Guidelines on the diagnosis and treatment of primary liver cancer (2011 edition)
1. Introduction
Primary liver cancer (PLC) is one of the common malignant tumors. Due to its insidious onset, lack of symptoms (or nonspecific presenting symptoms) in its early stage, and quick progression, PLC usually has been in its locally advanced stage or develops distant metastasis when it is confirmed; as a result, the management becomes extremely difficult and the prognosis usually is poor. If only symptomatic treatment is applied, the natural survival can be extremely short. In fact, PLC remains a major life-threatening disease in China.
PLC can be divided into several histological types including hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and mixed HCC and ICC. These types differ dramatically in terms of pathogenesis, biologic behaviors, histological features, clinical manifestations, treatment, and prognosis. Since HCC accounts for up to 90% of all PLC cases, PLC mainly refers to HCC in this document.
2. Diagnostic techniques and their applications
2.1 Screening and surveillance for PLC among high-risk populations
The main causes of PLC include chronic infection with hepatitis virus, dietary ingestion of high levels of aflatoxin, alcohol-induced liver injury, and algal-contaminated drinking water in rural areas. Other liver metabolic disorders, autoimmune diseases, and underlying liver diseases/cirrhosis can also play a role. Since the early diagnosis of PLC is particularly critical for the treatment efficacy and long-term survival, early screening and early surveillance should be emphasized. The routine monitoring/screening indicators/means include alpha-fetoprotein (AFP) and liver ultrasound (US). Examination should be arranged every six months for men ≥40 years and women ≥50 years who belong to the high-risk populations that have a history of HBV/HCV infection, alcoholism, and/or diabetes and a family history of liver cancer. It is generally believed that AFP is a relatively specific tumor marker for HCC; the continued AFP increase usually is a risk factor for HCC. In recent years, some western authors argue that AFP is not sensitive and specific enough; as a result, the 2010 American Association for the Study of Liver Diseases guidelines has excluded AFP as a screening indicator. However, most HCC cases in China are associated with hepatitis B virus infection, which differs from the pathogenic factors (mainly, hepatitis C virus infection, alcoholism, and metastatic factors) of HCC in western countries. Based on the findings of many domestic randomized and controlled trials (RCT) and the real conditions of China, it is recommended that AFP can still be used for the routine surveillance and screening of HCC.
2.2 Clinical manifestations
2.2.1. Symptoms
The pre-subclinical stage of PLC refers to the period from the onset of lesion to the diagnosis of subclinical liver cancer. This stage usually lasts ten months, during which the patients have no clinically-detectable symptoms or signs. During the subclinical stage (early stage) of PLC, the tumors, usually 3-5 cm in diameter, are still difficult to diagnose because most patients still have no typical symptoms. The disease usually is identified during serum AFP screening, typically 8 months after the onset of the lesion, during which a small proportion of patients may experience chronic underlying liver disease-related symptoms such as upper abdominal fullness, abdominal pain, fatigue, and loss of appetite. Therefore, high-risk individuals with the above conditions should be regularly screened for liver cancer. Once the typical symptoms of PLC occur, the disease often has been in its intermediate and late stages (typically 3-6 months), during which the disease progresses rapidly, with main clinical manifestations including:
(I) Pain in hepatic region. Right upper quadrant pain is most common, and also is a key symptom of this disease. It is often presented as intermittent or persistent insidious pain, blunt pain, or distending pain, which can be aggravated as the disease progresses. Lesion location is closely related to the development of pain: Lesions in the right lobe of liver can be accompanied with pain in the right hypochondriac region, and lesions in the left lobe with pain in the subcostal area; when the tumor invades the diaphragm, the pain can radiate to the right shoulder or right back; tumor grows to the right-back can cause the right flank pain. The main cause of pain is that tumor grows and thus tightens liver capsule. Sudden onset of severe abdominal pain and peritoneal irritation is usually due to the rupture and bleeding of cancer nodules under liver capsule.
(II) Loss of appetite. The patients may experience symptoms including epigastric fullness, indigestion, nausea, vomiting and diarrhea after meals. These non-specific symptoms are often neglected.
(III) Maransis and weakness. The patients can be systemically debilitated, and cachexia can occur in a few patients with advanced disease.
(IV) Fever; and Fever is common. Patients typically develop persistent low-grade fever ranging 37.5-38 ℃. In some cases, it can be irregular/intermittent, persistent, or remittent high-grade fever. Although the high fever is clinically similar to liver abscess, it is not accompanied with chills before its onset and is refractory to antibiotic therapy. In most cases, the fever is presented as cancerous fever, which is due to the absorption of tumor necrosis products; in fewer cases, the fever can also be caused by cholangitis, which occurs due to the oppression or invasion of the tumor mass, or other infections that may easily develop after the patient’s immune system is severely weakened.
(V) Symptoms related to the extrahepatic metastases for exmaple, pulmonary metastasis can cause cough or hemoptysis, pleural metastasis can cause chest pain and bloody pleural effusion, and bone metastases can cause bone pain or pathologic fractures.
(VI) Patients with advanced disease often develop jaundice, bleeding tendency (e.g., gum/nasal bleeding and subcutaneous ecchymosis), upper gastrointestinal bleeding, hepatic encephalopathy, and liver and kidney failure.
(VII) Paraneoplastic syndrome refers to a group of signs and symptoms caused by the metabolic disorder of a liver cancer tissue itself or by the disordered endocrine or metabolism due to the multiple effects of tumor tissue on human body. The clinical manifestations are diverse and non-specific. The common clinical manifestations include spontaneous hypoglycemia and polycythemia; other relatively rare manifestations include hyperlipidemia, hypercalcemia, precocious puberty, gonadotropin secretion syndrome, cutaneous porphyria, abnormal fibrinogen, and carcinoid syndrome.
2.2.2. Signs
At the early stage, patients with PLC may have few, if any, obvious signs. Mild hepatomegaly, jaundice and skin itching may be found in a few patients during routine health check-up. These signs may be the non-specific manifestations of the underlying liver diseases. The common signs of advanced PLC included jaundice, enlargement of the liver (firm consistency, uneven surface, and associated with or without nodules/vascular murmur) and peritoneal effusions. In patients with underlying hepatitis and/or cirrhosis, liver palms, spider telangiectasia, red moles, abdominal wall varices, and splenomegaly can be observed.
(I) Enlargement of the liver: The swollen liver shows progressive enlargement, hardness, uneven surface, and well-defined nodules (or even masses) of unequal size. Tenderness to touch is common. Prominent in the liver under the right costal arch or xiphoid, the upper abdomen may show partial or full uplift. Liver surface tumor located under the diaphragm is often featured by the localized diaphragmatic elevation, whereas the lower edge of the liver will not become swollen. Nodules located in the liver surface and close to the its lower edge are most palpable.
(II) Vascular murmur: Since liver cancer has rich and tortuous blood vessels, the sudden thinning of arteries or the crushing of tumor mass on liver arteries and abdominal aorta can result in the vascular blowing murmurs in the corresponding sites in nearly half of patients. This sign is valuable for diagnosis, but is less useful for early screening.
(III) Jaundice: Yellow staining of the skin and sclerae, often appears in the late stage, is mostly due to the obstruction of the bile duct resulted from the oppression of cancer or enlarged lymph nodes, and can also be caused by liver cell damage.
(IV) Portal hypertension: Most PLC patients have underlying cirrhosis, which is manifested as portal hypertension and splenomegaly. As a late manifestation, peritoneal effusions are typically transudate. The bloody effusion is often caused by the rupture of cancer into the abdominal cavity or by peritoneal metastasis. Portal vein and hepatic vein tumor thrombus can speed up the growth of peritoneal effusions.
2.2.3. Infiltration and metastasis
(I) Intrahepatic metastasis: Intrahepatic metastasis is the first way of liver cancer. The portal vein and its branches is frequently infringed, in which the tumor thrombi form. When shed, these tumor thrombi can cause multiple metastases in the liver. If portal vein is blocked by the tumor thrombi, the portal hypertension can be triggered or become worse.
(II) Extrahepatic metastasis:
(i) Hematogenous metastasis. Pulmonary metastasis is most common, and other metastatic sites include pleura, adrenal gland, kidney, and bone.
(ii) Lymphatic metastasis: Liver portal lymph node metastasis is most common. Other sites include pancreas, spleen, para-aortic lymph node, and, occasionally, supraclavicular lymph nodes.
(iii) Implantation metastasis: Implantation metastasis is relatively uncommon. The liver cancer cells shed from the surface for planting in the peritoneum, diaphragm, and thoracic cavity, causing bloody abdominal/pleural effusions. Ovarian metastasis may occur in female patients, forming relative large masses.
2.2.4. Common complications
(I) Upper gastrointestinal bleeding: PLC is often accompanied with hepatitis, underlying cirrhosis, and portal vein hypertension, whereas tumor thrombus in portal vein and hepatic vein can often intensify portal hypertension, and thus cause the variceal bleeding of the middle and lower portions of the esophagus and the gastric vein. After the cancer cells invade bile duct, the patient will develop biliary tract bleeding, hematemesis, and melena. Some patients may suffer from extensive bleeding due to gastrointestinal mucosal erosions, ulcers, and coagulation disorders. Bleeding can result in shock and hepatic coma.
(II) Hepatic nephropathy and hepatic encephalopathy (hepatic coma): The advanced liver cancer (especially diffuse liver cancer) can cause liver dysfunction or even liver failure, resulting in hepatorenal syndrome (HRS) (or functional acute renal failure, FARF), featured by oliguria and lower blood pressure, which are often associated with progressive hyponatremia, hypokalemia, and azotemia, Hepatic encephalopathy (HE) (or, hepatic coma) is often a manifestation of end-stage liver cancer, and is often triggered by gastrointestinal bleeding, massive use of diuretics, electrolyte imbalance, and secondary infection.
(III) Rupture and bleeding of liver nodules: Rupture and bleeding of liver nodules is the most urgent and severe complication of liver cancer. At the late stage, the cancer foci can develop spontaneously after necrosis and liquefaction or due to external forces. Therefore, palpation during physical examination should be performed gently. Avoid touching the abdominal region forcefully. The rupture of nodules can be localized within liver capsule, causing abrupt pain and rapid enlargement of the liver; A localized soft mass was palpable; once the ruptured mass penetrates into the abdominal cavity, it can cause acute abdominal pain and peritoneal irritation. Slight bleeding can be presented as bloody peritoneal effusion, whereas massive bleeding can cause shock even sudden death.
(IV) Secondary infections: Patients with liver cancer often have weakened immune function due to prolonged exertion and bed rest; therefore, they are susceptible to multiple infections including pneumonia, intestinal infections, fungal infections and sepsis, particularly when the white blood cells decrease after chemotherapy or radiotherapy.
2.3. Auxiliary examinations
2.3.1. Blood biochemistry
Abnormal liver function including elevated aspartate aminotransferase (AST or GOT), alanine aminotransferase (ALT or GPT), serum alkaline phosphatase (AKP), lactate dehydrogenase (LDH) and bilirubin and decreased albumin can occur in PLC patients; meanwhile, immunological indicators including lymphocyte subsets can also change. Positive hepatitis B surface antigen (HBsAg), positive results of routine detection of HBV (including HBsAg, HBeAg, HBeAb and anti-HBc), and/or positive hepatitis C antibody (anti-HCV IgG, anti-HCVst, anti HCVns, and anti HCV IgM) are key markers of hepatitis virus infection, whereas HBV DNA and HCV mRNA can reflect the hepatitis C viral load.
2.3.2. Tests for tumor markers
Serum AFP and lectin reactive AFP are key indicators and the most specific tumor markers for the diagnosis of PLC. They have been widely used for the screening, early diagnosis, post-operative monitoring, and follow-up of PLC. For patients with AFP ≥400 μg/L for more than one month or ≥200 μg/L for over two months and the possibility of pregnancy, embryonal carcinoma of genital gland, or active liver disease is ruled out, PLC should be highly suspected. More importantly, concurrent imaging examinations (CT/MRI) should be performed to identify PLC-specific lesions. Still, AFP can be negative in 30-40% of PLC, which include ICC, well-differentiated and poorly differentiated HCC, or HCC filled with liquefied necrotic tissues. Therefore, AFP alone is insufficient to diagnose all PLC. The positive rate of AFP is 60 to 70 percent in PLC patients, and sometimes can show even more diverse results. Therefore, routine detection and dynamic observation should be enhanced; meanwhile, the diagnosis should be confirmed by imaging examination or ultrasound-guided biopsy.
Other markers that can be used for the auxiliary diagnosis of HCC include many serum enzymes such as γ-glutamyltransferase (GGT) and its isoenzymes, α-L-fucose glycosidase (AFU), abnormal Des-gamma-carboxy prothrombin (DCP), Golgi protein 73 (GP73), 5-nucleotide phosphodiesterase (5’NPD) isozyme, aldolase isozyme A (ALD-A), and placental glutathione S-transferase (GST). Meanwhile, ferritin (FT) and acidic ferritin (AIF) can also be useful. Carcinoembryonic antigen (CEA) and carbohydrate antigen CA19-9 can also abnormally increase in some HCC patients.
2.3.3. Radiological examinations
(I) Abdominal ultrasound (US) US has became the most important examination for liver disease because it is simple, intuitive, non-invasive, and inexpensive. By identifying the intrahepatic space-occupying lesion, suggesting its nature, identifying the liquid or solid lesions, locating the foci in the liver, and confirming their relationship with the key intrahepatic vessels, US is valuable for the treatment decision-making and guiding the surgical operation. Furthermore, US can also display the dissemination and infiltration of liver cancer within the liver and its neighboring tissues/organs. The real-time contrast-enhanced US (CEUS) can be used to observe the hemodynamics of the lesion and thus is helpful for qualitative diagnosis; however, it may yield false positive results in ICC patients. On the contrary, intra-operative US can directly explore the liver surface and avoid the ultrasound attenuation and the signal interference from abdominal wall and ribs, and thus can identify small intrahepatic lesions that are missed by pre-operative radiological examinations.
(II) Computed tomography (CT): CT is the most important radiological modelity for the diagnosis and differential diagnosis of HCC. It can be applied for the observation of the morphology and blood supply of tumors, for the detection, characterization, and staging of HCC, and for the follow-up examination after treatment of HCC. CT has high resolution. In particular, the multi-slice spiral CT has extremely fast scanning speed, which enables it to complete whole liver scanning within seconds, avoiding respiratory motion artifacts. CT is capable of performing multi-phase dynamic contrast-enhanced scan with the minimum scan thickness of 0.5 mm, significantly improving the detection rate of small HCC lesions and the accuracy of characterization. HCC usually is shown as hypodense space-occupying enhancement with clear or indistinct borders on unenhanced scan, occasionally with Halo signs. Large hepatic carcinomas commonly have central liquefaction/necrosis, which is suggestive of the nature of a lesion, helps to understand the existence of lesions in surrounding tissues or organs, and facilitates positioning during radiotherapy. enhanced scan can not only clearly display the number, size, shape and enhancement patterns of a lesion, but also definitely determine the relationship between the lesion and major blood vessels, the existence of lymph node enlargement in the hepatic hilum and abdomen as well as invasion of adjacent organs, which can provide a reliable basis for accurate staging in clinical practice and be helpful in identifying hepatic hemangiomas. Typical imaging manifestations of HCC include significant enhancement in the arterial phase, enhancement inferior to adjacent liver tissues in the venous phase and continuous subsidizing of the contrast agent in the delayed phase. Thus, enhanced scan is highly specific.
(III) Magnetic resonance imaging (MRI or MR): MRI has no radioactive radiation, high tissue resolution, and capability of multi-faceted and multi-sequence imaging, and therefore is better than CT or US in displaying and distinguishing structure changes within the HCC lesion, such as hemorrhagic necrosis and fatty degeneration as well as the capsule. MRI is superior to CT in identifying malignant or benign intrahepatic space-occupying carcinomas, in particular hemangiomas; meanwhile, it can display the portal vein or hepatic vein branches without enhancement; many evidences show that MRI is superior to CT in identifying small HCC. Especially, with the further development and application of the high field-strength MR equipment, the speed of MR scan has been greatly accelerated and now MRI can complete thin-layer, multi-phase dynamic enhanced scan, just as CT does, to fully demonstrate the enhancement patterns of a lesion and improve the lesion detection rate and qualitative accuracy. In addition, functional MR imaging techniques (such as diffusion-weighted imaging, perfusion-weighted imaging and spectral analysis) as well as the application of hepatocyte-specific contrast agents can all provide valuable additional information for lesion detection and characterization, which can further help to improve the detection sensitivity rate and qualitative accuracy of HCC as well as assess the efficacy of a variety of local treatments comprehensively and accurately.
The above three radiological modalities have their own features and advantages and therefore should be applied in an integrated and coordinated manner.
(IV) Elective hepatic artery digital subtraction angiography (DSA): DSA is most frequently adopted to clearly demonstrate small hepatic lesions and their blood supply in parallel with chemotherapy, lipiodol embolism and other treatments. The main manifestations of HCC on DSA include:
(i) Tumor blood vessels, found in the early arterial phase;
(ii) Tumor staining, found in the substantive phase;
(iii) Intrahepatic arterial shift, straightening and twisting, etc. visible in larger tumors;
(iv) Liver tumor’s invasion of intrahepatic arteries, manifested as the serrated, beaded or rigid status;
(v) Arteriovenous fistula; “pool-like” or “lake-like” contrast agent filling area, etc.
DSA can be applied not only in the diagnosis and differential diagnosis but also in preoperative or pre-treatment estimation of the extent of a lesion, especially of intrahepatic spread of sub-nodules. DSA can also provide correct and objective information including the vascular anatomic variations and the anatomical relations of major blood vessels as well as portal vein infiltration, which is of great value for judging the possibility of surgical resection and its thoroughness and determining appropriate treatment options. DSA is an invasive and traumatic technique that can be used for patients not yet confirmed after undergoing other tests. In addition, pre-surgical DSA has been proposed for resectable HCC, even those with limited imaging performance; by doing so, DSA may detect lesions non-detectable by other imaging tools and identify any potential vascular invasion.
(V) Positron emission tomography-computed tomography (PET-CT): PET-CT is a functional molecular imaging system integrating PET and CT, which can not only reflect the biochemical metabolic information of space occupation in liver through functional PET imaging, but also provide precise anatomical positioning of a lesion by CT morphological imaging. Meanwhile, whole body scan can understand the general conditions and assess the metastasis, so as to achieve the early detection of lesions and the understanding of the size and metabolic changes before and after tumor treatment. However, the sensitivity and specificity of PET-CT for clinical diagnosis of HCC still needs to be further improved. Since it has not yet universally applied in most hospitals in China, it is not recommended as a routine examination in diagnosis of HCC. Rather, it can serve as an alternative tool for other radiological examinations.
(VI) Single photon emission computed tomography (ECT): ECT whole-body bone imaging can contribute to the diagnosis of bone metastasis of HCC. Compared with X-ray and CT, it can detect bone metastasis 3-6 months earlier.
2.3.4. Needle biopsy of liver
Ultrasound-guided percutaneous liver puncture core needle biopsy (Core biopsy) or fine needle aspiration (FNA) for histological or cytological examination can obtain the pathological diagnostic evidence of HCC and determine the molecular markers, and therefore is especially useful for the definitive diagnosis, pathological classification, disease assessment, treatment decision-making, and prognosis. It has been increasingly adopted in recent years. However, there are still certain limitations or risks. Liver bleeding or needle tract implantation of HCC should be avoided during needle biopsy of liver. It should not be performed in patients with severe heart, lung, brain or kidney disorders and/or systemic failure and those with an obvious tendency of bleeding.
2.4. Diagnostic criteria for liver cancer
2.4.1. Pathological diagnosis criteria
The pathohistologically and/or cytologically confirmed malignancy in the biopsy or resection specimens of intrahepatic space-occupying lesion or extrahepatic metastastic lesion remains the “gold standards” for the diagnosis of HCC.
2.4.2. Clinical diagnostic criteria
HCC is the only solid tumor that can be diagnosed using clinical diagnostic criteria that are widely recognized in China and abroad and featured by non-invasiveness, simplicity, and operability. The diagnosis usually is depended on three major factors: the underlying chronic liver disease, results of imaging examinations, and serum AFP level. However, the knowledge and specific requirements about the above factors change from time to time and their applications in clinical practice also have certain errors. Therefore, based on the real conditions in China as well as the domestic standards and clinical practices, the expert panel proposed that a relatively stricter criteria should be implemented. Specifically, a clinical diagnosis of HCC can be established if the following two items [(I) and (II) (i)] or three items [(I) + (II) (ii) + (III)] are fulfilled:
(I) With the evidences of cirrhosis and HBV and/or HCV infections (positive HBV and/or HCV antigen);
(II) Typical imaging findings of HCC: multi-slice helical computed tomography and/or dynamic contrast-enhanced MRI shows the arterial hypervascularity and venous or delayed phase washout of the focal liver lesion.
(i) If the focal liver lesion is ≥2 cm in diameter and either CR or MRI shows that the lesion has the above features of liver cancer, a diagnosis of HCC can be made;
(ii) For a space-occupying lesion about 1-2 cm in diameter in the liver, only if both two radiological modalities (CT and MRI) show that the lesion has the features of liver cancer can a diagnosis of HCC can be made, so as to ensure the specificity of the diagnosis.
(III) AFP level ≥400 μg/L lasts for at least one month or ≥200 μg/L for at least two months, and meanwhile the other causes (e.g., pregnancy, genital/embryonic tumors, active liver disease, and secondary liver cancer) of high AFP level are ruled out.
2.4.3. Other considerations
(I) Many foreign guidelines, including the clinical practice guidelines developed by American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL) and National Comprehensive Cancer Network (NCCN) of the United States, emphasize the importance of multi-slice CT and/or dynamic contrast-enhanced MRI, conducted in experienced radiological centers, for space-occupying lesions in the liver. Meanwhile, these guidelines argue that a quadruple-phase protocol that included unenhanced, hepatic arterial, portal venous, and delayed phases should be performed to ensure the accuracy of the radiological diagnosis of HCC. A repeat dynamic CT scan using a 5-mm thin slice method may be preferred for local lesions. The role of enhancement in the arterial phase should never be neglected during radiological examinatins. HCC is featured by the obviously enhanced lesion during the early arterial phase; the lesion usually is hypertense in relation to the normal liver tissue. On the contrary, such enhancement rapidly disappears during the venous phase, and the lesion become hypotense in relation to the surrounding liver tissues. If the radiological features of a space-occupying lesion in liver are not typical, or if CT and MRI show non-consistent findings, liver biopsy should be considered; nevertheless, even a negative result of biopsy can not thoroughly rule out the possibility of HCC, and further follow-up and observation is warranted.
(II) In recent years, clinical observational studies and trials both in China and abroad have demonstrated that serum AFP level also increases in some ICC patients or patients with “liver metastasis from gastric cancer”, and a large proportion of ICC patients can also be accompanied with cirrhosis. Although the incidence of ICC is far lower than that of HCC, both of them are commonly seen in cirrhotic patients. Therefore, space-occupying lesion in liver accompanied with elevated AFP does not necessarily mean HCC, which should be cautiously differentiated from other conditions. In China and many other Asia-Pacific countries, elevated AFP level is mainly due to HCC, and therefore is still valuable in differentiating HCC from ICC. Therefore, it is still used as a diagnostic indicator in these guidelines.
(III) In patients with serum AFP ≥400 μg/L, if ultrasound shows no space-occupying lesion in liver, the possibility of pregnancy, genital/embryonic tumors, active liver disease, and hepatoid adenocarcinomas of the gastrointestinal tract should be carefully ruled out. After these conditions are ruled out, multi-slice CT and/or dynamic contrast-enhanced MRI should be timely performed. If typical radiological features of HCC (the blood supply is rich during arterial phase and subsides during portal venous phase or delayed phase) is present, a diagnosis of HCC can be made. If the radiological findings are atypical, other imaging modalities may be adopted for contrast-enahnced examination; or, a biopsy may be performed on the liver lesion. It is insufficient to diagnose an HCC if only enhancement is found during arterial phase while no subsidence is observed during the venous phase. If AFP increases but does not reach the diagnostic threshold, similar efforts should be made to rule out the above conditions that may cause the increase of AFP. Furthermore, the changes of AFP should be closely monitored and tracked; meanwhile, the ultrasound surveillance interval should be decreased to 1-2 months, and, when necessary, CT and/or MRI dynamic observation should be arranged. If a liver cancer is highly suspected, an elective digital subtraction angiography (DSA) is recommended; liver biopsy should be conducted if necessary.
(IV) Patients should be closely monitored if the following conditions exist: space-occupying lesion(s) in liver is found; serum AFP level does not increase; radiological examinations show no typical radiological features of HCC; and the lesion is <1 cm in diameter. If the space-occupying lesion in the liver shows no vascular enhancement during dynamic imaging, the possibility of malignancy can be low. If the lesion gradually grows or becomes ≥2 cm in diameter, ultrasound-guided liver biopsy should be further conducted. Even if the biopsy shows negative results, the possibility of HCC should not be ruled out easily. Follow-up should be carefully arranged: the patient should receive radiological follow-up every 6 months until the lesion disappears, grows, or presents with the typical features of HCC. If the lesion grows but still shows not typical features of HCC, a repeated liver biopsy may be considered.
(V) Notably, among Chinese HCC patients, 5-20% of them have no history of cirrhosis, about 10% have no evidence of HBV/HCV infection, and about 30% have serum AFP lower than 200 μg/L throughout the disease course. Meanwhile, although the majority of HCC have rich blood supply radiologically, a small proportion of them do lack blood vessels. In addition, literature from western countries have reported that nonalcoholic steatohepatitis (NASH) can progress to cirrhosis and the HCC (i.e. NASH-related HCC); however, no relevant data have been available in China.
2.5. Differential diagnosis
2.5.1. HCC should be carefully differentiated from the following conditions when serum AFP is positive
(I) Chronic liver diseases such as hepatitis and cirrhosis. The serum AFP level should be dynamically observed. In patients with active liver disease, the change of AFP synchronize with that of ALT; it is featured by transient elevation or repeated fluctuation, but usually does not exceed 400 μg/L. Based on liver function tests, comprehensive observation and analysis should be performed. Sometimes the curves of AFP and ALT are separated: AFP increases whereas SCPT decreases (i.e. AFP changes in different direction with ALT); or, AFP remains at a high level. Under such conditions, the possibility of HCC should be considered.
(II) Tumors occur during pregnancy or at genital glands or embryonal tumors: the differentiation is mainly based on medical history, physical examination, abdominal/pelvic cavity ultrasound, and computed tomography.
(III) Gastrointestinal cancer: some adenocarcinoma in gastrointestinal tract and pancreas, known as hepatoid adenocarcinoma, can also cause the increase of AFP. Differential diagnosis should be based on detailed medical history, physical examination and imaging findings; meanwhile, determination of serum AFP variant levels are helpful to identify the origin of the tumor. In patients with hepatoid adenocarcinoma, the percentage of AFP reactive with Lens culinaris agglutinin (LCA) is high.
2.5.2. HCC should be carefully differentiated from the following conditions when serum AFP is negative
(I) Secondary liver cancer: secondary liver cancer is seen more commonly in patients with gastrointestinal metastasis, and also in those with lung cancer and breast cancer. Patients can have no underlying liver disease. The patients may present with the manifestations (e.g., hemafecia, abdominal discomfort, anemia, and weight loss) of a gastrointestinal tumor; their serum AFP may be normal, while gastrointestinal tumor markers including CEA, CA199, CA50, CA724, and CA242 may increase. Findings of imaging examinations:
(i) It is usually presented as multiple space-occupying lesions, whereas HCC is often solitary;
(ii) There is typical radiological findings of a metastatic tumor such as Bull’s-eye sign (the center lacks blood supply and shows hypoechoic or hypodense lesion, with a peripheral rim of enhancement);
(iii) Contrast-enhanced CT or DSA shows that the tumor has relatively few blood vessels, and its blood supply is not as rich as HCC;
(iv) Primary tumor in the gastrointestinal tract may be visible under gastrointestinal endoscopy or X-ray examination.
(II) Intrahepatic cholangiocarcinoma (ICC): It is a rare pathologic type of PLC, with 30-50 years as the most risky age group. The clinical symptoms are non-specific, and the patients usually have no history of liver disease. AFP does not increase in most patients, whereas CEA, CA199, and some other tumor markers may also increase. Unenhanced CT often shows lobular or oval hypodense areas of different sizes. These lesions have uneven density, with blurred or unclear margin. More importantly, enhanced CT showes that the blood supply in the space-occupying lesion in the liver is not as rich as HCC; meanwhile, there is relatively higher proportion of fiber components, resulting in delayed enhancement, which is featured by “fast-in and slow-out”. Irregular expansion of intrahepatic bile duct can sometimes be found in the surrounding tissues. Other findings may include atrophy of hepatic lobe, retraction of liver capsule, and sometimes string sign (linear high density lesion inside liver tumor and/or the surrounding parenchyma. However, the confirmation of this disease is mainly based on post-operative pathological findings, and only a small proportion is confirmed by radiological examinations.
(III) Hepatic sarcoma: Patients of this disease often have no history of liver disease. Radiological examinations can display homogeneous solid space-occupying lesions with rich blood supply, which is difficult to be differentiated from AFP-negative HCC.
(IV) Liver benign lesions: they may include:
(i) Hepatic adenomas: patients of this disease often have no history of liver disease. Women are more susceptible to hepatic adenomas, espeically in those who use oral contraceptives frequently. It is difficult to distinguish from well-differentiated HCC. Radionuclide imaging (99mTc hepatic scan) is useful for the differential diagnosis: the hepatic adenoma can uptake radionuclide, and highly positive imaging is visible during the delayed phase.
(ii) hepatic hemangioma: patients of this disease often have no history of liver disease. Women are more susceptible to this disease. Contrast-enhanced CT may show the enhancement of the lesion associated with opacification of its border, which is featured by “fast-in and slow-out” (which distinguishes from the “fast-in and fast-out” in HCC). The typical “light bulb” sign can be seen under MRI.
(iii) Liver abscess: patients with liver abscess often have a history of dysentery or suppurative disease but without a history of liver disease. They may have (or previously had) the manifestations of infection including fever and increased white blood cells and neutrophils. Localized edema as well as right upper quadrant tenderness and muscle tension may be present on the chest wall where the abscess is nearest to the surface. Liver abscess, when it is not liquefied or contains thick pus, is easily misdiagnosed as “liver cancer” under ultrasound; after having been liquefied, the liver abscess shows fluid sonolucent area, which should be distinguished from the area of necrosis in the center of the liver cancer. DSA shows no tumor vessel or staining. Fine needle aspiration may be performed at the tenderness point, if required. The empirical anti-amebic treatment is usually provides a good chance for differential diagnosis.
(iv) Hepatic echinococcosis: the liver is progressively enlarged, together with firm consistency a sense of nodules. In its advanced stage, most of the liver has been damaged, with clinical manifestations extremely similar to liver cancer. Nevertheless, hepatic echinococcosis typically has a long disease course and progresses slowly. A peculiar and very characteristic tremor (hydatid fremitus) may be felt in diagnosis by percussion. Patients often are residents in endemic pastoral areas and/or have a history of living with dog sheep, goat, etc. The Casoni test is a highly specific skin test used in the diagnosis of hydatid disease, with a detection rate up to 90-95%. Ultrasound can show hyperechoic floating ascus in the cystic space- occupying cavity, whereas CT sometimes can detect the scolex surrounded by a calcified cyst wall. Biopsy may trigger severe allergic reactions and therefore should not be performed.
2.6. Pathologic diagnosis
Histopathology and/or cytology provides the basis of the gold standard for the diagnosis of liver cancer. However, pathological diagnosis should still be combined with clinical evidences including the HBV/HCV infection, the results of serum AFP and other tumor markers, and the imaging findings of liver lesions. Currently, examinations based on modern molecular biology including genomics, proteomics, and metabolic enzymology are under development. These approaches will have higher specificity and accuracy, and can provide more information for predicting the treatment response, the trend of metastasis and relapse, and the prognosis. Pathological diagnosis for PLC should differentiate the following three major pathologic types and pay attention to other less common types:
(I) Hepatocellular carcinoma (HCC): HCC accounts for over 90% of PLC and is the most common pathologic type.
(i) Morphological typing: HCC can be morphologically divided into nodular, massive, and diffuse types. Also, the “Five Types-Six Subtypes”, which was defined by Chinese Pathological Research Cooperation Panel for Liver Cancer in 1977, can also be adopted. The liver tumor <1 cm in diameter is called as microcancer, 1-3 cm as small liver cancer, 3-5 cm as intermediate liver cancer, 5-10 cm as large liver cancer, and >10 cm as massive liver cancer. Currently, the Chinese diagnostic criteria for small liver cancer is as follows: the maximal diameter of a single nodule is ≤3 cm; or, the number of multiple nodules is up to 2, with the sum of maximal diameters ≤3 cm. In addition to their small sizes, most small liver cancers are featured by single nodule and expansive growth. They often have clear border with the neighboring liver tissues or have capsule formation. These small cancers grow slowly and have relatively low malignancy and low possibility of metastasis, and therefore the prognosis tends to be good.
(ii) Histological features: The majority of cancer cells shows trabecular lining. The cancer cells were polygonal in shape, with eosinophilic cytoplasm and round nucleus. Sinusoidal spaces exists within the trabeculae. Many other cytological and histological types can be found. Pseudoglandular structure can mimic intrahepatic cholangiocarcinoma and metastatic adenocarcinoma, and therefore should be carefully differentiated. The differentiation of cancer cells can be classified into four grades using Edmondson-Steiner grading system, or as three levels (well, moderately, and poorly).
(iii) Representative immunohistochemical markers: Hepatocyte Specific Antigen (Hep-Par1) may be positive expressed in the cytoplasma of liver cancer cells. Polyclonal carcinoembryonic antigen (pCEA) may be positively expressed in the capillary bile duct of membrane. CD34 can be diffusely distributed along the liver sinusoidal microvascular channels. Glypican-3 (GPC-3) is often expressed in the cytoplasm of HCC cells. Histopathological assessment of liver biopsy for small lesions should be conducted by experienced pathologists. Immunohistochemical staining for GPC-3, heat shock protein 70 (HSP70), and glutamine synthetase (GS) has been suggested, and a diagnosis of HCC can be made if two of them are confirmed to be positive.
(II) Intrahepatic cholangiocarcinoma (ICC): ICC is relatively rare. Accounting for about ≤5% of PLC, it originate from the epithelial cells in branches of bile ducts and intrahepatic bile duct.
(i) Morphological typing: ICC can be morphologically classified as nodular, peritubular infiltration, nodular infiltration, and intratubular growth types.
(ii) Histological features: the majority of ICC has the histological structure of adenocarcinoma. Usually the tumor forms a glandular pattern, which mimics the lumen of the bile ducts. Mucus may be secreted but not bile. The cancer cells usually are cuboidal or low columnar, with slightly stained and/or clear cytoplasm. An abundant fibroblastic stroma is usually present; in other words, there are a large number of fibrous tissues around the cancer cells. Many other cytological and histological types can be found. If trabecular lining exists, the disease should be cautiously differentiated from HCC. The differentiation of ICC can also be classified as “well”, “moderately”, and “poorly”.
(iii) Representative markers: Immunohistochemical examinations for cytokeratin 19 (CK19) and mucin 1 (MUC-1) show positive expressions in cytoplasma.
(III) The mixed type of liver cancer: cancer tissue has both HCC and ICC structure. This type is relatively rare. Two components (HCC and ICC) exist simultaneously in a same liver tumor nodule. Both of them are mixed, with ill-defined border, expressing their own representative immunohistochemical markers.
(IV) Other types. PLC also includes some rare types including clear cell carcinoma, giant cell carcinoma, nodular sclerosis carcinoma, and fibrolamellar carcinoma of liver (FLC). Notably, FLC is a special and rare histological subtype of HCC. It is often seen in young patients (<35 years) without underlying hepatitis B virus infection or cirrhosis. Usually being localized, the tumor is not as malignant as HCC and can be surgically resected. The prognosis is relatively good. The tumor (usually single) is typically located at the left lobe of liver. It has clear border, fan-shaped margin, and firm consistency. Fibrous septa going through the tumor can be observed under dissection pictures. Microscopy shows that tumor cells were nest-shaped, some of which are arranged in anastomosing cords, together with the enveloping of dense fibrous tissues. The tumor cells were cubic or polygonal, relatively large; the cytoplasm is abundant and highly acidophilic; in addition, these cells have prominent nucleoli and the sinusoids inside the tumor tissue are rich.
(V) Main content of a histological report The histological report for liver cancer should be standardized. The content should include the size and number of tumor(s), growth pattern, pathological type, vessel cancer embolus, histological type, degree of differentiation, capsular invasion, satellite lesions, surgical margin, and para-cancerous liver tissue (grading and staging of chronic viral hepatitis and types of cirrhosis), immunohistochemistry, and molecular pathological indicators. In addition, determination of the molecular markers for the targeted therapy, biological behavior, and prognosis of liver cancer can also provide useful evidences for clinical decision-making (Appendix 1).
3. Typing and staging of liver cancer
3.1. WHO histological classification of tumors of the liver and intrahepatic bile ducts (2005)
Epithelial tumors
Benign
Hepatocellular adenoma 8170/0
Focal nodular hyperplasia
Intrahepatic bile duct adenoma 8160/0
Intrahepatic bile duct cystadenoma 8161/0
Biliary papillomatosis 8264/0
Malignant
Hepatocellular carcinoma (liver cell carcinoma) 8170/3
Intrahepatic cholangiocarcinoma 8160/3
Bile duct cystadenocarcinoma 8161/3
Combined hepatocellular and cholangiocarcinoma 8180/3
Hepatoblastoma 8970/3
Undifferentiated carcinoma 8020/3
Non-epithelial tumours
Benign
Angiomyolipoma 8860/0
Lymphangioma and lymphangiomatosis 9170/3
Hemangioma 9120/0
Infantile hemangioendothelioma 9130/0
Malignant
Epithelioid hemangioendothelioma 9133/1
Angiosarcoma 9120/3
Embryonal sarcoma (undifferentiated sarcoma) 8991±3
Rhabdomyosarcoma 8900/3
Others
Miscellaneous tumors
Solitary fibrous tumor 8815/0
Teratoma 9080/1
Yolk sac tumour (endodermal sinus tumour) 9071/3
Carcinosarcoma 8980/3
Kaposi sarcoma 9140/3
Rhabdoid tumor 8963/3
Others
Hematopoietic and lymphoid tumors
Secondary tumours
Epithelial abnormalities
Liver cell dysplasia (liver cell change)
Large cell type (large cell change)
Small cell type (small cell change)
Dysplastic nodules (adenomatous hyperplasia)
Low-grade
High-grade (atypical adenomatous hyperplasia)
Bile duct abnormalities
Hyperplasia (bile duct epithelium and peribiliary glands)
Dysplasia (bile duct epithelium and peribiliary glands)
Intraepithelial carcinoma (carcinoma in situ) 8500/211
Miscellaneous lesions
Mesenchymal hamartoma
Nodular transformation
Inflammatory pseudotumor
3.2. Staging of lung cancer
3.2.1. TNM classification (UICC/AJCC, 2010)
T - Primary Tumor
TX: Primary tumor cannot be evaluated
T0: No evidence of primary tumor
T1: Solitary tumor without vascular invasion
Solitary tumor with vascularinvasion; or multiple tumors, none more than 5 cm in greatest dimension.
T3a: There is more than one tumor, and at least one is larger than 5 cm.
T3b: Solitary tumor or multiple tumors involve amajor branch of the portal or hepatic vein(s)
T4: Tumour(s) withdirect invasion of adjacent organs; ortumour(s) with perforation of gallbladder or other organs
N-Regional lymph nodes
NX: Regional lymph nodes cannot be evaluated.
N0: Without lymph node metastasis
N1: The cancer has spread to the regional lymph nodes.
M-Distant metastasis
MX: Distant metastasis can not be evaluated
M0: No distant metastasis
M1: Distant metastasis.
Stage grouping:
Stage I: T1N0M0
Stage II: T2N0M0
Stage IIIA: T3aN0M0
Stage IIIB: T3bN0M0
Stage IIIC: T4N0M0
Stage IVA: Any T, N1M0
Stage IVB: Any T, any N, M1
TNM staging system divides the solid tumors into four stages (from benign to potentially fatal) using the size, number and extension of the primary tumor, its lymphatic involvement, and the presence of metastases. Although it provide a detailed (and most standardized) description of the progression of cancer, it is less recognized globally due to the following reasons:
(I) Although most PLC patients are accompanied with severe cirrhosis, the TNM staging system does not describe the liver function. In fact, compensation of liver function is critical for HCC treatment, and liver function can dramatically affect prognosis and treatment choice.
(II) Although vascular invasion is highly important for the treatment and prognosis of HCC, it is usually difficult to accurately judge before treatment (particularly before surgery).
(III) The TNM stages have changed from time to time among different editions, making the comparison and assessment more difficult.
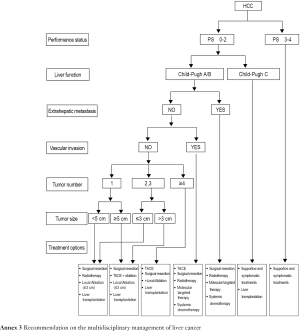
3.2.2. Barcelona Clinic Liver Cancer (BCLC) Staging Classification (Table 1)
The BCLC staging and treatment strategy is now adopted worldwide because it comprehensively assesses the tumor, liver function, and systemic conditions, is appropriately linked with treatment principles, and has been supported with high-grade evidences. However, HCC in Asia (except Japan and Indonesia) is highly heterogeneous, and is remarkably different from that in Western countries, in terms of etiology, stage, malignant biological behavior, diagnosis and treatment (therapeutic concepts and clinical practices), and prognosis. Meanwhile, many Chinese surgeons believe that the indications for surgery are too strict in BCLC staging and treatment strategy and therefore do not suit the real conditions and clinical practices in China. Therefore, this strategy is for reference only in China.

Full table
3.2.3. Performance status (PS) scales
By assessing the performance status (PS), these scales are used to assess the general health and treatment tolerance of a patient from the perspective of physical performance. For HCC patients, the PS is usually scored using Eastern Cooperative Oncology Group (ECOG) scoring system as follows:
Grade 0: fully active, able to carry on all pre-disease performance without restriction.
Grade 1: restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light house work, office work.
Grade 2: ambulatory and capable of all selfcare but unable to carry out any work activities. Up and about more than 50% of waking hours.
Grade 3: capable of only limited selfcare, confined to bed or chair more than 50% of waking hours.
Grade 4: completely disabled. Cannot carry on any selfcare. Totally confined to bed or chair.
Grade 5: dead.
3.2.4. Assessment of liver function reserve
The liver parenchymal functions are assessed using Child-Pugh grading system (Table 2) and indocyanine green (ICG) clearance test. Liver size can be used as an important indicator for the liver functional reserve. It can facilitate the selection of surgical procedure by objectively reflecting the liver size and liver parenchymal volume, indirectly reflecting the blood perfusion and hepatic metabolic capacity, and objectively assessing the patient's tolerance of the procedure. For liver cancer larger than 3 cm in diameter, CT and/or MRI can be performed to estimate the size of the liver remaining after resection. Standard remnant liver volume (SRLV) is an effective and simple indicator for assessing the liver function reserve after liver resection. Clinically, it can be used to predict the severity of the impaired liver function and avoid post-operative liver failure. Research has shown that the incidence of moderately or severely incomplete compensation of liver function is high among Chinese patients with an SRLV <416 mL/m2.

Full table
ICG clearance test has good reproducibility and mainly reflects the uptake capacity of the hepatocytes (the volume of functional hepatocytes) and hepatic blood flow. A dose of 0.5 mg/kg ICG is administered intravenously, and the retention rate of ICG in blood after 15 minutes (ICG-R15) is then calculated. An ICG-R15 lower than 12% is considered to indicate normal liver function. Furthermore, the liver blood flow can be determined using the clearance curve.
4. Surgical treatment
Liver cancer surgery includes mainly liver resection and liver transplantation.
4.1. Liver resection (hepatectomy)
4.1.1. Basic principles of liver resection
(I) Thoroughness: remove the tumor as completely as possible so that no residual tumor is detected on the surgical margin;
(II) Safety: spare normal hepatic tissues for reduced postoperative mortality and complications. The preoperative selection and evaluation, improvement of surgical techniques and prevention of postoperative recurrence and metastasis are the key points for the surgical treatment of advanced liver cancer. A comprehensive evaluation of the hepatic functional reserve is required before surgery. The functionality of liver parenchyma if commonly assessed by the Child-Pugh classification and ICG clearance test, and the residual liver volume is calculated with the use of CT and/or MRI.
Most cases of intermediate-advanced HCC are characterized by a single tumor >10 cm in diameter, multiple tumors, and tumors with portal vein, hepatic vein, or bile duct thrombus. Since a good general condition and adequate hepatic functional reserve are often required for liver resection, only a few of advanced HCC cases are eligible for surgical treatment regardless of the staging criteria. The Child-Pugh score and indocyanine green retention rate at 15 min (ICG15) are commonly used in assessment of hepatic functional reserve. BCLC group also suggests the use of the hepatic venous pressure gradient (HVPG) to assess the portal hypertension. For intermediate-advanced HCC, Child-Pugh Class A, HVPG <12 mmHg and ICG15 <20% often suggest good hepatic functional reserve with portal hypertension in the acceptable range. On this basis, imaging techniques are employed to estimate the expected residual liver volume following resection. A residue of 40% or more of the standard liver volume is required to ensure the safety of operation. A significantly higher long-term survival is observed in patients with resectable intermediate-advanced HCC undergoing surgical treatment, compared to non-surgical management or palliative care.
4.1.2. Classification of liver resection approaches
Liver resection includes radical resection and palliative resection. It is generally accepted that curative resection can be classified into three grades according to the completion of surgery: Grade I: the visible tumor is completely removed without residual findings on margins; Grade II: four additional conditions are introduced on the basis of Grade I: (1) Number of tumors ≤2; (2) No tumor thrombus is found in the portal vein trunk and its first-order branches, the hepatic duct and its first-order branches, or the hepatic vein trunk and the inferior vena cava; (3) No lymph node metastasis in the hepatic portal area; and (4) No extrahepatic metastasis. Grade III: negative postoperative follow-up results are required on the basis of Grade II, that is, increased preoperative serum AFP levels should return to normal within 2 months after surgery, and no residual tumor is found in imaging results.
4.1.3. Indications for liver resection
(I) Basic conditions: a patient's general conditions should allow him/her to tolerate surgery; the liver lesions are resectable; and the reserved liver function can serve in compensation. Specifically, a patient in generally good condition should have: no major disorders of the heart, lung, kidney and other vital organs; normal liver function, or with only mild damage (Child-Pugh class A), or once Class B liver function that has recovered to Class A after short-term routine therapy; hepatic functional reserve (such as ICGR15) basically within the normal range; and no unresectable, extrahepatic metastatic lesions. An ICG15 <14% is generally regarded as the lowest acceptable threshold for safe en bloc liver resection with rare possibility of liver failure.
(II) For radical hepatectomy of local tumor, the patient must have either of the following:
(i) Single liver with smooth surface and clearly defined boundaries or pseudocapsule, <30% liver tissue damaged by tumor, or damaged tissue >30% but with significantly compensatory enlargement of the contralateral lobes that exceed 50% or more of the standard liver volume;
(ii) Multiple tumors with less than three nodules that are confined to a single segment or lobe of the liver. For multiple liver tumors, studies have shown a significant benefit from surgery as long as the above conditions are met among patients with less than three tumors. When the number of lesions is more than three, the effect of surgical resection is at most comparable to hepatic artery interventional embolization and other non-surgical approaches.
(iii) Laparoscopic liver resection: widely applied these days, this technique is mainly indicated for isolated foci of <5 cm in liver segments 2-6. As a minimally invasive operation, it is associated with less blood loss and reduced surgical mortality. Therefore, investigators suggest that for liver tumors at a suitable location, particularly those in the early stage, a satisfying outcome can be achieved with laparoscopic liver resection. However, studies are still needed for prospective comparison between the approach and traditional open surgery.
(iv) For palliative hepatectomy of local tumor, the following requirements should be met:
a) Three to five multiple tumors beyond a half of the liver should be treated with multiple localized resections;
b) The lesion(s) should be confined to two to three adjacent liver segments or in the same half of the liver, with significantly compensatory enlargement of the tumor-free liver tissue of more than 50% of the standard liver volume;
c) For tumors in the central area (middle lobe or segments IV, V and VIII), liver cancer, the tumor-free liver tissue should present with significantly compensatory enlargement of more than 50% of the standard liver volume;
d) In the case of hilar lymph node metastasis, tumor resection is performed in conjunction with lymph node dissection or postoperative treatment;
e) Surrounding organs are removed together if involved.
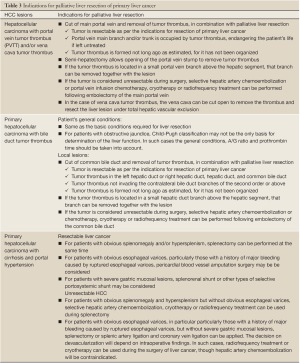
(v) Palliative liver resection is also used in the following conditions: liver cancer with portal vein tumor thrombus (PVTT) and/or vena cava tumor thrombus, liver cancer with bile duct thrombus, liver cancer with cirrhosis and portal hypertension, and other resectable liver cancer. Each of them has the corresponding surgical indications (Table 3). Portal vein tumor thrombus is a common complication of intermediate-advanced HCC. In such patients, if the tumor is confined to a half of the liver, and tumor thrombi are expected to be completely removed intraoperatively, surgical treatment and thrombus removal via the portal vein can be conducted, followed by interventional embolization and portal vein chemotherapy after the surgery. Violation of the bile duct and the resultant bile duct tumor thrombus is also common; these patients are present with noticeable jaundice. The nature of jaundice should be identified with caution. For obstructive jaundice due to tumor thrombus, if the tumor can be resected and thrombi completely be removed, the symptom will subside rapidly. Hence, jaundice is not a major surgical contraindication. In addition, when palliative resection is contraindicated, palliative non-resection surgical treatment can be considered, such as intraoperative hepatic artery ligation and/or hepatic arterial and portal venous infusion chemotherapy. Caution should be given to the treatment of small intrahepatic lesions. Some small lesions, undetectable by imaging studies or even intraoperative exploration, can be a cause of increased recurrence following liver resection. If incomplete resection is suspected, TACE is the ideal choice after surgery for both therapeutic purposes and detection of residual diseases. Remedial measures should be taken immediately if any residual lesion is found. In addition, test of viral load (HBV DNA and/or HCV RNA) should be performed postoperatively. If indicated, anti-viral treatment should be initiated to reduce the possibility of liver cancer recurrence.
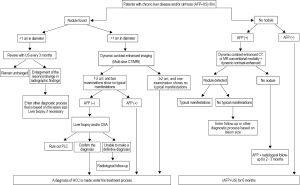
Full table
4.1.4. Modified surgical techniques
In principle, liver resection should be considered for single-lesion patients with adequate liver function reserve and free of extrahepatic metastasis, large vascular invasion or portal vein tumor thrombus. For multiple lesions, this technique can also be applied, if technically feasible, as long as these criteria are met. For intermediate-advanced hepatocellular carcinoma, particularly huge or multiple tumors, however, the surgery can be complicated despite a low rate of radical resection.
Methods of improving the resectablity of liver tumors include: preoperative chemo-embolization via the hepatic artery, which makes tumor shrink in some patients and enables resection; embolization of the liver lobe where the tumor is located via the portal vein, to induce compensatory enlargement of the remaining liver before resection. The latter method is relatively safer and more effective as few toxic adverse effects have been reported. For a huge tumor, the anterior approach liver resection can be performed without dividing perihepatic ligaments, in which the liver parenchyma and intrahepatic ducts are directly dissected before the ligaments are finally freed and the lesion removed. For multiple tumors, intraoperative ablation (e.g., radio frequency ablation) can be carried out in combination with the surgery to treat deep lesions after those along the liver edges are resected. For portal vein or hepatic vein tumor thrombi, the flow of the contralateral portal vein must be blocked during embolectomy to prevent spread of the tumor thrombus. For hepatic vein tumor thrombi, total hepatic vascular exclusion can be used for en bloc removal of tumor thrombi as completely as possible. For those complicated with with bile duct thrombi, if partial invasion of the bile duct wall is observed during the removal of tumor thrombi, the violated bile duct should be resected and reconstructed to reduce local recurrence.
4.1.5. Prevention of postoperative recurrence and metastasis
Intermediate-advanced liver cancer has a high recurrence rate after surgical resection, which may be due to the presence of small disseminated lesions or multi-center lesions before surgery. When recurrence is confirmed, the possibility of a successful second resection is rare. At this stage, local non-surgical treatment and systemic therapy can be employed to control tumor development and prolong survival. For high risk patients, clinical research has confirmed a certain effect of prophylactic embolization in detecting and managing intrahepatic small residual tumor after surgery. Although clinical randomized trials suggest alpha interferon can prevent the recurrence of liver cancer, the effect on long-term recurrence and patients with different types of hepatitis is still controversial, and thus the agent is not regarded as the current standard treatment for prevention of recurrence.
4.1.6. Contraindications
(I) Patients who have poor heart and lung function or are complicated with severe disorders of the other vital organs, and thus can not tolerate surgery;
(II) Patients with severe cirrhosis and poor liver function (Child-Pugh Class C); and
(III) Patients who have presented extrahepatic metastasis.
4.2. Liver transplantation
4.2.1. Selection criteria for liver transplantation
Currently, liver transplant is mostly used as a supplementary therapy for patients with unresectable liver cancer who are ineligible for or can not tolerate microwave ablation and TACE due to poor liver function. Selection of adequately indicated cases is key to improving the efficacy of liver transplant and ensuring the fair and effective use of the extremely valuable donor resources. The Milan Criteria are regarded as the international standard for the use of liver transplantation, in addition to the University of California, San Francisco (UCSF) criteria and Pittsburgh modified TNM criteria.
(I) Milan Criteria: these standards were put forward by Mazzaferro et al. in Italia in 1996. Specific criteria: a single tumor no larger than 5 cm in diameter; number of multiple lesions ≤3 with the maximum diameter ≤3 cm; and no violation of blood vessels and lymph nodes. In 1998, the United States for Organ Sharing (UNOS) Network adopted the Milan criteria (plus the MELD/PELD score, also known as the UNOS standard) as the main standards for screening liver transplant recipients. Since then, the Milan criteria have gradually become the world’s most recognized screening criteria of liver transplantation for liver cancer patients. It is well accepted as having definite effects with a 5-year survival of ≥75% and recurrence rate of <10%, as well as ease of clinical operations because only the size and number of tumors need to be taken into consideration. However, the Milan criteria are so strict that many patients with hepatocellular carcinoma who can potentially benefit from liver transplantation are screened out. Due to increasing shortage of donors, patients with liver cancer who used to meet the Milan criteria may likely be screened out when their lesions grow and exceed the upper limit during the wait for a donor liver. Moreover, no significantly increased overall survival has been associated with liver transplant for patients with small hepatocellular carcinoma that meets the Milan criteria compared to liver resection, though significantly higher tumor-free survival is observed in the former group. Considering factors such as donor shortage and high cost, the decision on liver transplant has been highly controversial for patients who meet those criteria, particularly in many developing countries. In addition, it is difficult to apply the Milan criteria to the screening of recipients for living donor liver transplant and patients whose previously intermediate-advanced hepatocellular carcinoma has downstaged.
(II) University of California, San Francisco (UCSF) criteria: these criteria were introduced by Yao et al. in the U.S. in 2001 on the basis of the Milan criteria, in which the indications for liver transplant were partially expanded. They may include: a single tumor no larger than 6.5 cm in diameter; number of multiple tumors ≤3 with a maximum diameter ≤4.5 cm and total tumor diameter ≤8 cm; and no violation of blood vessels and lymph nodes. The UCSF criteria have also broadened the intended application of the indications in the Milan criteria, while not obviously reducing the survival rate. Therefore, in recent years, the application of UCSF criteria for screening liver transplant recipients is supported by an increasing number of studies. Controversy, however, still exists in terms of the confirmation of lymph node metastasis and tumor vascular invasion (particularly microvascular invasion) because a preoperative diagnosis is often difficult. Upon thorough discussion of the expert panel, these guidelines recommend the use of the UCSF criteria.
(III) Pittsburgh modified TNM criteria: introduced by Marsh et al. in the U.S. in 2000, these criteria regard the presence of any of the three factors -- large vascular invasion, lymph node involvement or distant metastases -- as the only contraindication for liver transplant, and do not use the size, number and distribution of tumors as the exclusion criteria. The application of liver transplant is thus remarkably broadened among patients with liver cancer, allowing potential long-term survival in nearly 50% of them. In recent years, study reports in support of the UCSF criteria are accumulating. However, major flaws have also been observed in the above criteria. For example, it is difficult to accurately assess the condition of invasion in microvessels or vessels of liver segment branches before surgery. Furthermore, many HCC patients with hepatitis history may have inflammatory hepatic portal lymph nodes, so a definite diagnosis will have to rely on the pathological findings of intraoperative frozen sections. In addition, due to deepening conflict between the supply and demand of donor livers, although an expanded indication for liver transplant may benefit some of the patients with intermediate-advanced hepatocellular carcinoma, it can also reduce the overall survival significantly and in turn lower the possibility for patients with benign liver lesions to obtain long-term survival through transplantation.
(IV) Domestic criteria: there is not a single, widely accepted set of criteria in China, though institutions and investigators have put forward a number of criteria, such as Hangzhou criteria, Shanghai Fudan criteria, Huaxi criteria and Sanya consensus. Despite consensus on the absence of large vascular violation, lymph node metastasis and liver metastasis, requirements for tumor size and number vary greatly. The expanded indication in the domestic criteria may bring benefits to more patients with hepatocellular carcinoma via liver transplant without significantly reducing postoperative cumulative survival and disease-free survival rate, and thus they may be more practical in line with China's national conditions and the actual situations of patients. However, a standardized multi-center collaborative study is still needed to generate high-level medical evidence so that a uniform set of criteria can be established and recognized.
4.2.2. Prevention of recurrence after liver transplant
The tumor size, a common indicator in both domestic and foreign criteria for screening liver transplant recipients, is relatively objective and readily practical, but it does not provide sufficient information as it is not linked with the biological characteristics of hepatocellular carcinoma. It is generally believed that the biological behavior of the tumor is the most critical factor that determines the prognosis of a patient. Hence, with the continuous development of molecular biology, molecular markers that better reflect the biological behavior of liver cancer and predict the prognosis of patients will be identified to help improve the existing criteria for liver transplant and improve the overall survival of such patients. It is suggested that proper drug therapies after liver transplant (including anti-viral therapy and chemotherapy) may reduce and delay recurrence and improve survival for liver cancer patients, but further research is needed to produce sufficient medical evidence in this regards.
4.2.3. Selection of liver transplant and liver resection
There are no uniform criteria for selecting between the two most common surgical options, liver resection and liver transplant surgery. It is generally accepted that liver resection is preferred when the disease is localized and not complicated with cirrhosis; if the patient is complicated with cirrhosis and hepatic decompensation (Child-Pugh Class C), and transplant criteria are met, liver transplant is preferred. However, it is highly controversial as to whether liver transplant is performed for patients with resectable localized hepatocellular carcinoma and well compensated liver function (Child-Pugh Class A). For example, European experts support the preferred use of liver transplant on the grounds that the recurrence rate of liver resection is high, and patients meeting the Milan criteria of liver transplant have significantly better long-term survival and disease-free survival compared with those undergoing liver resection. In the present guidelines, patients who have good liver function and can tolerate liver resection are not included in the indications for liver transplant. Nonetheless, comprehensive assessment and analysis will be required for each individual patient to develop the most proper surgical plan tailored to the actual situation.
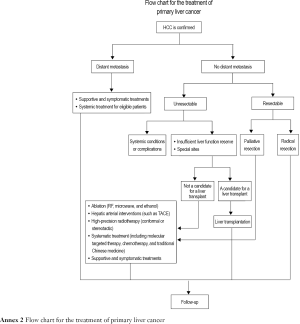
5. Local treatment
Although surgery is the preferred option of treatment for liver cancer, most patients are at the advanced stage at the time of diagnosis and thus ineligible for surgery. According to statistics, only about 20% of these patients are eligible for surgery. Therefore, intensive non-surgical treatment may play a vital role in relieving symptoms, improving the quality of life and prolonging survival for such patients.
5.1. Local ablation therapy
Local ablation therapies are procedures that, guided by medical imaging technology, localize the targeted-tumor and then kill tumor tissues through the local application of physical and/or chemical methods. These minimally invasive, safe and simple, easy-to-repeat approaches include radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, high-power focused ultrasound ablation (HIFU) and percutaneous ethanol injection (PEI). The imaging technology includes sonography, computed tomograpgh (CT), and magnetic resonance imaging (MRI), and the ablation can be performed through percataneous, laparoscopic, or open surgery.
5.1.1. Indications and contradictions
(I) Indications: this therapy is often indicated for a single tumor with a maximum diameter of <5 cm, or not more than three tumors with a maximum diameter of ≤3 cm. An eligible patient should be free of blood vessel, bile duct and adjacent organ involvement, or distant metastasis. A liver function of Child-Pugh Class A or B, or equivalent level after medical routine therapy is required. In some cases, for a single, unresectable tumor of >5 cm in diameter or multiple lesions with a maximum diameter >3 cm, local ablation can be used as a part in the palliative treatment with caution.
(II) Contraindications:
(i) Huge or diffuse hepatocellular carcinoma;
(ii) Tumor thrombi involving the portal vein trunk to the second-order branches, or hepatic vein tumor thrombus, adjacent organ involvement or distant metastasis;
(iii) Tumor located on the liver surface with over 1/3 exposed;
(iv) Liver function of Child-Pugh Class C that is not improved with the routine therapy;
(v) Esophageal variceal bleeding in one month prior to the treatment;
(vi) Uncorrectable coagulation disorders and clinically significant abnormalities in blood test with significant bleeding tendencies;
(vii) Refractory massive ascites and cachexia;
(viii) Active infection, particularly inflammation of the bile duct;
(ix) Failure of major organs such as the liver, kidney, heart, lung and brain;
(x) Disturbance of consciousness or inability to cooperate with the treatment.
Meanwhile, relative contraindications include tumors in the primary hilar region; and tumors close to the gall bladder, stomach and diaphragm or those exposed on the liver capsule in the way of the percutaneous puncture path. Intrahepatic lesions with extrahepatic metastasis should not be regarded as an absolute contraindication, as the development of local lesions may still be managed via ablation therapy in some cases.
5.1.2. Selection and application of common ablation means
(I) Radiofrequency ablation (RFA): a representative option of liver cancer minimally invasive treatment and also the most widely applied mean of thermal ablation. With definite effects, reduced hospital stay and lower costs, this easy-to-operate technique is an ideal alternative to open surgery. For patients with small hepatocellular carcinoma, the long-term efficacy of RFA is similar to that of liver transplant and liver resection, but better than that of TAE/TACE treatment. Compared with ethanol injection, RFA has a significantly higher cure rate, less number of treatments and higher long-term survival for tumors of 3-5 cm.
In essence, RFA treatment precisely destroy the tumor tissues as a whole while minimizing the injury to normal liver tissues based on an accurate definition of the tumor-infiltrating scope and satellite lesions. Therefore, accurate pre-treatment imaging studies are the vital part in the RFA treatment, and ultrasound is the preferred guiding method. In recent years, contrast-enhanced ultrasonography (CEUS) has played an important role; in confirming the actual tumor size and shape, defining the scope of tumor infiltration and detecting small hepatocellular carcinoma and satellite lesions. This technique provides a reliable foundation for the development of tumor ablation plans. There are, however, three major issues in the RFA treatment of intermediate-advanced HCC: Complete ablation of a large tumor is challenging; complications are likely to occur due to limited safety region when a tumor is located at the periphery close to the diaphragm surface, stomach, gall bladder and hilus; and, residual recurrence is common due to energy loss in the case of adjacent large vascular invasion or a tumor with rich blood supply (i.e. “heat sink effect”). For tumors larger than 5 cm, a radical therapeutic effect is rarely achieved with RFA. Small satellite lesions, which are likely to be missed during ablation, can lead to a high recurrence rate. Moreover, difficulties in controlling the transfer of radiofrequency ablation can be an issue during the treatment as the surrounding organs can be injured and liver cancer can occur due to the movement and penetration along the needle tract. In addition, this technique is not suitable for the treatment of lesions at the imaging blind spot.
(II) Microwave ablation (MWA): this is a method of thermal ablation commonly used in China, which has statistically comparable local therapeutic efficacy, complication rate and long-term survival to RFA. The modern MWA technology is capable of killing tumor tissues in a single procedure, and has an improved effectiveness against tumors with rich blood supply by coagulating and blocking the feeding vessels. A temperature monitoring system can be established to monitor the effective thermal field range and ensure the coagulation effect.
(III) Percutaneous ethanol injection (PEI): this method is used for the treatment of small hepatocellular carcinoma not larger than 3 cm in diameter and recurrence of small hepatocellular carcinoma. It can serve as a palliative approach to inoperable lesions or recurrence larger than 3 cm. Under clinical settings, thermal ablation (RFA and MWA) may cause injury to normal tissues if some lesions are close to the hepatic hilum, gallbladder and gastrointestinal tract. To prevent complications in such cases, PEI or PEI combined with thermal ablation can be used.
Both RFA and MWA can cause local tumor necrosis by the thermal effect. Despite potentially higher energy and larger ablation scope, MWA has similar local efficacy, complication morbidity and survival rate as the other technique. After ablation therapy, lesion necrosis should be regularly examined. In the case that any residual disease is identified, active treatment should be taken to improve the efficacy of ablation therapy.
5.1.3. Basic technical requirements
(I) The operating physician should be properly trained and be meticulously responsible for the procedure. Before treatment, the physician should order a comprehensive assessment of the patient's general condition, disease, tumor biological behavior (to predict the feasibility and outcome, and determine the treatment plan and combination therapy measures and procedures) and imaging studies, and develop a complete treatment protocol and strategy depending on the size, invasion and location of the tumor, to ensure adequate and safe coverage and a conformal ablation treatment that destroys the lesion through a single operation.
(II) The appropriate imaging technology should be employed to guide the procedures under monitoring to ensure the safety, accuracy and effectiveness of treatment.
(III) The distance between the tumor and the common hepatic duct of the hilum region and bilateral bile ducts should be at least 5 mm. The use of ablation therapy alone is not recommended for a lesion larger than 5 cm. For multiple lesions or larger tumors, TACE or TAE combined with radiofrequency therapy according to the status of liver function will be significantly more effective than radiofrequency treatment alone.
(IV) The ablation range should include all adjacent tissues 5 mm from the lesion as much as possible to achieve the “safety margins” and elimination of tumor cells. For irregularly shaped invasive tumors or metastatic foci without a clearly boundary, the range of ablation can be appropriately expanded if the adjacent liver tissue and structural conditions permit. For tumors with rich blood supply, the primary supplying vessels can be coagulated and blocked before ablation to improve the tumor-killing effect.
(V) Follow-up CT/MRI scan or contrast-enhanced ultrasound one month after ablation is the standard method to evaluate the local efficacy of the treatment. The efficacy are classified as: (i) complete response (CR), where follow-up imaging shows a low density (hyperechoic area in ultrasound) of the lesion area with no enhancement at the arterial phase; (ii) incomplete response (ICR), where follow-up scans show arterial-phase enhancement of the local lesion, suggestive of residual tumor. In the case of residual tumor after treatment, a second ablation therapy can be conducted. The presence of residual lesion following two ablation protocols is a sign of failure that warrants the application of alternative therapy in lieu of ablation.
(VI) A properly integrated treatment protocol and reasonable follow-up schedule are needed. Regular follow-up is required after treatment to identify potential local recurrence and new intrahepatic lesions, which can be effectively controlled with the safe and simple percutaneous ablation.
5.1.4. Selection between ablation therapy and surgical treatment for HCC of ≤5 cm
It is controversial as to whether ablation or surgery is the treatment of choice for live cancer not larger than 5 cm. Many prospective, randomized, controlled clinical trials and retrospective comparative studies have shown that regional ablation (mainly RFA and MWA) can acquire similar long-term survival to that of surgery for small hepatocellular carcinoma. However, compared with ablation measures, liver resection is more widely applied with a relatively lower recurrence rate due to the accumulation of experience over the years. It can be used to resect multiple lesions, small lesions and tumor thrombi in the same anatomical region. On the other hand, percutaneous local ablation has been associated with a low complication rate, faster recovery and shorter hospital stay. Two randomized controlled studies, finding no significant difference in the survival rate between ablation of surgical resection, have shown a disease-free survival (DFS) and relapse rate in favor of surgery.
In clinical settings, the option should be decided based on the patient’s physical readiness and liver function, the size, number and location of the tumor, technical maturity of the operating department as well as the patient’s willingness.
It is generally accepted that, if tolerable, liver resection should be the preferred option because it can remove small lesions at the corresponding liver segment or micrometastases to the liver lobe, thus effectively preventing the recurrence. Therefore, surgical treatment is still the first choice of treatment for liver cancer ≤5 cm. For patients with hepatocellular carcinoma ≤5 cm who meet both indications for local surgery and ablation treatment, surgical treatment will be preferred if at all possible, while ablation can be used as an alternative. For patients with two to three lesions at different locations who are ineligible for surgery due to poor liver function (including those with Child-Pugh Class B or equivalent liver function after routine care), regional ablation can be considered. For hepatocellular carcinoma ≤3 cm in a deeper location or central lesions, regional ablation can be the first choice because of its minimal invasiveness and comparable radical efficacy as surgical resection. For lesions between three to five centimeters, improved therapeutic effect can be achieved using the suitable needle and proper ablation techniques. Generally, comprehensive adjuvant therapy is still required after local ablation for most patients.
There are few comparative data between local ablation therapy and liver transplant or surgical resection. For larger HCC (>5 cm), there is little medical evidence on the feasibility of repeated ablations for different locations or laparotomic or laparoscopic ablation, so this approach is not recommended for such cases.
5.2. Hepatic arterial intervention
5.2.1. Basic principles
(I) This approach should be performed under digital subtraction angiography;
(II) Clinical indications must be strictly followed;
(III) Standardized and individualized chemotherapy protocols are key to successful treatment.
5.2.2. Eligible population
(I) Patients with unresectable intermediate-advanced primary liver cancer;
(II) Patients with resectable lesions who can not or will not undergo surgery for other reasons (e.g., older age, severe cirrhosis). For these patients, interventional treatment can be used as the first-line non-surgical option.
According to domestic clinical experience, hepatic arterial intervention can be effective against some bulky and huge lesions with a relatively intact envelope. However, surgical resection is always preferred if the tumor is operable. The following aspects should be taken into consideration when performing intervention:
(i) Serum AFP levels;
(ii) Whether the tumor has an intact envelope and a clear boundary;
(iii) Whether there are portal vein tumor thrombi.
5.2.3. Indications (Table 4)
(I) The main indication for TACE is unresectable intermediate-advanced HCC without no severe liver and kidney dysfunction. These conditions include:
(i) Bulky hepatocellular carcinoma: lesion accounting for <70% of the entire liver;
(ii) Multiple nodular hepatocellular carcinoma;
(iii) The main portal vein is not completely blocked, or it is completely blocked but the compensatory collateral blood vessels have formed between the hepatic artery and portal vein;
(iv) Surgical failure or postoperative recurrence;
(v) Liver function (Child-Pugh) Class A or B, ECOG score 0-2;
(vi) Portal hypertension bleeding as a result of rupture of the liver tumor and hepatic artery-portal venous static shunt.
(II) The use of TACE before liver resection can reduce the tumor volume to facilitate two-stage resection and help identify the number of lesions;
(III) Patients who have small hepatocellular carcinoma but are not eligible or not willing to undergo surgery, local radiofrequency or microwave ablation;
(IV) There is a need for management of local pain and bleeding, and blocking arteriovenous fistula;
(V) There is a need to prevent recurrence after liver resection.
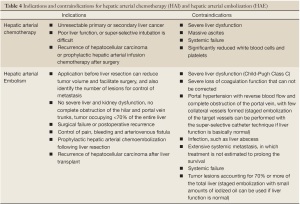
Full table
5.2.4. Contraindications (Table 4)
(I) Severe liver dysfunction (Child-Pugh Class C);
(II) Severe loss of coagulation function that can not be corrected;
(III) Tumor thrombi that have completely embolized the portal vein with few collateral blood vessels formed;
(IV) Active infections that complicate the condition and can not be treated concurrently;
(V) Wide distant metastasis with an estimated survival of <3 months;
(VI) Cachexia or multiple organ failure;
(VII) Liver tumors accounting for ≥70% of the entire liver; staged embolization with small amounts of iodized oil emulsion can be considered if liver function is normal;
(VIII) Significant reduction in peripheral blood leukocytes and platelets, white blood cells <3.0×109/L (non-absolute contraindication, such as hypersplenism, which is different from the decreased WBC count related to chemotherapy), platelets <60×109/L.
5.2.5. Key elements and classification of surgical procedures
Basic operations: the Seldinger method is often used for hepatic arteriography, in which the catheter is placed in the celiac artery or common hepatic artery following percutaneous femoral artery catheterization. Image acquisition should include the arterial phase, parenchymal phase and venous phase. Images of the superior mesenteric artery should be obtained with caution to look for the collateral blood supply.
Specific operations may include the following depending on the procedural options:
(I) Hepatic transcatheter arterial infusion (TAI): based on careful analysis of imaging findings and identification of the tumor size, number and feeding arteries, infusion chemotherapy is delivered through superselective catheterization in the supplying arteries. Adriamycin (ADM), epirubicin (EADM), cisplatin (PDD), 5-fluorouracil (5-FU), hydroxycamptothecin (HCPT) and mitomycin (MMC) are commonly used.
(II) Transcatheter arterial embolization (TAE): this is commonly used under clinical settings. Superselective catheterization is preferred whenever possible, in combination with proper embolization agents. An emulsion mixture of super-liquid lipiodol and chemotherapeutic agents is commonly used for this therapy. The dosage of iodized oil should depend on the size, blood supply, and tumor of feeding arteries of the tumor. Other embolic agents, such as gelatin sponge, permanent particles and microspheres can also be used. For hepatocellular carcinoma complicated with arteriovenous fistula, to prevent pulmonary embolism and other serious complications and to ensure that the effect of anti-tumor TAE, arteriovenous fistula should be effectively blocked before TAE. In the case severe arteriovenous fistula, the use of TAI alone is generally indicated.
(III) Transcatheter arterial chemoembolization (TACE): TAI and TAE are administered in combination to improve efficacy. As a first-line palliative treatment, TACE is the most commonly used approach in the clinical settings of China. The principle of TACE for HCC is mainly based on the difference in blood supply between liver tumors and normal liver tissues, i.e. 95-99% of blood supply to the lesions comes from the hepatic artery while 70-75% of that to normal liver tissues is from the portal vein and the hepatic arterial supply accounts for only 20-25%. While effectively blocking the arterial blood supply of liver cancer, TACE will release a continuous flow of high-concentration chemotherapy agents to combat cancer, causing ischemic necrosis and shrinkage but minimized damage to the normal liver tissues. Evidence-based medicine has demonstrated that TACE can effectively curb the growth of hepatocellular carcinoma growth and benefit liver cancer patients by significantly prolonging their survival. Hence it has the most effective treatment of choice for patients with unresectable intermediate-advanced hepatocellular carcinoma.
It is important to analyze the imaging findings, identify the location, size, number and supplying arteries of the tumor before the administration of TACE. Both the left and right hepatic arteries should be treated with infusion chemotherapy through superselective catheterization. The catheter tip should be threaded through the gall bladder and the right gastric artery, gastroepiploic artery and other blood vessels. Chemotherapy drugs should be properly diluted and slowly injected into the target vessel over an infusion time of less than 20 min. In most cases of HCC, more than 95% of the blood supply is from the hepatic artery, which is often thickened to form a rich supplying network to the densely stained tumor. Embolization should be conducted following infusion chemotherapy. It is recommended to slowly deliver an emulsion mixture of super-liquid lipiodol and chemotherapeutic agents into the target supplying vessel via a microcatheter through superselective catheterization. Entry of the embolic agent into normal liver tissues or non-target organs should be avoided during embolization. The dosage of lipiodol is 5-20 mL, and generally not larger than 30 mL as long as a dense lipiodol deposition is observed with shadows of small peritumoral branches of the portal vein under fluoroscopy. For significantly enlarged supply arteries of HCC, the use of particulate embolic agents (such as gelatin sponge or microsphere) after embolization with lipiodol emulsion is recommended. All supplying vessels should be embolized, if possible, to achieve devascularization of the tumor. The proper hepatic artery should be spared so that a second TACE may be possible when necessary.
Factors that affect the long-term effect of TACE include liver cirrhosis, liver function and tumor status (size, staging, pathological classification, portal vein tumor thrombus and arteriovenous fistula). Besides, there are several limitations of TACE treatment, including:
(i) Pathologically complete necrosis of lesions is difficult for TACE due to occasionally incomplete embolism, formation of collateral vessels or other factors;
(ii) After TACE, ischemia and hypoxia of the tumor tissues may give rise to elevation of the hypoxia-inducible factor (HIF) levels in residual tumor tissues, resulting in a high expression of the vascular endothelial growth factor (VEGF). These factors can lead to intrahepatic tumor recurrence and distant metastasis.
5.2.6. Common adverse reactions following TACE
Post-embolization syndrome is the most common adverse reaction after TACE, with fever, pain, nausea and vomiting as the major manifestations. Fever and pain are a result of local tissue ischemia and necrosis when the hepatic artery is embolized, and nausea and vomiting are associated with chemotherapy drugs. In addition, puncture site bleeding, decreased white blood cells, transient abnormal liver function, renal dysfunction and voiding difficulties are also commonly seen. In general, these adverse reactions will last 5-7 days after interventional treatment and will disappear after symptomatic treatment in most patients.
5.2.7. Follow-up and treatment interval
As a general recommendation, the first follow-up CT and/or MRI is carried out in 4-6 week after the initial hepatic arterial intervention, while the subsequent examinations will be scheduled depending on the specific conditions of the patient at an interval of 1-3 months. The frequency of interventional treatment should be determined based on the follow-up findings. The therapy can be ended if the imaging scan shows a dense lipiodol deposition and tissue necrosis inside the tumor and no evidence of enlargement or new lesion within 4-6 weeks after the procedure. After the two or three interventional procedures, subsequent therapy intervals should be prolonged if there is no evidence of tumor progression to ensure the recovery of liver function. During the intervals, CT and/or MRI dynamic contrast-enhanced scan can be employed to evaluate the survival of the liver tumor for determination of the need for repeat intervention treatment. After several interventional procedures, if the tumor continues to progress, an alternative therapy option or combination with other approaches such as surgery, local ablation and systemic treatment should be considered.
6. Radiation therapy
Although radiotherapy has been an essential treatment of malignant tumors nowadays, it is not commonly used for HCC patients until the 1990s due to poor effect and a high risk of liver damage. After the mid-1990s, new approaches have emerged with the rapid development of modern precision radiotherapy techniques, including three-dimensional conformal radiotherapy (3DCRT), intensity modulated conformal radiotherapy (IMRT) and stereotactic standardization of breast radiotherapy (SBRT). The sophisticated and wide application of those methods has brought a good prospect for the radiotherapy of liver cancer. Domestic and foreign investigators have reported many cases of unresectable HCC treated with modern precision radiotherapy, with a 3-year survival up to 25-30% in a select group of HCC patients. Radiotherapy is generally considered for the patients with confined but unresectable liver tumor due to poor liver function, or lesions located in an important anatomic structure for which resection is technically unfeasible; or patients who refuse to receive surgery. In addition, palliative care can be given to control pain or relieve pressure for some patients with distant metastasis.
6.1. Indications for radiotherapy of liver cancer
6.1.1. The therapy is mainly indicated for
(I) Patients with good general conditions, e.g., KPS ≥70, liver function Child-Pugh Class A, and single lesion;
(II) Patients with post-operative residual tumors;
(III) Local treatment of the liver tumor is required to avoid severe complications, such as hepatic portal obstruction, and portal vein or hepatic vein tumor thrombus;
(IV) Palliative care for distant metastases, such as lymph node metastases, adrenal metastases and bone metastases, which may reduce symptoms and improve the quality of life.
6.1.2. As an important part of the multi-disciplinary treatment for liver cancer, radiotherapy is indicated for
(I) Confined intrahepatic HCC: radiotherapy combined with transcatheter arterial intervention can significantly improve the efficiency and survival rates;
(II) HCC with tumor thrombus: radiotherapy can be targeted at the tumor thrombus emerging after surgical or interventional therapy or primary tumor thrombi (including those in the inferior vena cava) to prolong the survival;
(III) HCC with lymph node metastases: radiotherapy can significantly improve the survival of HCC patients with lymph node metastases;
(IV) HCC with adrenal metastases: radiotherapy can relieve the symptoms of adrenal metastases, though there is no evidence of prolonged survival;
(V) HCC with bone metastases: the goal of radiation therapy is to relieve symptoms, thus improving patients' quality of life, but there is no evidence of prolonged survival;
(VI) ICC: radiotherapy may be used to prolong the survival of ICC patients with positive surgical margin or unresectable lesions. Radiotherapy is used as more of a palliative means for the treatment of the above tumors. With little therapeutic effect, it can neither prolong the survival to a significant level nor replace the traditional treatment of liver cancer. However, since none of the other approaches is associated with stronger supportive evidence or better effects, radiotherapy is still listed as one of the important alternatives, particularly for extrahepatic metastases.
6.2. Techniques of radiotherapy for liver cancer
6.2.1. Split dose of radiation
According to clinical experience, large fractionated irradiation (for example, 5 Gy per course, three times a week, in a total dose of 50 Gy) has strong a tumor-killing effect but will cause considerable injury to normal liver tissues. Conventional fractionated radiation (for example, 2 Gy per day, five times a week, in a total dose of 50-62 Gy), on the other hand, is well tolerated by the normal liver tissue while still having a significantly inhibitory effect on tumor lesions. Further clinical practice and research will be needed to decide on a better dose-split solution between them. Nonetheless, for patients who require acute relief of symptoms, large fractionated radiotherapy is preferred because it allows faster tumor shrinkage and remarkable improvement of symptoms.
6.2.2. Radiation plan
(I) Approaches to radiotherapy: dosimetric comparison results have shown that, compared with 3DCRT, IMRT has a better conformal dosage distribution of the target area and lower exposure in normal liver tissues. Therefore, although 3DCRT is often considered as the treatment of choice, IMRT can be used when the dosimetric requirements can not be met with the former method. IMRT is more suitable for the following patients: patients with large liver tumor, making the normal tissues exposed to a large dose of irradiation; or patients with severe cirrhosis who can not tolerate high-dose irradiation.
(II) Control of breathing: breathing control techniques are recommended, such as active breath coordinator (ABC), to restrict tumor movement during radiotherapy, thereby reducing the radiation dose to normal tissues.
(III) Lesion target positioning: the CT and MRI image fusion technology is recommended to determine the gross tumor volume (GTV) based on the deposition of lipiodol after TACE. The clinical tumor volume (CTV) is calculated as GTV plus 5-10 mm, and the planned tumor volume (PTV) as CTV plus 6 mm when using the ABC device, or determined based on the patient's breathing situation.
At present, some investigators suggest the use of two TACE courses before radiotherapy, followed by an interval of 3-6 weeks before reassessment of the need for further radiation therapy. This protocol may have the following benefits:
(i) Small hepatocellular carcinoma lesions can be detected and treated during the treatment;
(ii) It is helpful for identifying the tumor target region;
(iii) It is useful for verifying the radiation plan prior to actual implementation;
(iv) It may delay intrahepatic local spread of the tumor and intrahepatic dissemination.
6.3. Complications of radiotherapy
Radiotherapy-related complications include toxic adverse effects at the acute stage (during radiotherapy) and liver injury at the post-radiotherapy stage (within 4 months).
6.3.1. Toxicity at the acute stage (during radiotherapy):
(I) Anorexia, nausea, vomiting, severe upper gastrointestinal bleeding, particularly in patients with a large radiation field, or with a relatively large volume of the duodenum, jejunum and stomach;
(II) Acute liver damage: increased bilirubin and serum ALT levels are observed;
(III) Bone marrow suppression, particularly in patients with a large volume of liver exposure or those complicated with hypersplenism.
6.3.2. Post-radiotherapy injury
Most are radiation induced liver disease (RILD). The clinical manifestations and diagnostic criteria include:
(I) High-dose radiotherapy has been delivered;
(II) Complications occur at the end of radiotherapy;
(III) Clinical manifestations can be divided into two categories: a) typical RILD: a fast-onset condition where massive ascites and liver enlargement are present very rapidly with AKP increased to more than two times normal, or ALT increased to more than five times normal; b) atypical RILD: only liver function damage is present, with AKP higher than two times normal or ALT increased to more than five times normal, without liver enlargement or ascites;
(IV) Clinical symptoms and liver dysfunction as a result of the progression of liver tumor have been excluded.
RILD is a severe radiation-related complication with a mortality of higher than 70% due to acute liver failure after onset. It is mainly treated with symptomatic approaches, including the use of glucocorticosteroids and diuretics, as well as liver protection and supportive therapy when necessary. To prevent RILD, it is important to confine the exposure of normal liver tissues to a tolerable range when developing the radiation treatment regime. A significantly lower value of the maximum tolerated liver radiation dose is reported in China compared to foreign studies, as most HCC patients are complicated with cirrhosis in our country. According to the domestic statistics, the tolerated radiation dose (average whole liver dose) is: 23 Gy for patients of Chlild-Pugh Class A and 6 Gy for patients of Child-Pugh Class B. Caution should be given to patients at a higher risk of RILD, including those with a poor preoperative liver function (e.g., Child-Pugh Class B), a large radiation volume of the liver and a higher dose, and those complicated with vascular tumor thrombus, such as portal vein and inferior vena cava tumor thrombi. When TACE is concurrently administered, the interval with liver radiotherapy should not be longer than one month. In addition, when acute liver damage is observed during radiotherapy (e.g., liver injury ≥ RTOG grade II), continued treatment will raise the risk of RILD up to 60%. Therefore, radiotherapy should be discontinued for such patients to avoid the occurrence of RILD after the treatment.
In short, acute liver damage is often reversible and easy to recover, whereas post-therapy liver damage is often irreversible and a severe radiation injury that has a mortality of up to 80% after onset. The main causes of this complication include severe underlying liver disorders (Child-Pugh Class B or C), excessive radiation volume of the normal liver and excessive doses. It is important to prevent the condition by limiting the radiation dose within the tolerance range (22 Gy as generally accepted in China).
7. Systemic treatment
One of the major challenges for HCC treatment is that there are two conditions, completely different in nature, at the same organ of a given patient -- malignant lesion and chronic liver disease, and the interaction of them forms a vicious cycle. HCC is prevalent in China, while most of the patients are complicated with hepatitis B and cirrhosis. Due to insidious onset and rapid progress, this condition is often diagnosed at an advanced, inoperable stage. Hence, many patients are treated with ablation or TACE, resulting in short survival and poor outcomes. Surgical resection, even though applicable in some of them, has high recurrence and low long-term survival rates. Therefore, multi-disciplinary approaches incorporating systemic therapy have become necessary for the treatment.
In most cases, patients often have varying degrees of abnormal liver function at the time of diagnosis. Supportive symptomatic treatment is the most common and only option for patients with severe hepatic liver dysfunction (Child-Pugh Class C), while systemic therapy is applicable for those with normal or near-normal liver function (Child-Pugh Class A or Class B) but without indications for surgery, ablation or TACE. The latest evidence favors the use of systemic therapy versus symptomatic treatment for advanced HCC patients who have no contraindication, because the former approach does not only reduce tumor burden, improve tumor-related symptoms and the quality of life, but prolong the survival and overall benefits.
In general, systemic treatment is applied for: advanced patients with extrahepatic metastases; patients with local lesions that are ineligible for surgical resection, radiofrequency or microwave ablation and TACE, or those presenting local progression after failure of treatment; patients with disseminated liver cancer; and patients complicated with tumor thrombus of the portal vein and/or inferior vena cava.
7.1. Molecular targeted therapy
The known pathogenesis of liver cancer is complicated as its occurrence, progression and metastasis are closely related to a variety of gene mutations, cell signaling pathways and abnormal neovascularization. The multiple critical links in the pathogenetic chain can be the theoretical basis and important potential targets for molecularly targeted therapy. Molecularly targeted drug therapy has unique advantages in controlling the proliferation of HCC, preventing and delaying recurrence and metastasis and improving the quality of life of patients. In recent years, the application of molecularly targeted drug therapy for HCC has become a new hotspot, attracting considerable concern and attention.
Sorafenib is an oral multi-target, multi-kinase inhibitor that can not only block the formation of tumor blood vessels by inhibiting vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptors (PDGFRs), but also inhibit tumor cell proliferation by blocking the Raf/MEK/ERK signaling pathway. This agent serves as a dual inhibitor and multiple-target blocker for the treatment of HCC. Many international multi-center Phase III clinical trials have shown that sorafenib can safely slow HCC progression and significantly prolong the survival of advanced patients. Meanwhile, clinical benefits have been shown in HCC patients in different geographical regions with different baseline parameters and different prognostic factors, suggesting similar therapeutic effects. Currently, sorafenib has been successively approved by the European EMEA, U.S. FDA and China's SFDA for the treatment of unresectable HCC and HCC with distant metastases. The conventional usage is 400 mg, po. Bid. Caution should be given to its impact on the liver function, for it requires a liver function of Child-Pugh Class A or Class B with satisfying functionality. Patients with better liver function at earlier stages can have more benefits from early medication. Clinical observation and research has confirmed that the combination of sorafenib and the hepatic arterial intervention or systemic chemotherapy can be more beneficial such patients. Studies on the combined therapy with other approaches (surgery, radiofrequency ablation and radiation) are underway. There are more ongoing clinical trials of other new molecularly targeted drugs, either used alone or in combination with surgery, interventional therapy and systemic chemotherapy, for the treatment of liver cancer.
7.2. Systemic chemotherapy
Systemic chemotherapy refers to the chemical therapy administered orally, intramuscularly or intravenously. As early as the 1950s, systemic chemotherapy has been applied in the treatment of liver cancer as a commonly used palliative approach under clinical settings. Most traditional cytotoxic drugs, such as ADM/EADM, 5-Fu, PDD and MMC, have been tested for the treatment of liver cancer, but the single-agent efficiency is relatively low (generally <10%). Although the survival benefit is not supported by any high-level evidence, a handful of studies have suggested that, compared with BSC, ADM-containing systemic chemotherapy may prolong the overall survival of patients with advanced HCC. However, the clinical application of this therapy is severely compromised by its poor reproducibility and significant toxicity. Therefore, there are few studies and little advancement in this regard over the years.
7.2.1. Arsenious acid injection
Arsenic trioxide (As2O3, arsenious acid) is the main component of the Chinese medicine “white arsenic”. It has first been used (in the form of arsenious acid injection) for the treatment of early promyelocytic leukemia in China, resulting in breakthrough achievements. In 2004, a Chinese multi-center clinical trial concluded that the arsenious acid injection was effective as a palliative treatment for advanced primary liver cancer. The medication was found to be able to control disease progression, improve patients' quality of life, alleviate pain and prolong survival while having mild adverse reactions and good tolerability. Since then, the arsenious acid injection has been approved by the State Food and Drug Administration (SFDA) with expanded indications for advanced hepatocellular carcinoma. It is the first systemic chemotherapy drug approved based on evidence of a multi-center clinical study. For clinical application, care should be given to select the suitable group of patients, and prevent and treat adverse reactions in a timely manner, particularly those related to the liver and kidney toxicity.
7.2.2. FOLFOX regimen
In recent years, with the introduction of a new generation of chemotherapy drugs such as oxaliplatin (OXA), there has been obvious progress in gastrointestinal cancer chemotherapy and a resultant improvement in the study of liver cancer chemotherapy. The traditional principle that systemic chemotherapy is not suitable for treatment of liver cancer has been questioned. A series of clinical studies and Phase II trials at home and abroad have indicated the efficacy of OXA-containing regimes against liver cancer, which demonstrate an increased objective efficiency in controlling progression and relieving symptoms. The possibility of improved survival has also been stressed. According to the newly published results of the international multi-center Phase III clinical study comparing FOLFOX4 and ADM alone as palliative chemotherapy for advanced liver cancer in patients unsuitable for surgery (EACH) in 2010, OXA-containing combination chemotherapy was found to have provided a better objective response, disease control, survival benefit and safety for patients with advanced HCC. Attracting great attention of the international and domestic communities, the study has been a turning point for the long-term lack of a standard systemic chemotherapy guideline for advanced HCC, causing a major change of the concept in the treatment of liver cancer.
At present, HCC is regarded as being largely responsive to the new OXA-contaning chemotherapy regimens. For patients with advanced HCC that have no contraindications, systemic chemotherapy is superior to general supportive care and can be considered for the following indications:
(I) Advanced patients with extrahepatic metastases;
(II) Patients with local lesions who are ineligible for surgical treatment and hepatic arterial interventional embolization chemotherapy due to such conditions as diffuse liver diseases or abnormal liver blood vessels;
(III) Patients with tumor thrombi in the portal vein trunk or the inferior vena cava;
(IV) Patients with liver vascular obstruction following multiple transcatheter arterial chemoembolization (TACE) or recurrence after interventional treatment.
Of course, the indications for systemic chemotherapy should be strictly followed in conjunction with regular assessment of the efficacy, close monitoring and prevention of adverse reactions. In principle, patients are not suitable for systemic chemotherapy if they have any of the following:
(i) ECOG >2, Child-Pugh >7;
(ii) White blood cells <3.0×109/L or neutrophils <1.5×109/L, platelets <60×109/L and hemoglobin <90 g/L;
(iii) Evident liver or renal function abnormalities, aminotransferase (AST or ALT) significantly increased >5 times normal and/or bilirubin >2 times normal, serum albumin <28 g/L, creatinine (Cr) ≥ normal upper limit, and creatinine clearance rate (CCr) ≥50 mL/min;
(iv) Infectious fever, bleeding tendencies, intermediate to large amount of peritoneal effusion, and hepatic encephalopathy.
7.2.3. Other drugs
Tamoxifen, anti-androgen agents and octreotide are not recommended to be used in the systemic therapy against liver cancer because the survival benefit has not been demonstrated by any international randomized clinical trial (RCT). However, octreotide can be used to control gastrointestinal bleeding and relieve intestinal obstruction in patients with hepatocellular carcinoma.
7.3.Traditional Chinese medication
Traditional Chinese medicine can help reduce the toxicity of radiotherapy and chemotherapy, improve cancer-related symptoms and the quality of life, and may prolong survival. Those agents can be used as an important supplementary therapy to the treatment of liver cancer. In addition to the traditional dialectical theory of syndrome differentiation, treatment and pharmaceutical medication, a number of modern Chinese medicine preparations, including Xiaoaiping Injection, Kanglaite Injection, Cinobufacini, Elemene and Delisheng Injections, as well as their oral formulations, have been approved by the regulatory authority and widely applied for the treatment of liver cancer. They have been well tolerated and shown certain effects and unique therapeutic modes in clinical practice, with satisfying compliance and safety. Since these drugs have been in the market for years, however, few laboratory and clinical studies have been conducted to yield sufficient high-level medical evidence. Further research will be needed in this regard.
7.4. Other treatment
It is generally believed that biological treatment can improve the quality of life of patients with liver cancer, and help improve the antitumor efficacy and reduce postoperative recurrence. The application of thymosin α1 may enhance immune function and support the anti-viral and anti- tumor effects. For patients with viral hepatitis B-related HCC, the long-term dosage of IFN-α and its long-acting agents as adjuvant therapy after resection can effectively delay recurrence and reduce the risk of recurrence.
For HCC patients with hepatitis B and/or hepatitis C, the viral load (HBV DNA/HCV RNA) and activity of hepatitis should be closely monitored. The above anticancer drug treatment (including TAI/TACE, molecularly targeted therapy and chemotherapy) are known to potentially activate hepatitis virus, while active viral replication and active hepatitis can often damage the liver function and significantly compromise the anti-tumor therapy. When active hepatitis C virus replication is observed, timely and intensive antiviral therapy should be performed with nucleoside analogues, alpha interferon and its long-acting formulations, and thymosin α1. In addition, overall consideration should be given to strengthen the supportive symptomatic therapy throughout the entire course of treatment for liver cancer, including pain relief, protection of liver function, improvement of gallbladder function, treatment of anemia, nutritional improvement, control of blood glucose in patients with diabetes mellitus, correction of low protein blood, management of abdominal effusion and prevention of gastrointestinal bleeding and other complications. Such measures are critical in the symptomatic treatment to relieve suffering, improve the quality of life and ensure the implementation and effects of anti-tumor therapy.
8. Recommendation for multi-disciplinary treatment of liver cancer
HCC is often seen in patients with underlying chronic liver disease or cirrhosis, making adequate treatment challenging and complicated. Hence, a multi-disciplinary standardized treatment will be needed with individualized plan for different patients or a single patient at different stages. Chinese investigators have suggested two categories of treatment strategies based on the physical conditions and ECOG scores (0-2 and 3-4) of HCC patients.
8.1. ECOG score of 3-4
For patients with an ECOG score of 3-4, intensive anti-tumor therapy is often unfeasible due to a poor general health status. In such cases, symptomatic and traditional Chinese medical regimes are often administered;
8.2. ECOG score of 0-2
Patients with an ECOG score of 0-2 can be divided into two groups according to the Child-Pugh classification system:
(I) Patients of Child-Pugh Class C are treated similarly as above. For patients with liver decompensation due to the end-stage liver disease, liver transplant is the standard treatment option if indicated. At present, the Milan criteria are the most widely applied indications of liver transplant around the world. However, the Milan criteria are so strict that many patients with hepatocellular carcinoma who can potentially benefit from liver transplantation are screened out. There are also some expanded or modified criteria, such as UCSF in foreign countries, though a consensus has not been reached among the many criteria in China, which are mostly consistent regarding the absence of large vascular infringement, lymph node metastasis and extrahepatic metastasis but vary in terms of the tumor size and number. Upon thorough discussion of the expert panel, the UCSF criteria are recommended, that is, a single tumor not larger than 6.5 cm in diameter; or number of multiple tumors ≤3 with a maximum diameter ≤4.5 cm and total tumor diameter ≤8 cm;.
(II) Patients of Child-Pugh Class A or B are divided as those without extrahepatic metastasis (including distant and lymph node metastases, N0M0) and those with extrahepatic metastasis (N1 or M1). The former group is subdivided into two groups based on the vascular invasion: one with tumor thrombi in the main branches of the portal vein or the inferior vena cava, and one free of large vascular invasion. The main portal branches are defined as the portal trunk and the first- and second-order branches, where tumor thrombi are visible in general imaging studies. Microvascular tumor thrombi are not included as a differentiation indicator because it is not as useful in developing the treatment strategy and has less effect on the outcomes. Systemic therapy is recommended for patients with extrahepatic metastases, including molecularly targeted therapy (sorafenib), systemic chemotherapy (FOLFOX4 regime or arsenious acid injection), biological therapy and traditional Chinese medicine. Palliative radiotherapy can be performed if necessary (to relieve pain from bone metastases).
(III) For patients with tumor thrombi in the main portal branches (portal vein trunk and 1st and 2nd order branches), chemotherapy and/or portal stent implantation plus TACE is recommended when complete resection of the tumor and visible tumor thrombi is not possible. Otherwise, surgical resection of hepatocellular carcinoma, portal vein thrombectomy, chemotherapy pump placement plus portal vein heparin flush and continuous infusion chemotherapy plus TACE can be applied as a surgery-oriented multi-disciplinary treatment to significantly improve the survival of such patients and reduce postoperative metastasis and recurrence. For patients with tumor thrombi in the inferior vena cava caused by pressure from the enlarged tumor who are asymptomatic, TACE can be delivered alone without stent placement, and tumor shrinkage should be monitored. If the tumor thrombi are a result of tumor invasion of the inferior vena cava, it is recommended to deliver TACE and place stent in the inferior vena cava, or perform stent placement before TACE. Radiotherapy can also be combined. A combined or sequential systemic treatment (such as sorafenib, FOLFOX4 chemotherapy, arsenious acid injection and traditional Chinese medicine) is always recommended when these patients are able to tolerate.
(IV) Patients without evidence of vascular invasion are further divided according to the number and maximum diameter of their tumors (based on pre-operative imaging findings). TACE is recommended to manage the lesions for patients with four or more tumors, for whom surgical resection is not considered as the first-line option. The above treatment can also be delivered with ablation therapy in combination.
(V) For patients with two or three tumors with a maximum diameter of >3 cm or a single tumor of >5 cm, the survival following surgical resection is higher the TACE. However, some patients are ineligible for resection because of poor liver function reserve or incomplete envelope. In such cases, TACE is recommended. A decision on surgical approaches should be based on both the techniques and liver function reserve. In general, patients eligible for surgery should have a Child-Pugh score of ≤7. For patients who can not tolerate or are ineligible for operations or other anti-cancer treatment, liver transplant can be used if justified by the UCSF criteria. To date, there is no evidence of reduced recurrence or prolonged survival with TACE. On the other hand, TACE may give rise to complications such as severe adhesions, gallbladder gangrene, bile duct necrosis and liver abscess, and also increase the difficulty of liver resection. Therefore, preoperative TACE is not recommended before surgical resection of operable liver tumors.
(VI) For patients with a single tumor <5 cm in diameter or two to three tumors with a maximum diameter of ≤3 cm, surgical resection is preferred. The existing medical evidence also suggests the use of ablation for patients whose largest tumor is ≤3 cm in diameter. Surgical resection is associated with lower recurrence and longer metastasis-free survival, while percutaneous ablation has lower incidence of complications, quicker recovery and shorter hospital stay. Radiation therapy may also be considered for patients who refuse surgery, are complicated with disorders of the heart, lung and other organs, or are contraindicated for anesthesia or surgery. For patients who can not tolerate or are ineligible for operations or other anti-cancer treatment, liver transplant can be used if justified by the UCSF criteria (see Appendix 2, 3).
8.3. Treatment of underlying diseases
When considering the treatment option for HCC, emphasis should be placed on the treatment of underlying liver disorders (chronic hepatitis B, cirrhosis and liver dysfunction). During surgical resection or liver transplant, local ablation, TAI/TACE, radiotherapy and systemic treatment (molecularly target to drug therapy and chemotherapy), test and close monitoring of the viral load should be conducted; prophylactic use of antiviral drugs can be considered. Meanwhile, after hepatectomy, standard antiviral therapy is also recommended.
In summary, early detection, diagnosis and treatment of HCC is the key to good outcomes. A standardized comprehensive treatment should incorporate the mode with a multi-disciplinary team (MDT) to develop the optimal individual treatment plan upon extensive discussion and cooperation after considering the underlying diseases, pathological type, location and extent of invasion of tumor (clinical stage), portal vein or inferior vena cava tumor thrombus and distant metastases, as well as the general condition (PSECOG scores) and organ function status (particularly the degree of decompensated liver function) of a patient. A combination of surgery, hepatic arterial intervention, ablation, radiation therapy, systemic therapy (molecularly targeted therapy, chemotherapy, biological therapy, traditional Chinese medicine and anti-viral treatment, etc.) as well as supportive symptomatic treatment and other means can be taken into account while avoiding inappropriate therapy or over-treatment, so as to maximize the anti-tumor effects, increase the overall efficacy, improve the quality of life and prolong survival or even cure the disease for the patient. At the same time, individual treatment based on liver cancer molecular typing may be an important topic of concern in future.
9. Follow-up
PLC Patients should receive routine follow-up by the dynamic observation of their symptoms and signs and the auxiliary examinations (including serum AFP and imaging examinations). The progression and relapse of the disease and treatment-related adverse reactions should be carefully monitored. Generally, the follow-up can be arranged every 3-4 months during the first 3 years after treatment, every 4-6 months in years 3-5, and every 6-12 months after 5 years (if the condition remains normal).
Acknowledgements
Guidelines on the Diagnosis and Treatment of Primary Liver Cancer (2011 edition) Expert Panel
Consultants: Meng-Chao Wu, Zhao-You Tang, Yan Sun, Shu-Sen Zheng
Team leader: Shu-Kui Qin
Deputy team leader: Jia Fan, Feng Shen, Jian-Qiang Cai, Jia-Hong Dong, Wei-Lin Wang, Gao-Jun Teng
Members (in alphabetical order): Min-Hua Chen, Min-Shan Chen, Xiao-Ping Chen, Wen-Ming Cong, Zhao-Liu Dai, Xiang Du, Guo-Hong Han, Guo-Liang Jiang, Huai Li, Jun Liang, Lian-Xin Liu, Li-Gong Lu, Sheng-Lin Ma, Yi-Lei Mao, Hai-Bin Shi, Jian-Hua Wang, Mao-Qiang Wang, Bao-Cai Xing, Jian-Ming Xu, Jia-Mei Yang, Jian-Yong Yang, Sheng-Long Ye, Jian Zhou, Ji-Ye Zhu
Disclosure: The author declares no conflict of interest.
Note: 1 The dynamic contrast-enhanced radiological modalities included dynamic contrast-enhanced multi-slice CT and dynamic contrast-enhanced MRI; 2. The typical radiological findings refer to the remarkably enhanced lesion(s) during the dynamic contrast-enhanced arterial phase (which reflects the rich blood supply of tumors) and the rapid clearance of a hyperdense lesion during the portal venous and delayed phases (the latter is then shown as hypodense on CT or low-signal intensity on MRI). Nevertheless, a few HCC lesions (particularly those small lesions ≤2 cm in diameter) can be remarkably enhanced during the arterial phase and equal density/signal intensity during the portal venous and delayed phases; or, the enhancement is not obvious during the arterial phase. These findings may reflect that the blood supply is poor inside the tumor, and must be cautiously differentiated from the benign lesions; 3. AFP(+) refers to the AFP lever ≥400 μg/L for at least one month or ≥200 μg/L for at least two months; 4. This flow chart is suitable for the diagnosis of HCC in patients with a history of chronic hepatitis B, hepatitis C, and/or cirrhosis.