Endocrine therapy as adjuvant or neoadjuvant therapy for breast cancer: selecting the best agents, the timing and duration of treatment
Introduction
Breast cancer is the most frequently occurring cancer among women worldwide, which can be divided into four intrinsic subtypes defined by gene expression profiling (1). In clinical practice, surrogate approaches have been developed using more widely available immunohistochemical (IHC) tests, among which Luminal type, known as hormone receptor (HR)-positive breast cancer, defined by estrogen receptor (ER) and/or progesterone receptor (PgR) expression by IHC, account for 75% of all breast cancer, summing up to 85% in women over 70 years old (2). The therapeutic manipulation of endogenous estrogen levels and the interaction of estrogen with its receptor is a cornerstone of adjuvant therapy in female patients with HR positive breast cancer. First of all, accurate assessment of HR status is critical for the use of adjuvant endocrine therapy in breast cancer, however, results have often been inaccurate and irreproducible. Thresholds for determining positivity also vary (for example, ≥1%, ≥10%, any). Most studies used an IHC cut-off value of 10% or greater. It is not known whether using a cut-off value of 1% (as recommended in current guidelines) to give the chance to receive adjuvant endocrine therapy is accurate enough, as a large single-institution experience suggests that equivocal ER staining between 1% and 9% (more commonly seen among young patients, with higher grade or HER2-positive or PgR-negative tumors), tracks prognostically more closely with ER absent disease in terms of recurrence-free survival (3). Determination of menopausal status is another important factor in deciding on treatment, and an accurate assessment of the menopausal status of each individual patient is essential (4).
Adjuvant tamoxifen (TAM) is a major endocrine treatment option, which has been found to be effective in both pre- and postmenopausal patients. The outcomes of patients with early breast cancer have improved with the use of adjuvant systemic treatments, which include chemotherapy, endocrine therapy, and targeted agents for eligible subgroups of patients, along with new treatment strategy (5). While TAM has been the mainstay of endocrine therapy for breast cancer for over 30 years, we now have a range of therapeutic manipulations. In particular premenopausal patients, the ovaries are the main site of hormone production, and therefore the value of ovarian ablation (OA) by surgical removal and/or ovarian irradiation, or temporary ovarian suppression (OS) by administration of luteinizing hormone-releasing hormone (LHRH) agonists have been tested in adjuvant treatment. In postmenopausal women, aromatization of androgens, which are produced by the adrenal glands, serve as the main source of estrogen production after cessation of ovarian function, the value of aromatase inhibitors (AIs) prevent the action of aromatase in the synthesis of estrogen has been tested as well. Meanwhile, increasing evidence supports the use of extended endocrine therapy with either TAM or an AI after five years of initial adjuvant TAM to reduce breast cancer recurrence and mortality. Clinical practice guidelines should keep changing with developing evidence-based practice guidelines pertaining to breast cancer care. The present publication conducted a comprehensive systematic review of the literature addressing the use of adjuvant endocrine therapy for early breast cancer, which can be used as a standalone reference to the extensive data on this important area of breast cancer care.
Adjuvant endocrine therapy
Definition of menopause
According to NCCN (6), menopause is generally the permanent cessation of menses, and as the term is utilized in breast cancer management includes a profound and permanent decrease in ovarian estrogen synthesis. Reasonable criteria for determine menopause include any of the following:
- Prior bilateral oophorectomy;
- Age ≥60 y;
- Age <60 y and amenorrhea for 12 or more months in the absence of chemotherapy, TAM, toremifene, or OS and follicle-stimulation hormone (FSH) and estradiol in the postmenopausal range;
- If taking TAM or toremifene, and age <60 y, then FSH and plasma estradiol level in postmenopausal ranges.
Of note, it is not possible to assign menopausal status to women who are receiving an LHRH agonist or antagonist. In women premenopausal at the beginning of adjuvant chemotherapy, amenorrhea is not a reliable indicator for menopausal status. For women with therapy-induced amenorrhea, serial measurements of FSH and/or estradiol are needed to ensure postmenopausal status.
Premenopausal at diagnosis
Choice of initial five years adjuvant endocrine therapy
- TAM (20 mg daily) for 5 years;
- OA/S + Exemestane* (25 mg daily) for 5 years;
- OA/S +TAM (20 mg daily) for 5 years.
*Other AIs, such as Anastrozole (1 mg daily) or Letrozole (2.5 mg daily) can also be used.
Tamoxifen
TAM is a selective estrogen receptor modulator that blocks the effect of estrogen, which has been used in adjuvant setting for HR positive patients for more than 30 years. The most robust evidence for the benefit of adjuvant endocrine therapy for both pre- and postmenopausal women comes from the extensive evaluation of TAM. The results of a Swedish trial, a phase III trial to compare two years with five years of adjuvant TAM in the treatment postmenopausal women younger than 75 years of age, showed five years TAM experienced statistically significant improvements in event-free survival [relative hazard =0.82; 95% confidence interval (CI), 0.71–0.96] and overall survival (relative hazard =0.82; 95% CI, 0.69–0.99) (7). Then five years of TAM became the backbone of adjuvant hormonal therapy based on a series of randomized trials. The most recent EBCTCG meta-analysis included all trials worldwide on early breast cancer (excluding ductal carcinoma in situ) that compared adjuvant five years of adjuvant TAM with no therapy with over 15 years of follow-up (8). In patients with ER negative cancer, TAM did not improve the rate of recurrence or survival. Estrogen receptor positivity at the level of 10 fmol/mg or more was enough to yield a positive TAM effect. In women with ER positive breast cancer, TAM substantially improved overall outcome in all subgroups of patients, the benefits were seen independent of age, chemotherapy use, menopausal status, and nodal status. Given a known ER status, PR status was not significantly predictive of response. A greater effect on 10-year breast cancer mortality rates was observed with five years than with one or two years of TAM. For patients with ER positive cancer who received five years of TAM, the 15-year recurrence rate was 33% (compared with 46.2% without), and the breast cancer mortality rate was 23.9% (compared with 33.1%), indicating the benefit of TAM was thus observed to persist after its use was discontinued. In fact, the Oxford overview showed the use of five years TAM reduced breast cancer mortality rate by about a third throughout the first 15 years [RR 0.71 (0.05) during years 0–4, 0.66 (0.05) during years 5–9, and 0.68 (0.08) during years 10–14]. The risk of contralateral breast cancer was also lowered by 38% over all time periods, independent of age. These data support TAM to be the standard adjuvant treatment in premenopausal patients with ER+ disease.
Ovarian ablation or ovarian suppression (OA/OS)
In premenopausal patients, the ovaries are the main site of hormone production, therefore, treatment suppress the ovarian function may have a positive effect in adjuvant setting. In fact, OA is the oldest form of systemic therapy for breast cancer. The term is often used to refer to surgical oophorectomy or ovarian irradiation with permanent inactivation. Recently, temporary OS by administration of LHRH agonists have been used in treatment. The hormonal maneuver of OA/S benefits only female patients with HR positive breast cancer. In addition, OA/S has endocrine effects only in premenopausal patients and thus should be considered a therapeutic strategy only in that age group. Although, recent trials also evaluated OS as a method of preserving fertility during chemotherapy in HR negative patients; those studies are beyond the scope of the present review (9,10).
The efficacy of using OA/S in adjuvant stetting is difficult to evaluate. First, studies can use OA (by radiotherapy or surgery), OS, or both. Second, the comparisons can include no treatment with only OA/S, and in combination with every other systemic therapy (TAM, AIs, chemotherapy). Along with the large body of evidence about OA/S in early-stage breast cancer, its current role as a treatment strategy becomes clear.
One EBCTCG meta-analysis completely compared of OA/S or not and of OA/S plus chemotherapy or chemotherapy alone. A total of 7,725 female breast cancer patients less than 50 years of age were included (11). OA/S were associated with significantly decreased rates of recurrence (15-year recurrence rate was 47.3% for the OA/S group and 51.6% for the control group, P=0.00001) and survival (15-year breast cancer mortality rates were 40.3% and 43.5% respectively, P=0.004). However, subgroup analyses found the effect appeared smaller in which chemotherapy was also administered, and the analysis included both ER positive and ER unknown cancers (<50% patients on OA/S were confirmed to be HR positive) and it does not answer key questions of whether OA/S is better than not when combine with endocrine therapy, which is the best combination, which subgroups of patients benefit most from OA/S? Afterwards, three large phase III trials tested the efficacy of OS in combination with either TAM or AIs in premenopausal women.
SOFT trial is the only trial was reported to address the question whether the combination is superior to a single agent TAM (12). A total of 3,066 premenopausal women were enrolled and randomized to receive single agent TAM, TAM plus OS, or Exemestane plus OS. The primary endpoint, DFS (disease free survival) between TAM along or TAM plus OS, did not provide a significant benefit over single agent (HR 0.83, P=0.10) after median 5.6 years follow-up. No difference in BCFI (breast cancer free interval) or DRFI (distant recurrence free interval) was observed at five years in the subgroup of patients who had received no prior chemotherapy (low recurrence risk patient). However, the benefit of the combinations was more pronounced among patients with higher risks that warrant adjuvant chemotherapy. The rate of BCFI at five years was 78% with TAM alone, 82.5% with TAM + OS, and 85.7% with Exemestane + OS, and 5-year DRFI were 83.6% with TAM, 84.8% with TAM + OS (HR: 0.87; 95% CI: 0.64 to 1.17), and 87.8% with Exemestane + OS (HR: 0.72; 95% CI: 0.52 to 0.98). The benefit is much more pronounced in women younger than 35 years of age (totally 350 patients, 94% received chemotherapy), the rate of BCFI at five years was 67.7% with TAM alone, 78.9% with TAM + OS, and 83.4% with Exemestane + OS. Compared with TAM alone, combine ovarian function suppression was associated with more toxicity and adverse effects.
Other two reports evaluated the better combination regimen with OS in this setting, TAM or Exemestane. The first trial was ABCSG12 trial (13), premenopausal patients with endocrine-responsive early breast cancer randomized to receive goserelin plus TAM or goserelin plus Anastrozole, and in a second randomization, patients either receive zoledronic acid or not. After a median follow-up of 47.8 months, DFS rates of 92.8% in TAM group, 92.0% in the Anastrozole group (HR 1.10, P=0.59). TAM experienced a significantly better OS rate, and Anastrozole experienced less serious adverse events. It was rather a small trial that enrolled 1,803 patients and the duration of adjuvant endocrine therapy was only three years.
The other report is the joint analysis of the two phase III trials, SOFT and TEXT trial, included a total of 4,690 premenopausal patients with HR positive breast cancer who were randomized to receive either OS plus Exemestane or OS plus TAM for five years (12). With the median follow-up of 68 months, this combined analysis demonstrated a significant improvement in DFS in the Exemestane group (91.1% vs. 87.3%, HR 0.72, P<0.001), although the overall survival was similar in both groups (96%). Compared with ABCSG-12, the joint analysis has more patients and the duration of adjuvant endocrine therapy was five years. The profile of adverse effects was different for Exemestane plus OS (greater loss of sexual interest and arousal difficulties, vaginal dryness, bone pain) compared with TAM plus OS (more hot flushes and sweats) (14). With Exemestane plus OS, median reductions from baseline E2, E1, and E1S levels were significantly lower than with TAM plus OS at all time points, however, 25%, 24%, and 17% patients on Exemestane had an E2 level greater than 2.72 pg/mL (threshold) at 3, 6, and 12 months, respectively (15).
Converse results existing among the above studies, we believe the choice of AI does not matter, but the duration of OS and the basic characteristic of patients matter. Taking these results together, AI in combination with OS may represent the new standard of care option for the adjuvant treatment in premenopausal women with HR positive breast cancer, particularly in patients with higher risk that warrant chemotherapy and younger patients less than 35 years of age (16). In the absence of chemotherapy, there is no evidence of overall benefit for OA/S plus endocrine, TAM is still the standard.
Postmenopause at diagnosis
Choice of initial five years adjuvant endocrine therapy
- TAM (20 mg daily) for five years;
- Anastrozole (1 mg daily) or letrozole (2.5 mg daily) or exemestane (25 mg daily) for five years;
- TAM (20 mg daily) for 2–3 years, then a switch to AIs for a total of five years of endocrine therapy;
- AIs for 2–3 years, then a switch to TAM (20 mg daily) for a total of five years of endocrine therapy.
Aromatase inhibitors (AIs)
In postmenopausal women, aromatization of androgens, which are produced by the adrenal glands, serve as the main source of estrogen production after cessation of ovarian function. Aromatase enzyme is involved in the last step of estrogen biosynthesis that converts testosterones to estrogens via aromatization process. Since aromatase has high specificity and is only involved in the last step steroid biosynthesis, inhibition of this enzyme does not affect levels of other biologically critical steroids, and may have positive effect in clinical practice. In postmenopausal women, peripheral conversion of androgens to estrogens serves as the sole source of estrogen production after cessation of ovarian function, AIs focus on this pathway can decrease aromatization of androgens and deplete estrogens (17). Currently, there are three AIs in clinical use, classified in two distinct subclasses according to chemical structures, non-steroidal AIs as Anastrozole or Letrozole, and Steroidal AI as Exemestane.
Clinical effective of AIs compared with TAM
The third-generation AIs are currently considered standard of care for adjuvant treatment of postmenopausal women with HR positive breast cancer, and several strategies has been investigated, including upfront AIs for five years, switch to TAM after 2–3 years up front AIs, or switch to AIs after 2–3 years of TAM (17).
For the upfront strategy, the ATAC trial enrolled HR positive patients randomized to Anastrozole (n=2,618) and TAM (n=2,598), the results were significantly in favor of Anastrozole for DFS (HR 0.86, 95% CI: 0.78–0.95; P=0.003), time to recurrence (0.79, 0.70–0.89; P=0.0002), and time to distant recurrence (0.85, 0.73–0.98; P=0.02) after a median of 120 months follow-up (18). Similar, in the BIG 1–98 trial, 2,459 patients were randomly assigned to five years monotherapy with TAM and 2,463 to monotherapy with Letrozole. With a median follow-up of 8·7 years, Letrozole was significantly better, whether by IPCW or intention-to-treat analysis [IPCW: DFS HR 0.82 (95% CI: 0.74–0.92), overall survival HR 0.79 (0.69–0.90), DRFI HR 0.79 (0.68–0.92), BCFI HR 0.80 (0.70–0.92)] (19); Although, there was no significant improvement in overall survival observed in these trials, which might be due to crossover in considerable numbers of patients, AIs appeared to be better tolerated with less treatment-related serious adverse events. Of note, concurrent administration of Anastrozole and TAM in the ATAC trial showed detrimental effects in DFS compared to Anastrozole single agent (18).
For switching strategy, there were five clinical trials that compared fve years of TAM to sequential treatment of TAM for 2–3 years followed by AIs. These trials include ABCSG-8, BIG 1–98, ARNO 95, Italian Tamoxifen Anastrozole (ITA), and Intergroup Exemestane Study (IES) trials. All of these trials demonstrated significant improvement in DFS among patients who received sequential treatment compared to TAM alone with the HR ranging from 0.57–0.76. The BIG 1–98 trial was the only trial that directly compared the sequential treatment of AI followed by TAM [1,540], the reverse sequence [1,548], and upfront treatment of AI [1,546] or TAM [1,548] in the four-arm option. Eight-year intention-to-treat estimates for Letrozole monotherapy, Letrozole followed by TAM, and TAM followed by Letrozole were 78.6%, 77.8%, 77.3% for DFS; 87.5%, 87.7%, 85.9% for overall survival; 89.9%, 88.7%, 88.1% for DRFI; and 86.1%, 85.3%, 84.3% for BCFI. There was no statistically significant difference between the two sequential arms compared to single agent Letrozole, while there were numerically more relapses within the first few years in TAM followed by AI arm (particularly in patients with lymph node involvement) (19).
A previous EBCTCG meta-analysis encompassed all randomized trials started by year 2000 and data to September 30, 2006 (20), in cohort 1 (TAM vs. AI as monotherapy for five years) included 9,856 patients with a mean of 5.8 years’ follow-up showed AI therapy was associated with an absolute 3% decrease in recurrence (9.6% for AI vs. 12.6% for TAM) and a nonsignificant absolute 1.1% decrease in breast cancer mortality (4.8% for AI vs. 5.9% for TAM). In Cohort 2 (TAM vs. AI after 2–3 years of TAM for 5 years) included 9,015 patients with a mean follow-up of 3.9 years calculated from the time of treatment divergence, AI therapy was associated with an absolute 3.1% decrease in recurrence (5.0% for AI vs. 8.1% for TAM since divergence) and an absolute 0.7% decrease in breast cancer mortality (1.7% for AI vs. 2.4% for TAM since divergence). The absolute gain was greater in patients with a poorer prognosis. A recently published meta-analysis included individual data on 31,920 ER positive postmenopausal patients evaluated different treatment strategies, 5 years of AI versus 5 years of TAM, or versus 2–3 years of TAM then AI to year 5 (21). Aggregating all three types of comparison, recurrence RRs favored AI during periods when treatments differed (RR 0.70, 0.64–0.77), but not significantly thereafter (RR 0.93, 0.86–1.01; P=0.08). Breast cancer mortality was reduced both while treatments differed (RR 0.79, 0.67–0.92), and subsequently (RR 0.89, 0.81–0.99), and for all periods combined (RR 0.86, 0.80–0.94; P=0.0005). All-cause mortality was also reduced (RR 0.88, 0.82–0.94; P=0.0003). RRs differed little by age, body-mass index, stage, grade, progesterone receptor status, or HER2 status. There were fewer endometrial cancers with AI than TAM (10-year incidence 0.4% vs. 1.2%; RR 0.33, 0.21–0.51) but more bone fractures (5-year risk 8.2% vs. 5.5%; RR 1.42, 1.28–1.57); non-breast-cancer mortality was similar.
Across the board, AIs were shown to be superior to TAM. The optimal sequence and duration of AI therapy, with or without TAM, is uncertain. Taking these data together, postmenopausal patients with HR positive disease consider incorporating an AI at some point during adjuvant therapy, either up front or sequentially high risk. Patients with axillary lymph node involvement should receive upfront AI. However, switching to TAM after 2–3 years of AI can be considered in case of intolerability since there was no statistically significant difference in DFS among patients who received five years of AI compared to 2–3 years of AI followed by TAM.
Clinical effective of different AIs
The MA 27 trial provide a direct comparison of Exemestane and Anastrozole. 7,576 women were enrolled, 4-year EFS was 91% for Exemestane and 91.2% for Anastrozole (HR, 1.02; 95% CI, 0.87 to 1.18; P=0.85) (22). Overall, DDFS and DFS were also similar. Two additional publications (abstracts only) emerging from BIG 1–98 and ATAC provide an indirect comparison of Anastrozole and Letrozole, suggesting that Letrozole could be more effective than Anastrozole in reducing early distant recurrence and mortality rates at five years (23,24). However, this finding is based on trends, which requires validation of the recent FACE trial (25). FACE trial was designed to evaluate the efficacy and safety of adjuvant Letrozole versus Anastrozole in postmenopausal patients with HR postive, node-positive breast cancer. 4,170 patients were enrolled, 5-year estimated DFS rate was 84.9% for Letrozole vs. 82.9% for Anastrozole (HR =0.93, 95% CI: 0.80–1.07; P=0.3150). Five-year estimated overall survival rate was 89.9% and 89.2%, respectively (HR =0.98, 95% CI: 0.82–1.17; P=0.7916). Safety profiles were also similar between two treatment arms. The studies concluded that three AIs were comparable and can be used equally in adjuvant setting.
Duration
Choice of adjuvant endocrine therapy duration
- TAM (20 mg daily) or AIs for five years;
- TAM (20 mg daily) for ten years, premenopausal or postmenopausal;/
- TAM (20 mg daily) for five years, if postmenopausal, followed by letrozole (2.5 mg daily) for five years.
Patients with HR positive breast cancer initially have lower rates of recurrence compared with HR negative disease, however, they have a constant and unrelenting risk of relapse that extends up to 15 years despite the use of five years adjuvant TAM (8). Clinical investigation has focused on the optimal duration of adjuvant endocrine therapy (Table 1), potential strategies differ depending on a woman’s menopausal status.

Full table
Ten years of TAM
The first larger phase III trial was NSABP B-14 study, 1,152 patients randomized with either placebo or TAM after completion of five years of adjuvant TAM therapy (26). With seven years of follow-up, patients who discontinued TAM had a slight advantage (DFS, 82% vs. 78%, respectively; P=0.03), (RFS, 94% vs. 92%, respectively; P=0.13). And continued TAM use showed cumulative toxicity, endometrial cancer rate in the continued arm of 2.1%, compared with 1.1% in the placebo arm (RR, 2.0; 95% CI, 0.7–6.6). This study supported that five years of adjuvant TAM be the standard of care for all women of any age with invasive HR-positive breast cancer.
Newer data from two larger studies, the ATLAS trial and the aTTom trial, respectively randomized 12,894 and 6,953 patients who had received five years of TAM to another five years TAM or not, supported longer-term TAM use. The aTTom trial included 2,755 ER positive and 4,198 ER untested women, reported that the extension of TAM to ten years was associated with a reduced recurrence rate (32% vs. 28%; 580 vs. 672 events; RR, 0.85; 95% CI, 0.76–0.95; P=0.003), breast cancer mortality rate (24% vs. 21%; 392 vs. 443 deaths; RR, 0.88; 95% CI, 0.77–1.01; P=0.05). And the greatest decreases in risk of recurrence and breast cancer mortality were seen after year 10. Ten years TAM also increases the risk of endometrial cancer (102 vs. 45 cases; RR: 2.2; P<0.0001) and death related to those cancers (37 vs. 20, 1.1% vs. 0.6%, P=0.02) (27,28).
In the ATLAS trial, continued TAM resulted in an absolute reduction in breast cancer recurrence of 3.7% (21.4% vs. 25.1%, 617 vs. 711 events; P=0.002), breast cancer mortality (331 vs. 397 deaths; P=0.01) and overall mortality (639 vs. 722 deaths; P=0.01) (29). Continued TAM showed time-dependent recurrence-rate- reduction effects, with minor reductions during years 5 through 9 (RR, 0.90; 95% CI, 0.79–1.02; P=0.10), but a substantial carryover benefit seen during late follow-up (after year 10 of TAM) (RR, 0.75; 95% CI, 0.62–0.90; P=0.003). The mortality reduction was also significant only after year 10 (RR, 0.71; 95% CI, 0.58–0.88; P=0.0016). Extended TAM showed small increased incidences of pulmonary embolism (41 vs. 21 cases; P=0.01) and of endometrial cancer (116 vs. 63 cases; P=0.0002). A decrease in ischemic heart disease was noted (127 vs. 163 cases, P=0.02), and no increased risk of stroke was noted (P=0.63).
The survival benefit of prolonged TAM is independent of age, nodal status, tumor size, previous TAM duration, extent of surgery, menopausal status, previous hysterectomy, or geography. A combined analysis of the ATLAS and aTTom studies showed ten years of adjuvant TAM decreases breast cancer mortality by approximately one-third in the first ten years of therapy, and by one-half thereafter. Patients should be informed of the risk of abnormal vaginal bleeding, endometrial proliferation and/or hyperplasia, and endometrial cancer. Thus, TAM be used for up to ten years is recommended.
Five years of TAM followed by five years of AIS
For postmenopausal patients, TAM for five years followed by an AI for up to five years is an alternative. In the largest study, the MA.17/BIG 1-97 trial, patients received five years of either Letrozole or placebo after five years TAM (30). With 2.4 years follow-up, extended of Letrozole had significantly reduced recurrence risk (HR, 0.57; 95% CI, 0.43–0.75; P=0.00008), after an early unblinding of the study, more than 60% patients in the control group crossover to receive Letrozole, despite this, exploratory analysis still showed both a DFS and an overall survival benefit for Letrozole compared with placebo, supporting the use of extended AIs therapy after TAM.
The ABCSG 6a trial showed that extended Anastrozole after TAM reduced total recurrences (HR, 0.62; 95% CI, 0.40–0.96; P=0.031) and distant recurrences (HR, 0.53; 95% CI, 0.29–0.96; P=0.034) (31). The NSABP B-33 trial tested extended Exemestane for postmenopausal women who were disease-free after five years of adjuvant TAM, showed higher-risk patients (less than 60 years, with larger tumors, positive nodes, or prior adjuvant chemotherapy) benefit more from extended adjuvant therapy (32).
A recent meta-analysis showed that for ER-positive breast cancer, extended adjuvant therapy after five years of TAM lead to a 36% reduction in local regional relapse and a 13% reduction in distant relapse, with significant improvements in RFS (OR, 0.72; 95% CI, 0.56–0.92; P=0.01), breast cancer-specific survival (OR, 0.78; 95% CI, 0.69–0.90; P=0.0003), and overall survival (OR, 0.89; 95% CI, 0.80–0.99; P=0.03) (33).A definitive signal has emerged in favor of extended endocrine therapy beyond five years for women with early-stage breast cancer. However, uncertainty remains regarding the best total duration and strategies for premenopausal and postmenopausal women. As mentioned before, the ATALS study enrolled only about 9% premenopausal patients, less powerful to indicate the efficacy of extended TAM therapy in this subgroup. On the other hand, after the results of SOFT/TEXT, more and higher risk premenopausal patients will receive OA/S based endocrine therapy during the initial five years, we are lacking evidence to guide. For postmenopausal patients, current evidence of extended therapy all after initial five years of TAM adjuvant therapy. However, in current clinical practice, majority postmenopausal patients receive an AI into their adjuvant care at some point in the first five years, we are also lacking evidence to guide, so we are waiting for ongoing studies of longer durations of AI therapy (Table 2)for postmenopausal women to guide our clinical practice. Besides, biomarkers that may be able to predict late recurrence at diagnosis will assist us to make clinical decision in the future. Such as Breast Cancer Index (BCI), a multi-gene assay includes the two-gene ratio HOXB13:IL17BR (H/I) and the Molecular Grade Index (MGI), can define patients with high DRFS at 0–5 years and at >5 years, and thus seemed to have prognostic value for late recurrence in postmenopausal women with ER-positive breast cancer (34). Further investigations are needed to validate these biomarkers’ potential for prediction of extended adjuvant treatment benefit in both premenopausal and postmenopausal patients.

Full table
Adverse effects
TAM and AIS are generally well tolerated, but have specific adverse effects, including effects on bone, cardiovascular, and gynecologic health. TAM is associated with multiple concerning side effects, particularly risks of endometrial cancer and venous thromboembolism, which is the result of its partial estrogenic effects in the uterus and vascular system (35,36). TAM is associated with an increased risk of benign endometrial pathology (bleeding, polyps, hyperplasia), hysterectomy, vaginal discharge, and endometrial cancer as well (approximately 1% of patients).
In contrast to TAM, AIs are not associated with increased risk of thromboembolism and endometrial cancer, seem to be less frequently associated with hot flashes. Common side effects of AIs include vasomotor symptoms, musculoskeletal symptoms, and loss of bone mineral density and fractures. Female patients receiving AIs should be monitored for changes in bone mineral density, as well as cardiac outcomes and changes in lipid profile. There are several medications that can help alleviate vasomotor symptoms. These medications include venlafaxine, gabapentin, and Clonidine (37). A musculoskeletal or arthralgia syndrome characterized by bone and joint symptoms, including pain, stiffness, or achiness that is symmetric and not associated with other signs of rheumatologic disorders. Musculoskeletal symptoms have been reported in up to 50% of women taking AIs and up to 20% of these patients discontinue the treatment due to this side effect (38), and duloxetine appeared to help alleviate these musculoskeletal symptoms (39). AIs are associated with increased cardiovascular disease, possibly including ischemic cardiac disease, although differences are small. Some studies have found an effect on lipid metabolism, including an increased risk of hypercholesterolemia.
For bone loss, it is crucial to monitor bone density test yearly in women who take AIs. Greater loss of bone mineral density and fractures induced by AI can be mitigated with the use of bisphosphonate therapy. Bisphosphonates have profound effect on osteoclasts, and affect T-cell function, so could also be effective as adjuvant treatments, particularly in preventing or delaying bone recurrence. Recently, a system review received data on 18,766 women with median follow-up 5.6 woman-years, overall, the reductions in recurrence (RR 0.94; 95% CI, 0.87–1.01; 2P=0.08), distant recurrence (0.92; 0.8–0.99; 2P=0.03), and breast cancer mortality (0.91; 0.83–0.99; 2P=0.04) were of only borderline significance, but the reduction in bone recurrence was more definite (0.83; 0.73–0.94; 2P=0.004). Among postmenopausal women bisphosphonates produced highly significant reductions in recurrence (RR 0.86; 95% CI, 0.78–0.94; 2P=0.002), distant recurrence (0.82; 0.74–0.92; 2P=0.0003), bone recurrence (0.72; 0.60–0.86; 2P=0.0002), and breast cancer mortality (0.82; 0.73–0.93; 2P=0.002) (40). Hence, Bisphosphonates can be added to the AI treatment of postmenopausal breast cancer to reduce the rate of breast cancer recurrence in the bone and improve breast cancer survival. Currently, denosumab, the anti-RANK ligand antibody, is also approved specifically for AI-induced bone loss. IBCSG18 randomized 3,420 postmenopausal, AI treated patients with either denosumab 60 or placebo mg every six months, showed denosumab group had a significantly delayed time to first clinical fracture (HR 0.50; 95% CI, 0.39–0.65; P<0.0001) (41).
Neoadjuvant endocrine therapy (NET)
Neoadjuvant therapy induces tumour downstaging and increases rates of breast-conserving surgery (BCS) (42). In Luminal tumors, adjuvant endocrine therapy is likely to account for most of the gains obtained with the administration of adjuvant systemic treatment and the need for additional adjuvant chemotherapy in these patients remains debatable. Hence, NET becomes an attractive option for selected patients with hormonal-receptor positive locally advanced breast cancer.
Efficacy of endocrine therapy (NET)
In a phase II trial, 239 patients postmenopausal women with stage IIA–IIIB HR+ BC were randomly assigned to receive neoadjuvant Anastrozole 1 mg/day (n=61) or Exemestane 25 mg/day (n=60) for 3 months or doxorubicin 60 mg/m2 with paclitaxel 200 mg/m2 (four 3-week cycles). There was no statistically significant difference between AI and chemotherapy in terms of clinical response rate, time to response, or pathologic complete response (pCR). Endocrine treatment was well tolerated, and with slightly higher Rates of BCS (33% vs. 24%; P=0.058) (43). GEICAM/2006-03 randomised randomised 97 patients with IHC-defined luminal disease (ER+/PR+/HER−2−/cytokeratin 8/18+) to receive neoadjuvant Exemestane for 24 weeks or chemotherapy (four, three-week cycles of epirubicin 90 mg/m2 plus cyclophosphamide 600 mg/m2 followed by four, three–week cycles of docetaxel 100 mg/m2), no statistically significant difference was found between the two arms in terms of clinical response rate (48% vs. 66% respectively; P=0.075), there was a trend for a worse outcome in the exemestane arm for premenopausal patients and those with high tumour Ki67 expression (44).
Efficacy of different endocrine agents in endocrine therapy (NET)
Table 3 list four trials compare AIs with TAM in neoadjuvant setting. P024 (45) and a Russian study (46) found AIs were superior to TAM in terms of clinical response rate and also in terms of breast conservation rate. While the IMPACT trial (47) and The PROACT trial (48) found no significant difference in overall response rate between patients who received Anastrozole or TAM, indicated that Anastrozole proved to be at least as effective as TAM and was probably more effective in certain subgroups. A meta-analysis of these studies, including a total of 1,160 patients indicated superior outcomes in terms of clinical objective response rate (RR 1.29; 95% CI, 1.14–1.47; P<0.001) and breast conservation rate (RR, 1.36; 95% CI, 1.16–1.59; P<0.001) with AI as compared to TAM. Furthermore, there was no difference in clinically relevant toxicities between the two treatments.
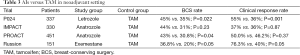
Full table
ACOSOG-Z1031 was the only study prospectively designed to compare three AIs head-to-head in neoadjuvant setting (49). This was a phase II trial that recruited 377 postmenopausal women with clinical stage II or III, HR+ disease to receive one of the three AI for four months before surgery. No statistically significant differences in clinical response (Exemestane 62.9%; Letrozole 74.8%; Anastrozole 69.1%) or surgical outcomes. These results suggest that the effectiveness of the three commercially available AIs are largely equivalent.
Hence, if we choose the best suitable “target population”, the efficacy of NET is similar to that of neoadjuvant chemotherapy. Usually, patients with less proliferative tumors will eventually be the greater responders to endocrine therapy as expected. Probably the most suitable patients are postmenopausal women, in particular (but not limited to) older women, ideally with low-grade HR-rich (Allred ≥6 for both ER and PR) luminal A cancers. In addition, patient preferences, geriatric assessments, and comorbidities should all be taken into consideration to ensure that NET is the most suitable treatment in a particular situation (50). Core biopsies can be used to assess ER and PR status before neoadjuvant therapy, but in view of the heterogeneity in tumor HR expression, it is preferable to test the tumor in the surgical excision specimen. According to St Gallen, less than four months duration of NET could be insufficient to achieve maximum reduction in tumor volume (51). The duration to the max response is the best, as a Spanish phase II NET trial revealed a median time to objective response was 3.9 months and a median time to maximal response was 4.2 months in postmenopausal patients (52). More than a third of the responders (37.1%) achieved maximal reduction in tumor volume after six months. treat patients for at least six months (or at least no less than four months). Beyond six months, continue NET until maximal response or up to the point where BCS becomes possible–always a decision to be taken in conjunction with the surgical team.
Future
Besides the improvement of new strategy of adjuvant endocrine therapy, more and more concerns about endocrine resistance. Hence, recently several novel compounds incorporated to the armamentarium of HR+ BC treatment. Such as everolimus (mTOR inhibitor) and palbociclib (CDK4-CDK6 kinases inhibitor) were approved by for the treatment of postmenopausal women with advanced HR+/HER2− disease in combination with AIs (53,54). Other drugs, such as pan-PI3k inhibitor, also showed success stories in the treatment of metastasis luminal BCs. Although these new agents can be associated with increased toxicity as compared to endocrine therapy alone, the incorporation of these agents in the early breast cancer scenario will eventually be a matter of careful evaluation of the risk/benefit ratio, and many studies are ongoing to evaluate these agents in adjuvant or neoadjuvant setting of luminal breast cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
References
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Grann VR, Troxel AB, Zojwalla NJ, et al. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer 2005;103:2241-51. [Crossref] [PubMed]
- Yi M, Huo L, Koenig KB, et al. Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann Oncol 2014;25:1004-11. [Crossref] [PubMed]
- Clemons M, Simmons C. Identifying menopause in breast cancer patients: considerations and implications. Breast Cancer Res Treat 2007;104:115-20. [Crossref] [PubMed]
- Cossetti RJ, Tyldesley SK, Speers CH, et al. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol 2015;33:65-73. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 1.2016. Available online: , accessed on 21 Mar, 2016.http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst 1996;88:1543-9. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771-84. [Crossref] [PubMed]
- Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med 2015;372:923-32. [Crossref] [PubMed]
- Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA 2011;306:269-76. [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014;371:107-18. [Crossref] [PubMed]
- Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009;360:679-91. [Crossref] [PubMed]
- Bernhard J, Luo W, Ribi K, et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol 2015;16:848-58. [Crossref] [PubMed]
- Bellet M, Gray KP, Francis PA, et al. Twelve-Month Estrogen Levels in Premenopausal Women With Hormone Receptor-Positive Breast Cancer Receiving Adjuvant Triptorelin Plus Exemestane or Tamoxifen in the Suppression of Ovarian Function Trial (SOFT): The SOFT-EST Substudy. J Clin Oncol 2016;34:1584-93. [Crossref] [PubMed]
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 2016;34:1689-701. [Crossref] [PubMed]
- Chumsri S. Clinical utilities of aromatase inhibitors in breast cancer. Int J Womens Health 2015;7:493-9. [Crossref] [PubMed]
- Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11:1135-41. [Crossref] [PubMed]
- Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol 2011;12:1101-8. [Crossref] [PubMed]
- Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010;28:509-18. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Dowsett M, Forbes JF, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-52. [Crossref] [PubMed]
- Goss PE, Ingle JN, Pritchard KI, et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol 2013;31:1398-404. [Crossref] [PubMed]
- Kaura S, Dranitsaris G. Letrozole or anastrozole for the treatment of hormone positive breast cancer: a clinical comparison using indirect statistical techniques. (abstract 224P). Ann Oncol 2010;21:viii81. Available online: ; cited March 21, 2016.http://annonc.oxfordjournals.org/content/21/suppl_8/viii78.full.pdf
- Rugo H, Kaura S, Dranitsaris G. Application of number needed to treat (nnt) to compare benefit: letrozole or anastrozole for the prevention of early recurrences in postmenopausal women with early stage breast cancer. (abstract 195P). Ann Oncol 2008;19:viii81. Available online: ; cited March 21, 2016.http://annonc.oxfordjournals.org/content/19/suppl_8/viii77.full.pdf
- O'Shaughnessy J, Yardley DA, Burris HA, et al. Abstract PD2-01: Randomized phase 3 trial of adjuvant letrozole versus anastrozole in postmenopausal patients with hormone receptor positive, node positive early breast cancer: Final efficacy and safety results of the femara versus anastrozole clinical evaluation (Face) trial. doi: . Cancer Res 2016;76:PD2-01. [Crossref]
- Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 2001;93:684-90. [Crossref] [PubMed]
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013;31:abstr 5.
- Rea DW, Gray RG, Bowden SJ, et al. Overall and subgroup findings of the aTTom trial: A randomised comparison of continuing adjuvant tamoxifen to 10 years compared to stopping after 5 years in 6953 women with ER positive or ER untested early breast cancer. Eur J Cancer 2013;49:S402. (abstract 1860).
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805-16. [Crossref] [PubMed]
- Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005;97:1262-71. [Crossref] [PubMed]
- Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 2007;99:1845-53. [Crossref] [PubMed]
- Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 2008;26:1965-71. [Crossref] [PubMed]
- Petrelli F, Coinu A, Cabiddu M, et al. Five or more years of adjuvant endocrine therapy in breast cancer: a meta-analysis of published randomised trials. Breast Cancer Res Treat 2013;140:233-40. [Crossref] [PubMed]
- Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196-205. [Crossref] [PubMed]
- Jordan VC. Tamoxifen: toxicities and drug resistance during the treatment and prevention of breast cancer. Annu Rev Pharmacol Toxicol 1995;35:195-211. [Crossref] [PubMed]
- Fisher B, Costantino JP, Redmond CK, et al. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst 1994;86:527-37. [Crossref] [PubMed]
- Loprinzi CL, Barton DL, Qin R. Nonestrogenic management of hot flashes. J Clin Oncol 2011;29:3842-6. [Crossref] [PubMed]
- Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol 2013;24:1443-9. [Crossref] [PubMed]
- Henry NL, Banerjee M, Wicha M, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer 2011;117:5469-75. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Coleman R, Powles T, et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015;386:1353-61. [Crossref] [PubMed]
- Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015;386:433-43. [Crossref] [PubMed]
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [PubMed]
- Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 2007;110:244-54. [Crossref] [PubMed]
- Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 2012;23:3069-74. [Crossref] [PubMed]
- Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat 2007;105 Suppl 1:33-43. [Crossref] [PubMed]
- Semiglazov VF, Semiglazov VV, Ivanov VG, et al. Neoadjuvant endocrine therapy: exemestane vs tamoxifen in postmenopausal ER+ breast cancer patients (T1-4, N1-2, M0). Proc Am Soc Clin Oncol 2005;23:abstr 530.
- Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 2005;23:5108-16. [Crossref] [PubMed]
- Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative "Arimidex" Compared to Tamoxifen (PROACT) trial. Cancer 2006;106:2095-103. [Crossref] [PubMed]
- Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol 2011;29:2342-9. [Crossref] [PubMed]
- Barroso-Sousa R, Silva DD, Alessi JV, et al. Neoadjuvant endocrine therapy in breast cancer: current role and future perspectives. Ecancermedicalscience 2016;10:609. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. -Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- Llombart-Cussac A, Guerrero Á, Galán A, et al. Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol 2012;14:125-31. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]


