
The challenging surgical management of hepatic epithelioid hemangioendothelioma: a narrative review
Introduction
Epithelioid hemangioendothelioma (EHE) is an uncommon vascular endothelial cell cancer composed of epithelioid and histiocyte-like vascular endothelial cells. EHE is now classified by the World Health Organization as a tumoral condition with the full potential of malignancy (1). EHE can occur in multiple body parts: soft tissue, neck, head, pleura, lungs, and bones. Hepatic EHE (HEHE) represents an even less commonly observed cancer involving the liver, with 1–2 cases reported every 1 million people (2). HEHE is predominant in females (M:F ratio 2:3) and typically occurs between 30 and 50 years (3). HEHE can be single, multiple, or diffuse. In many cases, the tumor is multifocal at the time of diagnosis.
HEHE represents a low-to-medium grade cancer (4). The HEHE malignancy degree is between hemangioma and hemangiosarcoma (5). In this tumor, epithelioid and dendritic-like cells infiltrate the hepatic sinuses (6). In a more advanced stage, hepatic/portal veins infiltration and distal metastases are often observed. Common sites of extrahepatic disease are bones, lymph nodes, spleen, lungs, and peritoneum (7). No specific clinical manifestations are present for this pathology (8). HEHE is very often initially misdiagnosed due to atypical clinical manifestations (3), being diagnosed typically only after pathological examination. Moreover, the HEHE radiological behavior is nonspecific. Differential diagnosis must be achieved from multifocal metastases, multifocal hepatocarcinoma, peripheral cholangiocarcinoma, abscess, or cavernous hemangioma. At second-level imaging, the main HEHE features are (I) the peripheral location of the lesions, (II) the merging aspect of the multifocal lesions, and (III) the capsule retraction (9,10).
However, capsule contraction has been observed only in lesions >2 cm (9). The etiology of HEHE is not fully clear. Different risk factors have been proposed, like the use of contraceptives, primary biliary cholangitis, alcoholic abuse, the presence of viral hepatitis, or the exposure to toxic substances like asbestos, polyurethane, chloroethylene, and silica (7). A specific genetic mutation, namely the translocation t(1/3)(P36/25), has been observed as a specific mutation in HEHE. However, it has not been fully elucidated how these fusion transcripts lead to tumorigenesis (11).
The most common signs and symptoms observed in HEHE patients are right upper abdominal pain (48.6%), hepatomegaly (20.4%), and anorexia (15.6%). Uncommonly, Kasabakh-Merritt syndrome and brucellosis have been described (12). Liver-specific blood tests and tumor markers are generally routine (3,13). We present the following article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-21-139/rc).
Methods
A review of the literature has been done with the intent to focus on the relevant studies exploring the diagnosis and surgical treatment of HEHE. Particular attention has been done on the role of the different surgical therapies. A search of these studies has been done using the electronic database MEDLINE-PubMed (Table 1). The identified studies are summarized on Table 2.
Table 1
| Items | Specification |
|---|---|
| Date of search | 13-05-2022 |
| Databases and other sources searched | PubMed |
| Search terms used | (Liver OR hepatic) AND (hemangioendothelioma OR haemangioendothelioma OR HEHE) |
| Timeframe | 2010–2022 |
| Inclusion and exclusion criteria | Inclusion criteria: study type = original article; language = English |
| Selection process | Authors independently performing the selection: F Giovanardi and Q Lai |
Table 2
| Author | Year | N | Diagnostic methods | Tumor size, mean, cm | Metastasis | Treatment | Recurrence rate, % | Survival, % |
|---|---|---|---|---|---|---|---|---|
| Thin et al. (14) | 2010 | 1 | Liver biopsy | NA | Intrahepatic metastasis | LT | 0 | 100 (5-year) |
| Grotz et al. (15) | 2010 | 22 | Liver biopsy | NA | Lung, peritoneum, bone, brain and skin | LR or LT | 40 | 62 (LR, 5-year), 46 (LT, 5-year) |
| Wang et al. (16) | 2012 | 21 | Surgical specimens | NA | Lung metastases, diaphragm/abdominal-wall metastases | LR, LR followed by TACE, or LT | NA | 74 (LR, 3-year), 33 (LR followed by TACE, 3-year), 0 (LT, 3-year) |
| Thomas et al. (17) | 2014 | 7 | Liver biopsy, diagnostic laparoscopy, surgical specimens | 3.6 | Lung | Hepatectomy or LT | 43 | 83 (5-year) |
| Theodosopoulos et al. (18) | 2013 | 5 | Liver biopsy | 4 | Intrahepatic metastasis | Surgical resection with a non-formal hepatectomy or wedge resection | 40 | 60 (2-year) |
| Orlando et al. (19) | 2013 | 108 | Percutaneous needle, surgical, or combined biopsies | NA | Osseous and peritoneal localizations | LT | NA | 72 (5-year) |
| Groeschl et al. (20) | 2014 | 12 | Liver biopsy, surgical specimens | NA | NA | Segmental resection, lobectomy/extended resection, LT | NA | 57 (LR, 1-year), 80 (LT, 1-year) |
| Remiszewski et al. (21) | 2014 | 10 | Liver biopsy | 5.1 | Lymph node metastases | LT | 0 | 90 (5-year) |
| Lin et al. (22) | 2015 | 1 | Surgical specimens | NA | No | LT | 0 | 100 (5-year) |
| Sundar Alagusundaramoorthy et al. (23) | 2015 | 11 | Liver biopsy | NA | NA | LT | NA | 79 (5-year) |
| Dong et al. (24) | 2015 | 3 | Liver biopsy | NA | NA | LR and RFA, or LT | 0 | 100 (3-year) |
| Jung et al. (25) | 2016 | 6 | Liver biopsy | NA | NA | LR or LT | 17 | 83 (5-year) |
| Abdoh et al. (26) | 2016 | 1 | Liver biopsy | NA | Intrahepatic metastasis | LT | 100 | 0 (1-year) |
| Samuk et al. (27) | 2016 | 1 | Liver biopsy | NA | Lung | LT | 0 | 100 (5-year) |
| Lai et al. (28) | 2017 | 149 | Percutaneous and/or surgical biopsy | NA | Lung, breast | LT and adjuvant therapy | 25 | 81 (5-year) |
| Konstantinidis et al. (29) | 2018 | 67 | NA | 14.8 | NA | LR or LT | NA | 83 (5-year) |
| Wang et al. (30) | 2018 | 1 | Liver biopsy | 4.7 | No | LR + chemotherapy | 0 | 100 (15-year) |
| Noh et al. (31) | 2020 | 19 | Liver biopsy | 3.5 | NA | LR, LR + chemotherapy, LT + radiation therapy, LT + chemotherapy | NA | 88 (5-year) |
| Sanduzzi-Zamparelli et al. (32) | 2020 | 11 | Surgical specimens, needle biopsy or “wedge-biopsy” | NA | Lung, lymph node metastases | LR or LT | 36 | 100 (5-year) |
| Krasnodębski et al. (33) | 2020 | 18 | Liver biopsy | NA | Hilar lymph nodes | LT | 0 | 41 (5-year) |
HEHE, hepatic epithelioid hemangioendothelioma; N, number; %, percentage; NA, not available, LT, liver transplantation; LR, liver resection; TACE, trans-arterial chemoembolization.
Diagnosis
The radiological diagnosis of HEHE is often challenging. Using magnetic resonance imaging (MRI), the most common radiological aspect of the tumor is observed in T2-weighted images, with peripherally distributed lesions with target appearance (34). Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MRI should also be functional (35). After positron emission tomography (PET), fluorodeoxyglucose (FDG) uptake was reported in only two-thirds of the HEHE cases (36).
Histopathology remains the best way to diagnose this tumor (37). Fine-needle aspiration or biopsy followed by immunohistochemical staining represent the best methods for the diagnosis (38). However, a false negative rate of 10% has been observed after the biopsy (39). Differential diagnosis must be made with focal nodular hyperplasia or hemangiosarcoma (40). At immunohistochemistry, positivity for CD31, CD34, or Factor VIII-related antigen are typical. Recently, an attempt to also identify a miRNA expression spectrum has been made to improve the diagnostic ability (41).
Liver resection
Due to its rarity, HEHE does not represent a good target for randomized controlled trials with multiple treatment strategies. Currently, several therapeutical strategies are used to manage HEHE, namely hepatic resection and liver transplantation (LT), radiotherapy/chemotherapy, anti-angiogenic drugs, locoregional radiological therapies, and the observation-and-waiting approach (42).
Using hepatic resection vs. LT represents a topic of debate, with some controversies existing due to the challenging possibility to compare these two strategies in rare cancer.
Liver resection may be a curative strategy in well-selected cases in which the lesion is single or the disease is oligonodular and monolobar. However, the HEHE diagnosis is often made when an extensive involvement of the liver is reported (43). Therefore, radical hepatic resection should relate to relevant comorbidities, like post-resection liver failure. On the opposite, a conservative resection should not eradicate cancer, thus being connected with local recurrence or distant metastases (5).
Recently, the segmental hepatic resection approach has been implemented due to improved knowledge of intrahepatic anatomy. Segment-oriented resection consents to reach a radical strategy with a maximum of liver parenchyma preservation. This surgical approach looks to have great potential for a HEHE cure because this tumor typically raises in a liver without an underlying cirrhotic disease. Therefore, this condition permits complex liver resections.
Both LT and hepatic resection should be considered successful strategies for obtaining reasonable long-term survival (39). However, the high rate of post-transplant mortality and morbidities observed, like infection (44,45) and graft failure (46), should be considered when the LT strategy is chosen. Moreover, more significant blood loss, prolonged operating procedures, and extended hospital stay are observed (47). In general, patients receiving LT have early (≤3 months) and late (>3 months) mortality rates ranging 1–5% and 22% (28), respectively, which are higher than the mortality rates reported after hepatic resection (0–3%) (48).
Laparoscopic vs. open resection
The potential advantage of mini-invasive liver resection (MILS) for HEHE management has not been fully explored. However, such an approach should be a reasonable approach in the setting of this tumor.
HEHE is often observed in its multifocal form, with several lesions near the liver surface (49). Therefore, MILS with multiple partial resections should be a possible strategy to use in this case. MILS correlates with minor intraoperative blood loss, shorter length of hospital stay, and limited postsurgical adhesions (50). Moreover, MILS is suitable for newly managing intrahepatic recurrence. Also in this case, repeat MILS relates to less intraoperative blood loss and shorter hospital stay than open repeat resection (51,52). Therefore, repeat MILS should be considered a valuable opportunity to treat potentially resectable recurred HEHE.
The appropriate surgical margin to reach for the cure of HEHE has not been clearly stated. Some experiences reported that tumor cells should be observed within 1 cm from the principal lesion. However, no studies correlating between surgical margins <1 cm and recurrence have been reported (53). On the opposite, a negative surgical margin has been demonstrated to be sufficient in all liver sarcomas, including HEHE (29). Therefore, a 1-cm margin looks not to be necessary, but HEHE resection should only reach a negative margin of resection.
Liver transplantation
LT represents the best therapeutic option for unresectable HEHE, namely in the conditions in which the diseases is multifocal and/or bilobar. However, to date, less than 300 procedures have been done, most of them being reported in three multicentre studies from Canada (N=11), the United States (N=110), and Europe (N=149) (28,54,55). To date patient selection criteria and an algorithm for the optimal management of HEHE are still missing. Therefore, the mindset of the LT community remains doubtful in managing this tumor.
So far, good outcomes have been reported. In the study from the United States (period: 1987–2005), the 5-year survival was 67% (54). The European study [1989–2017] (28) had 5- and 10-year survival percentages of 79.5% and 74.4%, respectively. Both studies demonstrated that the presence of the extrahepatic disease did not represent an absolute contraindication for LT.
The HEHE-LT score proposed by Lai et al. identified the presence of macrovascular involvement at pathology, the waiting time before transplantation shorter than four months, and hilar nodal metastases as three independent risk factors for HEHE recurrence after transplant (28).
Thus, considering these variables, three classes of post-LT recurrence risk have been identified, with a 5-year disease-free survival rate ranging from 93.9% in the low score group to 38.5% in the high score one (P<0.001). The limit of the HEHE-LT score is that two out of three variables can be confirmed only after surgery.
Systemic treatments
Several chemotherapeutic drugs have been shown to be effective for the treatment of HEHE. Considering the vascular nature of this tumor and the presence of the receptors for the vascular endothelial growth factor (VEGF) in HEHE cells, it is postulated that VEGF plays a role in the growth of this cancer. In combination with a cell cycle inhibitor (i.e., sorafenib, pazopanib, bevacizumab), VEGF inhibitors (i.e., capecitabine), consented to achieve sound curative effects. For patients treated with pazopanib, the change in tumor density after computed tomography (CT) without evident tumor contraction or calcification can be considered a strong indication of tumor response (56). In patients with metastatic disease, adjuvant chemotherapy should represent an effective alternative therapy to prevent tumor recurrence.
Other drugs have been adopted for curing HEHE, like mTOR inhibitors, thalidomide, pegylated liposomal doxorubicin, and metronomic cyclophosphamide, interferon-alpha, and 5-fluorouracil (57,58). A randomized multicenter study from China showed that the Huaier granule could reduce the risk of postoperative HEHE recurrence (6). Huaier granule, a water-based product of Huaier extract, has an anti-tumor response thanks to the inhibition of tumor angiogenesis and the induction of the cell-cycle arrest at the G0/G1 checkpoint (59). Huaier granule also regulates innate immunity by stimulating cytokine release and production of reactive oxygen species (60).
More studies based on more extensive databases are needed to provide guidelines for HEHE diagnosis and treatment.
Discussion
Because of its rarity, no standardized flowchart for the treatment of HEHE exists. Only recently, a flow chart for the management of HEHE patients undergoing LT has been proposed, but not yet prospectively validated (Figure 1) (28). In a large review collecting 253 HEHE patients, the reported 1-, 3-, and 5-year overall survival rates were 83.4%, 55.7%, and 41.1%, respectively (3). Among the different kinds of treatments proposed, hepatectomy was the most frequently chosen, followed by LT, chemotherapy, radiotherapy, locoregional therapies, and observational follow-up. Hepatic resection consented to reach the best 5-year survival (i.e., 75%) (61). However, this therapy is often not indicated because the tumor is multifocal at the diagnosis.
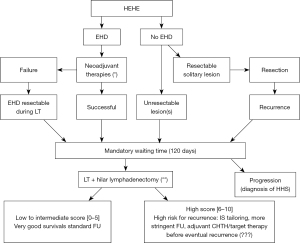
The clinical course of HEHE is hugely variegated, passing from complete spontaneous regression to aggressive rapid progression. This heterogeneity in the observed results is principally connected with the significant differences observed in the initial tumor characteristics of patients treated with surgical vs. non-surgical approaches.
For example, in the two Surveillance, Epidemiology, and End Results (SEER) studies published on HEHE, significant differences have been reported in terms of survival. In the first study (N=56, period 1973–2014), 1-year overall survival rates were 57% vs. 67% vs. 80% in patients undergoing resection, no surgical approaches, and LT, respectively (31). The more recent analysis (N=79, period 2004–2016) reported 1- and 5-year overall survival of 87% vs. 75% and 61% vs. 37% in the surgical vs. the non-surgical group, respectively (62).
Several surgical approaches, like hepatic artery ligation, hepatic resection, or LT, have been utilized to cure HEHE (Table 2) (14-28,30,32,33,50,63). Hepatectomy is adopted to cure localized single lesions, whilst LT represents the treatment of choice to treat multifocal diseases (51). In a retrospective study on 30 HEHE patients treated at the Mayo Clinic, most patients undergoing surgery had no extrahepatic involvement at the time of surgery. However, overall survival was not inferior in patients with extrahepatic involvement, arguing that metastatic disease may not be considered an absolute contraindication for surgery (46). This observation was also confirmed in the setting of LT (28). In detail, 40 patients transplanted with the extrahepatic disease had 5-year survivals of 72%, a remarkable survival rate in patients with metastases at the time of transplant (28).
Conclusions
HEHE is rare cancer for which different treatment strategies have been applied. Surgery often represents the first choice in terms of obtainable results. LT is indicated in multifocal and/or bilobar disease, while liver resection is indicated only in the less common condition of single or oligonodular monolobar disease. The minimally invasive surgical approach has shown comparable results to open surgery in terms of survival. Further studies are needed to establish the safety and reproducibility of minimally invasive surgery in treating this type of cancer. VEGF inhibitors, often in combination with other chemotherapeutic drugs, can be used for patients exceeding the surgical criteria.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Levi Sandri) for the series “Hepatobiliary and Pancreatic Cancers: An Update of Surgical Treatments” published in Chinese Clinical Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-21-139/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-21-139/coif). The series “Hepatobiliary and Pancreatic Cancers: An Update of Surgical Treatments” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moulai N, Chavanon O, Guillou L, et al. Atypical primary epithelioid hemangioendothelioma of the heart. J Thorac Oncol 2006;1:188-9. [Crossref] [PubMed]
- Elleuch N, Dahmani W, Aida Ben S, et al. Hepatic epithelioid hemangioendothelioma: A misdiagnosed rare liver tumor. Presse Med 2018;47:182-5. [Crossref] [PubMed]
- Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006;107:2108-21. [Crossref] [PubMed]
- Ishak KG, Sesterhenn IA, Goodman ZD, et al. Epithelioid hemangioendothelioma of the liver: a clinicopathologic and follow-up study of 32 cases. Hum Pathol 1984;15:839-52. [Crossref] [PubMed]
- Antonescu C. Malignant vascular tumors--an update. Mod Pathol 2014;27:S30-8. [Crossref] [PubMed]
- Bruegel M, Muenzel D, Waldt S, et al. Hepatic epithelioid hemangioendothelioma: findings at CT and MRI including preliminary observations at diffusion-weighted echo-planar imaging. Abdom Imaging 2011;36:415-24. [Crossref] [PubMed]
- Gurung S, Fu H, Zhang WW, et al. Hepatic epithelioid hemangioendothelioma metastasized to the peritoneum, omentum and mesentery: a case report. Int J Clin Exp Pathol 2015;8:5883-9. [PubMed]
- Earnest F 4th, Johnson CD. Case 96: Hepatic epithelioid hemangioendothelioma. Radiology 2006;240:295-8. [Crossref] [PubMed]
- Blachar A, Federle MP, Brancatelli G. Hepatic capsular retraction: spectrum of benign and malignant etiologies. Abdom Imaging 2002;27:690-9. [Crossref] [PubMed]
- Mamone G, Miraglia R. The "Target sign" and the "Lollipop sign" in hepatic epithelioid hemangioendothelioma. Abdom Radiol (NY) 2019;44:1617-20. [Crossref] [PubMed]
- Neofytou K, Chrysochos A, Charalambous N, et al. Hepatic epithelioid hemangioendothelioma and the danger of misdiagnosis: report of a case. Case Rep Oncol Med 2013;2013:243939. [Crossref] [PubMed]
- Mistry AM, Gorden DL, Busler JF, et al. Diagnostic and therapeutic challenges in hepatic epithelioid hemangioendothelioma. J Gastrointest Cancer 2012;43:521-5. [Crossref] [PubMed]
- Zhou L, Cui MY, Xiong J, et al. Spectrum of appearances on CT and MRI of hepatic epithelioid hemangioendothelioma. BMC Gastroenterol 2015;15:69. [Crossref] [PubMed]
- Thin LW, Wong DD, De Boer BW, et al. Hepatic epithelioid haemangioendothelioma: challenges in diagnosis and management. Intern Med J 2010;40:710-5. [Crossref] [PubMed]
- Grotz TE, Nagorney D, Donohue J, et al. Hepatic epithelioid haemangioendothelioma: is transplantation the only treatment option? HPB (Oxford) 2010;12:546-53. [Crossref] [PubMed]
- Wang LR, Zhou JM, Zhao YM, et al. Clinical experience with primary hepatic epithelioid hemangioendothelioma: retrospective study of 33 patients. World J Surg 2012;36:2677-83. [Crossref] [PubMed]
- Thomas RM, Aloia TA, Truty MJ, et al. Treatment sequencing strategy for hepatic epithelioid haemangioendothelioma. HPB (Oxford) 2014;16:677-85. [Crossref] [PubMed]
- Theodosopoulos T, Dellaportas D, Tsangkas A, et al. Clinicopathological features and management of hepatic vascular tumors. A 20-year experience in a Greek University Hospital. J BUON 2013;18:1026-31. [PubMed]
- Orlando G, Adam R, Mirza D, et al. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation 2013;95:872-7. [Crossref] [PubMed]
- Groeschl RT, Miura JT, Oshima K, et al. Does histology predict outcome for malignant vascular tumors of the liver? J Surg Oncol 2014;109:483-6. [Crossref] [PubMed]
- Remiszewski P, Szczerba E, Kalinowski P, et al. Epithelioid hemangioendothelioma of the liver as a rare indication for liver transplantation. World J Gastroenterol 2014;20:11333-9. [Crossref] [PubMed]
- Lin YH, Lin CC, Concejero AM, et al. Surgical experience of adult primary hepatic sarcomas. World J Surg Oncol 2015;13:87. [Crossref] [PubMed]
- Sundar Alagusundaramoorthy S, Vilchez V, Zanni A, et al. Role of transplantation in the treatment of benign solid tumors of the liver: a review of the United Network of Organ Sharing data set. JAMA Surg 2015;150:337-42. [Crossref] [PubMed]
- Dong K, Wang XX, Feng JL, et al. Pathological characteristics of liver biopsies in eight patients with hepatic epithelioid hemangioendothelioma. Int J Clin Exp Pathol 2015;8:11015-23. [PubMed]
- Jung DH, Hwang S, Hong SM, et al. Clinicopathological Features and Prognosis of Hepatic Epithelioid Hemangioendothelioma After Liver Resection and Transplantation. Ann Transplant 2016;21:784-90. [Crossref] [PubMed]
- Abdoh QA, Alnajjar AM, Abaalkhail FA, et al. Aggressive Recurrence of Primary Hepatic Epithelioid Haemangioendothelioma after Liver Transplantation. Can J Gastroenterol Hepatol 2016;2016:6135297. [Crossref] [PubMed]
- Samuk I, Tekin A, Tryphonopoulos P, et al. Abdominal transplantation for unresectable tumors in children: the zooming out principle. Pediatr Surg Int 2016;32:337-46. [Crossref] [PubMed]
- Lai Q, Feys E, Karam V, et al. Hepatic Epithelioid Hemangioendothelioma and Adult Liver Transplantation: Proposal for a Prognostic Score Based on the Analysis of the ELTR-ELITA Registry. Transplantation 2017;101:555-64. [Crossref] [PubMed]
- Konstantinidis IT, Nota C, Jutric Z, et al. Primary liver sarcomas in the modern era: Resection or transplantation? J Surg Oncol 2018;117:886-91. [Crossref] [PubMed]
- Wang JK, Wu ZR, Su F, et al. Resectable Single Hepatic Epithelioid Hemangioendothelioma in the Left Lobe of the Liver: A Case Report. Open Med (Wars) 2018;13:456-9. [Crossref] [PubMed]
- Noh OK, Kim SS, Yang MJ, et al. Treatment and prognosis of hepatic epithelioid hemangioendothelioma based on SEER data analysis from 1973 to 2014. Hepatobiliary Pancreat Dis Int 2020;19:29-35. [Crossref] [PubMed]
- Sanduzzi-Zamparelli M, Rimola J, Montironi C, et al. Hepatic epithelioid hemangioendothelioma: An international multicenter study. Dig Liver Dis 2020;52:1041-6. [Crossref] [PubMed]
- Krasnodębski M, Grąt M, Morawski M, et al. Hepatic Epithelioid Hemangioendothelioma: A Rare Disease With Favorable Outcomes After Liver Transplantation. Transplant Proc 2020;52:2447-9. [Crossref] [PubMed]
- Paolantonio P, Laghi A, Vanzulli A, et al. MRI of hepatic epithelioid hemangioendothelioma (HEH). J Magn Reson Imaging 2014;40:552-8. [Crossref] [PubMed]
- Kim EH, Rha SE, Lee YJ, et al. CT and MR imaging findings of hepatic epithelioid hemangioendotheliomas: emphasis on single nodular type. Abdom Imaging 2015;40:500-9. [Crossref] [PubMed]
- Dong A, Dong H, Wang Y, et al. MRI and FDG PET/CT findings of hepatic epithelioid hemangioendothelioma. Clin Nucl Med 2013;38:e66-73. [Crossref] [PubMed]
- Miller WJ, Dodd GD 3rd, Federle MP, et al. Epithelioid hemangioendothelioma of the liver: imaging findings with pathologic correlation. AJR Am J Roentgenol 1992;159:53-7. [Crossref] [PubMed]
- Campione S, Cozzolino I, Mainenti P, et al. Hepatic epithelioid hemangioendothelioma: Pitfalls in the diagnosis on fine needle cytology and "small biopsy" and review of the literature. Pathol Res Pract 2015;211:702-5. [Crossref] [PubMed]
- Venkatesh SK, Chandan V, Roberts LR. Liver masses: a clinical, radiologic, and pathologic perspective. Clin Gastroenterol Hepatol 2014;12:1414-29. [Crossref] [PubMed]
- Lerut JP, Orlando G, Adam R, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg 2007;246:949-57; discussion 957. [Crossref] [PubMed]
- Morishita A, Iwama H, Yoneyama H, et al. MicroRNA profile of hepatic epithelioid hemangioendothelioma: A case report. Oncol Lett 2017;13:1655-9. [Crossref] [PubMed]
- Weitz J, Klimstra DS, Cymes K, et al. Management of primary liver sarcomas. Cancer 2007;109:1391-6. [Crossref] [PubMed]
- Galvão FH, Bakonyi-Neto A, Machado MA, et al. Interferon alpha-2B and liver resection to treat multifocal hepatic epithelioid hemangioendothelioma: a relevant approach to avoid liver transplantation. Transplant Proc 2005;37:4354-8. [Crossref] [PubMed]
- Antunes M, Teixeira A, Fortuna P, et al. Infections After Liver Transplantation: A Retrospective, Single-center Study. Transplant Proc 2015;47:1019-24. [Crossref] [PubMed]
- Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013;19:3-26. [Crossref] [PubMed]
- Brahmbhatt M, Prenner S, Bittermann T. Liver Transplantation for Hepatic Epithelioid Hemangioendothelioma Is Facilitated by Exception Points With Acceptable Long-term Outcomes. Transplantation 2020;104:1187-92. [Crossref] [PubMed]
- Kostakis ID, Machairas N, Prodromidou A, et al. Comparison Between Salvage Liver Transplantation and Repeat Liver Resection for Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Transplant Proc 2019;51:433-6. [Crossref] [PubMed]
- Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Lazăr DC, Avram MF, Romoșan I, et al. Malignant hepatic vascular tumors in adults: Characteristics, diagnostic difficulties and current management. World J Clin Oncol 2019;10:110-35. [Crossref] [PubMed]
- Wakabayashi T, Felli E, Memeo R, et al. Short-term outcomes of laparoscopic repeat liver resection after open liver resection: a systematic review. Surg Endosc 2019;33:2083-92. [Crossref] [PubMed]
- Ome Y, Hashida K, Yokota M, et al. The feasibility and efficacy of pure laparoscopic repeat hepatectomy. Surg Endosc 2018;32:3474-9. [Crossref] [PubMed]
- Peng L, Zhou Z, Xiao W, et al. Systematic review and meta-analysis of laparoscopic versus open repeat hepatectomy for recurrent liver cancer. Surg Oncol 2019;28:19-30. [Crossref] [PubMed]
- Agostini-Vulaj D, Pehlivanoglu B, Weiss SW, et al. Intrasinusoidal Spread of Hepatic Epithelioid Hemangioendothelioma: Implications for the Diagnosis in Minimal Samples. Am J Surg Pathol 2019;43:573-9. [Crossref] [PubMed]
- Rodriguez JA, Becker NS, O'Mahony CA, et al. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg 2008;12:110-6. [Crossref] [PubMed]
- Nudo CG, Yoshida EM, Bain VG, et al. Liver transplantation for hepatic epithelioid hemangioendothelioma: the Canadian multicentre experience. Can J Gastroenterol 2008;22:821-4. [Crossref] [PubMed]
- Bally O, Tassy L, Richioud B, et al. Eight years tumor control with pazopanib for a metastatic resistant epithelioid hemangioendothelioma. Clin Sarcoma Res 2015;5:12. [Crossref] [PubMed]
- Lau A, Malangone S, Green M, et al. Combination capecitabine and bevacizumab in the treatment of metastatic hepatic epithelioid hemangioendothelioma. Ther Adv Med Oncol 2015;7:229-36. [Crossref] [PubMed]
- Chevreau C, Le Cesne A, Ray-Coquard I, et al. Sorafenib in patients with progressive epithelioid hemangioendothelioma: a phase 2 study by the French Sarcoma Group (GSF/GETO). Cancer 2013;119:2639-44. [Crossref] [PubMed]
- Wang X, Zhang N, Huo Q, et al. Anti-angiogenic and antitumor activities of Huaier aqueous extract. Oncol Rep 2012;28:1167-75. [Crossref] [PubMed]
- Chen Q, Shu C, Laurence AD, et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut 2018;67:2006-16. [Crossref] [PubMed]
- Xu J, Hu S, Li S, et al. Laparoscopic resection of hepatic epithelioid hemangioendothelioma: report of eleven rare cases and literature review. World J Surg Oncol 2020;18:282. [Crossref] [PubMed]
- Chahrour MA, Khachfe HH, Habib JR, et al. Treatment and Prognosis of Hepatic Epithelioid Hemangioendothelioma: A SEER Database Analysis. World J Surg 2021;45:2886-94. [Crossref] [PubMed]
- Gigante E, Lai Q, Lerut JP, et al. Hepatic haemangioendothelioma: A proteiform disease. Dig Liver Dis 2020;52:1039-40. [Crossref] [PubMed]


