N Am 2003;12:231-42. [Crossref] [PubMed]
- Morgan RE, Pommier SJ, Pommier RF. Expanded criteria for debulking of liver metastasis also apply to pancreatic neuroendocrine tumors. Surgery 2018;163:218-25. [Crossref] [PubMed]
- Keutgen XM, Schadde E, Pommier RF, et al. Metastatic neuroendocrine tumors of the gastrointestinal tract and pancreas: A surgeon’s plea to centering attention on the liver. Semin Oncol 2018;45:232-5. [Crossref] [PubMed]
- Kim J, Zimmerman MA, Hong JC. Liver transplantation in the treatment of unresectable hepatic metastasis from neuroendocrine tumors. J Gastrointest Oncol 2020;11:601-8. [Crossref] [PubMed]
- Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery 2017;162:525-36. [Crossref] [PubMed]
- Scoville SD, Xourafas D, Ejaz AM, et al. Contemporary indications for and outcomes of hepatic resection for neuroendocrine liver metastases. World J Gastrointest Surg 2020;12:159-70. [Crossref] [PubMed]
- Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129-36. [Crossref] [PubMed]
- Marchese U, Gaillard M, Pellat A, et al. Multimodal Management of Grade 1 and 2 Pancreatic Neuroendocrine Tumors. Cancers (Basel) 2022;14:433. [Crossref] [PubMed]
- Oberg K, Kvols L, Caplin M, et al. Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 2004;15:966-73. [Crossref] [PubMed]
- Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371:224-33. [Crossref] [PubMed]
- Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 2013;24:152-60. [Crossref] [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [Crossref] [PubMed]
- Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [Crossref] [PubMed]
- Kulke MH, Ou FS, Niedzwiecki D, et al. Everolimus with or without bevacizumab in advanced pNET: CALGB 80701 (Alliance). Endocr Relat Cancer 2022;29:335-44. [Crossref] [PubMed]
- Chan J, Geyer S, Ou FS, et al. LBA53 Alliance A021602: Phase III, double-blinded study of cabozantinib versus placebo for advanced neuroendocrine tumors (NET) after progression on prior therapy (CABINET). Ann Oncol 2023;34:S1292. [Crossref]
- Urso L, Nieri A, Rambaldi I, et al. Radioligand therapy (RLT) as neoadjuvant treatment for inoperable pancreatic neuroendocrine tumors: a literature review. Endocrine 2022;78:255-61. [Crossref] [PubMed]
- Hirmas N, Jadaan R, Al-Ibraheem A. Peptide Receptor Radionuclide Therapy and the Treatment of Gastroentero-pancreatic Neuroendocrine Tumors: Current Findings and Future Perspectives. Nucl Med Mol Imaging 2018;52:190-9. [Crossref] [PubMed]
- Hope TA, Abbott A, Colucci K, et al. NANETS/SNMMI Procedure Standard for Somatostatin Receptor-Based Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE. J Nucl Med 2019;60:937-43. [Crossref] [PubMed]
- Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011;29:2416-23. [Crossref] [PubMed]
- Cives M, Strosberg J. Treatment Strategies for Metastatic Neuroendocrine Tumors of the Gastrointestinal Tract. Curr Treat Options Oncol 2017;18:14. [Crossref] [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Baudin E, Walter T, Docao C, et al. First multicentric randomized phase II trial investigating the antitumor efficacy of peptide receptor radionuclide therapy with 177Lutetium – Octreotate (OCLU) in unresectable progressive neuroendocrine pancreatic tumor: Results of the OCLURANDOM trial, on behalf of the ENDOCAN RENATEN network and GTE. Annales d'Endocrinologie 2022;83:289-90. [Crossref]
- Satapathy S, Mittal BR. 177Lu-DOTATATE peptide receptor radionuclide therapy versus Everolimus in advanced pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Nucl Med Commun 2019;40:1195-203. [Crossref] [PubMed]
- Howe JR. Sequencing of Therapies in Progressive Neuroendocrine Tumors. Ann Surg Oncol 2022;29:6501-3. [Crossref] [PubMed]
- Kennedy A, Bester L, Salem R, et al. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford) 2015;17:29-37. [Crossref] [PubMed]
- Christante D, Pommier S, Givi B, et al. Hepatic artery chemoinfusion with chemoembolization for neuroendocrine cancer with progressive hepatic metastases despite octreotide therapy. Surgery 2008;144:885-93; discussion 893-4. [Crossref] [PubMed]
- Egger ME, Armstrong E, Martin RC 2nd, et al. Transarterial Chemoembolization vs Radioembolization for Neuroendocrine Liver Metastases: A Multi-Institutional Analysis. J Am Coll Surg 2020;230:363-70. [Crossref] [PubMed]
- Makary MS, Kapke J, Yildiz V, et al. Conventional versus Drug-Eluting Bead Transarterial Chemoembolization for Neuroendocrine Tumor Liver Metastases. J Vasc Interv Radiol 2016;27:1298-304. [Crossref] [PubMed]
- Fiore F, Del Prete M, Franco R, et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine 2014;47:177-82. [Crossref] [PubMed]
- Del Prete M, Fiore F, Modica R, et al. Hepatic arterial embolization in patients with neuroendocrine tumors. J Exp Clin Cancer Res 2014;33:43. [Crossref] [PubMed]
- Kanabar R, Barriuso J, McNamara MG, et al. Liver Embolisation for Patients with Neuroendocrine Neoplasms: Systematic Review. Neuroendocrinology 2021;111:354-69. [Crossref] [PubMed]
- Mayo SC, de Jong MC, Bloomston M, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol 2011;18:3657-65. [Crossref] [PubMed]
- Leung DA, Goin JE, Sickles C, et al. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol 2001;12:321-6. [Crossref] [PubMed]
- Bloomston M, Al-Saif O, Klemanski D, et al. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: lessons learned. J Gastrointest Surg 2007;11:264-71. [Crossref] [PubMed]
- Chen JX, Wileyto EP, Soulen MC. Randomized Embolization Trial for NeuroEndocrine Tumor Metastases to the Liver (RETNET): study protocol for a randomized controlled trial. Trials 2018;19:390. [Crossref] [PubMed]
- Soulen M, White S, Fidelman N, et al. 03:27 PM Abstract No. 105 Randomized Embolization Trial for NeuroEndocrine Tumors (RETNET): first safety report. J Vasc Interv Radiol 2019;30:S49-50. [Crossref]
- Springfeld C, Ferrone CR, Katz MHG, et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol 2023;20:318-37. [Crossref] [PubMed]
- Prakash L, Bhosale P, Cloyd J, et al. Role of Fluorouracil, Doxorubicin, and Streptozocin Therapy in the Preoperative Treatment of Localized Pancreatic Neuroendocrine Tumors. J Gastrointest Surg 2017;21:155-63. [Crossref] [PubMed]
- Cloyd JM, Omichi K, Mizuno T, et al. Preoperative Fluorouracil, Doxorubicin, and Streptozocin for the Treatment of Pancreatic Neuroendocrine Liver Metastases. Ann Surg Oncol 2018;25:1709-15. [Crossref] [PubMed]
- Squires MH, Worth PJ, Konda B, et al. Neoadjuvant Capecitabine/Temozolomide for Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:355-60. [Crossref] [PubMed]
- Murase Y, Kudo A, Akahoshi K, et al. Surgery after sunitinib administration to improve survival of patients with advanced pancreatic neuroendocrine neoplasms. Ann Gastroenterol Surg 2021;5:692-700. [Crossref] [PubMed]
- van Vliet EI, van Eijck CH, de Krijger RR, et al. Neoadjuvant Treatment of Nonfunctioning Pancreatic Neuroendocrine Tumors with [177Lu-DOTA0,Tyr3]Octreotate. J Nucl Med 2015;56:1647-53. [Crossref] [PubMed]
- Partelli S, Bertani E, Bartolomei M, et al. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery 2018;163:761-7. [Crossref] [PubMed]
- Minczeles NS, van Eijck CHJ, van Gils MJ, et al. Induction therapy with (177)Lu-DOTATATE procures long-term survival in locally advanced or oligometastatic pancreatic neuroendocrine neoplasm patients. Eur J Nucl Med Mol Imaging 2022;49:3203-14. [Crossref] [PubMed]
- Partelli S, Landoni L, Bartolomei M, et al. 1186MO A prospective phase II single-arm trial on neoadjuvant peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE followed by surgery for pancreatic neuroendocrine tumors (NeoLuPaNET). Ann Oncol 2023;34:S703. [Crossref]
- Ostwal V, Basu S, Bhargava P, et al. Capecitabine-Temozolomide in Advanced Grade 2 and Grade 3 Neuroendocrine Neoplasms: Benefits of Chemotherapy in Neuroendocrine Neoplasms with Significant 18FDG Uptake. Neuroendocrinology 2021;111:998-1004. [Crossref] [PubMed]
- Schiavo Lena M, Partelli S, Castelli P, et al. Histopathological and Immunophenotypic Changes of Pancreatic Neuroendocrine Tumors after Neoadjuvant Peptide Receptor Radionuclide Therapy (PRRT). Endocr Pathol 2020;31:119-31. [Crossref] [PubMed]
- Fisher AV, Lopez-Aguiar AG, Rendell VR, et al. Predictive Value of Chromogranin A and a Pre-Operative Risk Score to Predict Recurrence After Resection of Pancreatic Neuroendocrine Tumors. J Gastrointest Surg 2019;23:651-8. [Crossref] [PubMed]
- Kwon W, Jang JY, Song KB, et al. Risk Factors for Recurrence in Pancreatic Neuroendocrine Tumor and Size as a Surrogate in Determining the Treatment Strategy: A Korean Nationwide Study. Neuroendocrinology 2021;111:794-804. [Crossref] [PubMed]
- Li Y, Fan G, Yu F, et al. Meta-Analysis of Prognostic Factors for Recurrence of Resected Well-Differentiated Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2021;111:1231-7. [Crossref] [PubMed]
- Yamamoto Y, Okamura Y, Uemura S, et al. Vascularity and Tumor Size are Significant Predictors for Recurrence after Resection of a Pancreatic Neuroendocrine Tumor. Ann Surg Oncol 2017;24:2363-70. [Crossref] [PubMed]
- Nanno Y, Toyama H, Matsumoto I, et al. Baseline plasma chromogranin A levels in patients with well-differentiated neuroendocrine tumors of the pancreas: A potential predictor of postoperative recurrence. Pancreatology 2017;17:291-4. [Crossref] [PubMed]
- Pulvirenti A, Javed AA, Landoni L, et al. Multi-institutional Development and External Validation of a Nomogram to Predict Recurrence After Curative Resection of Pancreatic Neuroendocrine Tumors. Ann Surg 2021;274:1051-7. [Crossref] [PubMed]
- Andreasi V, Muffatti F, Guarneri G, et al. Surgical Principles in the Management of Pancreatic Neuroendocrine Neoplasms. Curr Treat Options Oncol 2020;21:48. [Crossref] [PubMed]
- Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:844-60. [Crossref] [PubMed]
- Arvold ND, Willett CG, Fernandez-del Castillo C, et al. Pancreatic neuroendocrine tumors with involved surgical margins: prognostic factors and the role of adjuvant radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:e337-43. [Crossref] [PubMed]
- Maire F, Hammel P, Kianmanesh R, et al. Is adjuvant therapy with streptozotocin and 5-fluorouracil useful after resection of liver metastases from digestive endocrine tumors? Surgery 2009;145:69-75. [Crossref] [PubMed]
- Soares HP, Guthrie KA, Ahmad SA, et al. Randomized phase II trial of postoperative adjuvant capecitabine and temozolomide versus observation in high-risk pancreatic neuroendocrine tumors: SWOG S2104. J Clin Oncol 2022;40:TPS515. [Crossref]
- Patel RK, Sutton TL, Schwantes IR, et al. Care at high-volume centers is associated with improved outcomes for patients with pancreatic neuroendocrine tumors: A population-level analysis. J Surg Oncol 2023;127:956-65. [Crossref] [PubMed]
- Baeg K, Harris C, Naparst MS, et al. Effect of treatment center volume on outcomes in gastroenteropancreatic neuroendocrine tumor patients. BMC Cancer 2021;21:146. [Crossref] [PubMed]


