Contemporary management of ductal carcinoma in situ and lobular carcinoma in situ
Introduction
Histopathologically, lobular carcinoma in situ (LCIS) and ductal carcinoma in situ (DCIS) are differentiated from invasive carcinoma by the confinement of malignant cells to the basement membrane (1). Historically, these two in situ carcinomas were considered obligate precursors to invasive lesions (1). However, recent genomic and transcriptomic analyses indicate molecular similarities in in situ and invasive cancers within the context of histologic grade as opposed to stage of progression (2,3). Specifically, comparisons of low grade DCIS and low grade invasive ductal cancer (IDC) to high grade DCIS and high grade IDC show genomic differences in ploidy level, karyotype and amplification (2). Estrogen receptor expression and activation among these lesions has been also been implicated in the progression to invasive disease (4). Moreover, the work of Hanahan et al. on the hallmarks of cancer have contributed to the evolution of our understanding of tumorigenesis and moved us towards consideration of both DCIS and LCIS as non-obligate precursors along the broad spectrum of malignant progression (5). Thus, the progression from DCIS and LCIS to invasive cancer is not assured, but rather a complex process involving interactions between genetics and the microenvironment at the molecular level (5,6).
Epidemiology
The implementation of population-based screening in North America and Europe has resulted in a marked increase in the incidence of in situ cancers (1,7). In developing countries, the practice of opportunistic breast screening has resulted in a dearth of information about the true incidence of in situ cancers (8), as in these settings, DCIS and LCIS are more likely to be diagnosed based on imaging and concomitant core needle biopsy (CNB) prompted by the presence of symptoms (9). As a result, it is difficult to determine the incidence and prevalence of these in situ lesions in countries in which breast cancer screening is not widely implemented.
In literature from the United States, the incidence of LCIS on open surgical biopsy is between 0.5% and 3.8% and ranges from 0.02% to 3.3% on core needle biopsies (10-12). Population screening data from South Australia detected LCIS in 5.3% of in situ specimens (13). Estimates from the Breast Cancer Surveillance Consortium (BCSC) in the United States reports that DCIS represents 24.9% of all cancers detected on screening (14). This figure corresponds with data from population-based screening programs in Turkey, Singapore and South Australia reporting 22%, 26% and 20% DCIS respectively (13,15,16). When compared to countries in Europe, such as Switzerland, the Netherlands and Italy, it appears that the US has the highest rates of DCIS (17). In China, where population-based screening is still not widely practiced, Si et al. in their 20-year review noted only 2.4% of the 4,968 sample population were diagnosed with DCIS/LCIS (18).
Risk factors
The risk factors for the development of in situ and invasive cancers are similar. These factors include family history and genetic predisposition, increased mammographic breast density and a history of atypia on breast biopsy (1). For women with a family history of breast cancer, Claus et al. calculated a 48% (OR 1.48) and 68% (OR 1.68) increased risk of DCIS and LCIS respectively compared to women with no family history of breast cancer. However, there was no association seen between alcohol consumption, smoking or oral contraceptive (OCP) use and risk of in situ carcinoma (19). A review of the data shows no consensus on the risk of developing in situ cancers and the use of hormone replacement therapy, although exogenous hormones are likely to contribute to incidence of DCIS as well as for invasive cancers (17,19).
Ductal carcinoma in situ (DCIS)
Imaging
The primary contributor to increased detection of DCIS is implementation of widespread screening mammography, starting in the United States in the 1980s. Whereas DCIS was rarely diagnosed before the use of mammography, it now accounts for an estimated 50,000 new breast cancers detected in women annually (20). DCIS most frequently presents as incidental microcalcifications on screening mammography. Only 10% of DCIS are associated with other imaging findings, including asymmetric density or mass (Figure 1) (21,22). BIRADS morphologic classification categorizes microcalcifications as amorphous, coarse heterogeneous, fine pleomorphic, fine linear, dystrophic or round, with the highest risk of DCIS seen with the fine pleomorphic and fine linear classifications (23,24). In some studies, digital mammography has been shown to have greater sensitivity for detection of DCIS than screen-film mammography, particularly among pre- and perimenopausal women (25,26). Interestingly, breast tomosynthesis, or “3-D mammography” while reducing call-backs, has not resulted in increased detection of DCIS (27).
The benefit of magnetic resonance imaging (MRI) in the routine management of DCIS has yet to be determined. MRI may have a possible role in preoperative workup in some women, especially in the setting of multifocal disease which can sometimes preclude breast conserving surgery (BCS). In this setting, MRI has been shown to have improved sensitivity over mammography in detecting multicentricity (28-30). In determining extent of disease, MRI can both underestimate DCIS compared to mammography (31) as well as overestimate DCIS. Therefore, MRI alone should not be used as an indication for mastectomy, although it may guide the need for further evaluation (32).
The potential advantages of MRI are reduced re-excision rates, identification of contralateral breast cancer at an earlier stage, and decreased local recurrence; however, these potential benefits have not been clearly established in published studies. Limited data have shown that preoperative MRI may have no significant impact on re-excision rate, margin status, or margin width (33-35). Moreover, increased sensitivity of breast MRI comes at the cost of increased resource utilization, heightened patient anxiety, and a propensity for more patients to opt for mastectomy, regardless of the results of the MRI (7,33,36). In one study only preoperative MRI and age were independent predictors for receipt of mastectomy (33). MRI can be useful for screening the contralateral breast, resulting in identification of contralateral breast cancer in 2.6% of patients (36). This may be one factor contributing to higher contralateral mastectomy in women diagnosed with unilateral DCIS (37). However, the majority of contralateral mastectomies are still performed in women with unilateral DCIS, underscoring the possible unintended consequences of increased MRI evaluation.
Pathology
The World Health Organization (WHO) defines DCIS as a neoplastic intraductal lesion characterized by increased epithelial proliferation, presence of subtle to marked cellular atypia and an inherent but not necessarily obligate tendency for progression to invasive breast cancer (38). In pure DCIS, the intraductal epithelial cells are separated from the breast stroma by an intact layer of basement membrane and myoepithelial cells. DCIS is further separated into comedo and non-comedo types, based on the presence of necrosis (Figure 2). In 2009, the College of American Pathologists (CAP) and the American Society for Clinical Oncology (ASCO) established guidelines for pathology reporting of DCIS, requiring defining DCIS lesions as low, intermediate or high grade, with nuclear grade determined using six morphologic features: pleomorphism, size, chromatin, nucleoli, mitoses and orientation (39).

The pathologic assessment of DCIS can often be difficult, with even experienced breast pathologists often disagreeing on which characteristics constitute a diagnosis of DCIS (40,41). The morphologic distinction between atypia and low grade DCIS can often be subtle, requiring specialized expertise in some cases to render a diagnosis. Multicentricity can be encountered in DCIS; moreover, skip lesions can be seen leading to difficulty in margin assessment (42,43).
For DCIS diagnosed on core biopsy, occult invasion may sometimes be identified. Despite the use of larger gauge stereotactic devices, the incidence of upstaging at the time of surgical excision remains 20–25% (44-46). The possible identification of invasive cancer at the time of definitive excision should be discussed with patients, who must be advised that findings on final pathology may result in additional treatment recommendations.
Important challenges remain in the pathologic assessment of DCIS, particularly in reporting size and margin status. In addition, there are known disagreements between pathologists in how best to distinguish some DCIS from other epithelial lesions such as atypical ductal hyperplasias. However, substantial work has resulted in consensus for pathologic assessment for DCIS reporting criteria, including synoptic data elements that have facilitated both research and treatment.
Surgical management
Surgery for DCIS is aimed to prevent progression to invasive cancer with the attendant risks of disease dissemination and cancer mortality. NCCN guidelines for treatment of DCIS recommend excision of all disease to negative margins, with either BCS or mastectomy (47). Radiation is often recommended as part of BCS to reduce risk of local recurrence, based on randomized trial data. Treatment trends for DCIS over the past 20 years in the United States demonstrate a reduction in unilateral mastectomy with a resulting increase in lumpectomy and radiation (1,48). The most striking trend however, is the increase in bilateral mastectomy for unilateral DCIS, which is currently used to treat almost 10% of all newly diagnosed cases of DCIS. Important to note is that the choice of surgery or radiation have not been shown to impact disease specific mortality, indicating that surgical options should be considered in the context of patient values, risk aversion, and competing comorbidities (48).
Mastectomy
Currently, almost a third of women diagnosed with DCIS in the United States will undergo mastectomy for their disease. Generally, mastectomy is indicated in patients with DCIS for extensive and/or multifocal DCIS involving more than one quadrant or in women with a contraindication for breast irradiation, such as prior irradiation or history of collagen vascular disease. However, patient preference remains the most important consideration in surgical treatment planning (49). In addition to simple mastectomy, skin-sparing mastectomy and nipple-sparing mastectomy are being used increasingly for DCIS. Overall, both skin-sparing and nipple sparing approaches have been shown to be oncologically safe with low recurrence rates (50-52). The good outcomes in these studies may be attributable to careful patient selection, involving exclusion of patients with centrally located disease, extensive DCIS, or radiographic abnormalities in close proximity to the nipple-areolar complex (53). Since the risk of distant metastasis with DCIS is negligible, the reason for performing mastectomy or BCS should be based on the extent of disease. The long-term risk of local recurrence following mastectomy is excellent at 1–2% in most series.
Lumpectomy
BCS is the most commonly employed surgical procedure in patients with DCIS. Since up to 90% of DCIS is nonpalpable and usually not visualized on ultrasound, various imaging-based localization techniques have been used to more precisely target the area of DCIS for excision. The most commonly used approach is that of wire localization, with either a single wire for focal calcifications or bracketed wires for more extensive regions of involvement. Recently, radioactive seed localization has been used in some institutions to allow for more focal targeting of nonpalpable lesions, with excellent results reported (54). Regardless of the localization technique used, confirmation of retrieval of the targeted lesion on a specimen radiograph is an essential and required component of the procedure.
BCS is more challenging for DCIS than for invasive cancer, due to the greater difficulty in obtaining negative margins secondary to the more discohesive growth pattern. In one study, only DCIS and whether additional shave margins were obtained were predictive of a positive lumpectomy margin (55). Although the optimal size of a negative margin for DCIS remains a matter of debate, it is clear that recurrence is highly associated with a positive margin, and most surgeons aim to achieve at least 1–2 mm margins for DCIS, even at the cost of multiple re-excisions.
Surgical management of the axilla
The American Society of Clinical Oncology (ASCO) clinical practice guideline update for sentinel lymph node biopsy (SLNB) in early stage breast cancer supports the use of SLNB for DCIS in women undergoing mastectomy. This recommendation is in part due to the known incidence of upstaging from core biopsy to surgical excision (56), as well as concerns regarding the potential for technical challenges in performing SLNB following disruption of lymphatics between the breast and axilla. In unselected DCIS, the rate of positive sentinel node involvement ranges between 5–10%, many of which consist of isolated tumor cells or micrometastases (57-61) (Table 1). Data support that the outcomes for those DCIS patients with small volume nodal disease do not differ from those with negative nodes, further advocating against routine SLNB for DCIS.

Full table
Radiation
In the United States, adjuvant radiation therapy (RT) following wide local excision for DCIS remains standard of care, although the NCCN guidelines include the option of excision only for low risk DCIS. There have now been five prospective randomized trials comparing lumpectomy alone to lumpectomy with radiation in women with DCIS (Table 2) (62-66). Overall, ipsilateral breast recurrences can be reduced by more than half with adjuvant radiation, with the absolute magnitude of benefit dependent on baseline recurrence risk. The benefit of radiation was seen in all subgroups regardless of age at diagnosis, extent of breast-conserving surgery, use of tamoxifen, method of DCIS detection, margin status, focality, grade, comedonecrosis, architecture, or tumor size. Based on these studies, most women with DCIS are recommended to consider adjuvant RT to reduce the risk of local recurrence.
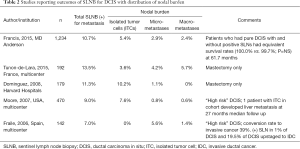
Full table
Despite a clear proportional benefit of RT in all subsets of patients undergoing lumpectomy for DCIS, wide excision alone has gained increasing attention as an alternative to lumpectomy with RT among some subgroups of women with low-risk DCIS who are at sufficiently low risk of recurrence that they may not derive meaningful clinical benefit from radiation. The most recently completed study was conducted by the Radiation Therapy Oncology Group (RTOG), which reported results from a prospective randomized trial that allocated low risk DCIS to radiotherapy or observation following lumpectomy for DCIS (62,67). Even in this low risk cohort, defined as low-intermediate grade DCIS smaller than 2.5 cm with at least 3 mm margins, local failure at 7 years was significantly improved when lumpectomy was followed by radiotherapy (0.9% vs. 6.7%, P≤0.01). However, it has been argued that this difference may be too small to be clinically meaningful and these data may in fact support lumpectomy alone in this favorable group.
Several single-arm studies of excision without radiation have also been reported. The largest of these was the Eastern Cooperative Oncology Group (ECOG) 5194 trial, a multi-center trial of lumpectomy alone in women with DCIS at low risk for recurrence, based on clinical and pathologic criteria (68,69). Eligible patients were required to have low- or intermediate-grade DCIS, tumor size of ≤25 mm (cohort 1), or high-grade DCIS, tumor size of ≤1.0 cm (cohort 2), and a minimum negative margin width of ≥3 mm or no tumor on re-excision. At a median follow-up of 12.3 years, the 12-year rates of developing an ipsilateral breast event (IBE) were 14.4% for cohort 1 and 24.6% for cohort 2 (P=0.003). Twelve-year rates of developing an invasive IBE were 7.5% and 13.4%, respectively (P=0.08). A smaller single arm study of 158 patients treated for DCIS with wide excision alone at the Harvard hospitals was recently updated (70). At a median follow up of 11 years, 19 patients (13%) had LR as a first event of which 13 were DCIS only and six were invasive. The 10-year estimated cumulative incidence of LR was 15.6%.
There has been recent interest in the use of accelerated partial breast irradiation (APBI) for DCIS. According to the ASTRO consensus statement, DCIS less than 3 cm can be treated with APBI with caution (71). To date, the largest cohort of women with DCIS treated with APBI demonstrated an ipsilateral breast tumor recurrence of 2.6% at 5 years with no regional recurrences (72). Other smaller studies (73-75) suggest that APBI in DCIS is equivalent to APBI in early breast cancer. At present, there is consensus that DCIS can be treated with caution using ABPI techniques; however longer follow-up will determine whether this benefit is durable.
Endocrine therapy
The use of adjuvant endocrine therapy for DCIS is aimed to reduce both ipsilateral breast recurrences in women undergoing lumpectomy as well as new contralateral breast events. However, the tradeoff between clinical benefit and side effects has not provided a clear advantage in favor of endocrine therapy for all patients, particularly in those with low risk disease or hormone receptor-negative DCIS. Two prospective randomized trials have provided some insight in how tamoxifen might be best applied: in NSABP B24 women treated for DCIS with BCS and radiation were randomized to either tamoxifen or placebo. Overall, women treated with tamoxifen had a 30% reduction in breast cancer-free survival, with the benefit restricted to ER(+) DCIS (76). In the UK, Australia, New Zealand study, patients with DCIS treated with lumpectomy were randomized to in a 2×2 design to either tamoxifen, radiation, both, or neither. At a median follow up of 12.7 years, women randomized to tamoxifen had a significant 29% reduction in both ipsilateral and contralateral events, but the benefit was limited to those women who did not have radiation (77). Aromatase inhibitors (AIs) are also emerging as potentially beneficial in this setting. NSABP B-35 randomized women after lumpectomy to either tamoxifen with radiation or anastrozole with radiation and found that anastrozole was superior to tamoxifen event-free survival in women younger than 60 years of age (78). Taken together, these studies suggest that endocrine therapy may play a role in patients with hormone receptor-positive disease who decline radiotherapy, and that future studies will help refine indications for AI in the management of DCIS.
Active surveillance
There has been interest in recent years in active surveillance for low risk DCIS, in part based upon the recognition of the tremendous biological heterogeneity in the group of conditions defined as “DCIS”. Although there is ongoing work on identifying those biomarkers which could help identify the lowest risk DCIS with sufficiently low risk to warrant surveillance rather than excision, no specific marker has emerged to provide sufficiently accurate risk stratification. The first such clinical trial randomizing patients with low risk DCIS (age >45, grade 1 or 2 DCIS) to active surveillance with or without endocrine therapy was activated in the UK in 2014. Named the “LORIS” study, the trial is aimed to determine how invasive cancer incidence, overall and breast cancer specific survival are impacted with surveillance alone (79). In the United States, the COMET study will aim to address this question in a cohort of low risk, ER-positive HER2-negative DCIS.
Lobular carcinoma in situ (LCIS)
Detection and diagnosis
Since first proposed by Haagensen et al. in 1978, and subsequently used in the WHO classification of breast tumors, the term lobular neoplasia (LN) has been used to encompass the histopathologic spectrum of atypical lobular hyperplasia (ALH) and LCIS (6,9). LCIS is further subdivided into classic lobular carcinoma in situ (CLCIS) and pleomorphic lobular carcinoma in situ (PLCIS) (80). Due to a lack of distinctive physical exam findings, or pathognomonic findings on imaging, ALH, CLCIS and PLCIS are usually found incidentally in biopsy specimens (81). Characteristically, LN has a propensity to be bilateral and multifocal (82).
Although the terminology of LN has been in the literature for many years it is not uniformly used (81). Specifically, some authors use LN without differentiating between the subtypes (ALH, LCIS, PLCIS) in their analysis while others make distinctions between the subtypes of LN. The importance of distinguishing between the subtypes of LN lies in the differences in risk of breast cancer. LCIS confers a much higher risk (9–10 times increased risk) of breast cancer than ALH (4–5 times increased risk) (83). Moreover, further distinction of LCIS as CLCIS or PLCIS is important due to the difference in recommendations for management (84).
In addition to being a risk factor, LN has also been shown to be a non-obligate precursor based on comparable histologic and molecular profiles to invasive lobular cancer (ILC) (85-87) and a higher risk of ipsilateral breast cancer compared to the contralateral breast (9). Furthermore, in contrast to women of average risk, individuals with LN have an increased probability of developing ILC (18-fold increase) as opposed to IDC (3- to 4-fold increase) (9,81,88).
Mammography is the most sensitive imaging modality for LN (9). Due to a transition from film screen mammography (FSM) to digital screen mammography (DSM) there has been a threefold increase in the detection of high risk lesions such as LN (89). Punctate microcalcifications are the most common radiologic finding of LCIS on mammography (90). However, it should be noted that there is a difference in the prevalence of mammographic findings between PLCIS and CLCIS. Specifically, PLCIS is more likely to present with microcalcifications, architectural distortion and/or density (Figures 3,4) (82).
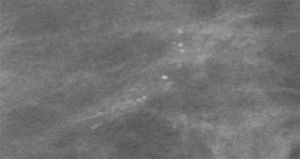
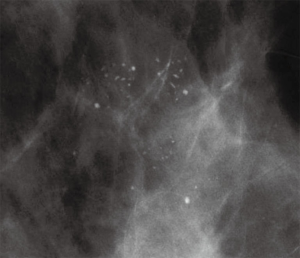
On MRI, LN typically appears as an area of non-mass-like enhancement (91). The use of MRI as a screening modality for patients with LCIS is somewhat controversial in light of differing recommendations from the American Cancer Society (ACS) and the NCCN (92). Current recommendations from the ACS states there is insufficient evidence to support or oppose the use of yearly MRI screening among patients with LCIS (93). Alternatively, the NCCN recommends consideration of annual MRI for patients with a diagnosis of LCIS after the age of 30 (94). Studies have estimated an incidental cancer detection rate of 4% in patients with LCIS undergoing MRI (92,95). These findings correspond to cancer detection rates for patients with genetic abnormalities (e.g., BRCA mutations) screened with MRI; thus leading some authors to argue for MRI screening of patients with LCIS (96). However, MRI as an adjunct to conventional screening has not been proven to increase detection of early stage breast cancer or result in higher cancer detection rates when compared to conventional screening alone among individuals with LCIS (97). Additionally, use of screening MRI results in a higher number of biopsies and more frequent follow up visits and evaluations (95). These inconsistencies in the literature underscore the persistent controversy around screening MRI in the LCIS population.
Despite these descriptions of image findings associated with LN it should be noted that the majority of LN, principally CLCIS, is not associated with any particular specific radiologic findings (9). The definitive diagnostic modalities for LN are CNB or open surgical biopsy. However, with a move away from surgical biopsy as the initial tissue acquisition modality image guided CNB is recommended as the first biopsy method used for diagnosis (98).
Pathology
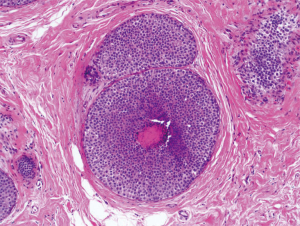
The difference between ALH and LCIS, which has been described as “quantitative rather than qualitative”, is dependent on the number of involved acini of a lobular unit (81). Specifically, CLCIS is defined as distension of more than half the acini of a lobular unit by a uniform and discohesive population of small atypical epithelial cells (80) (Figure 5). CLCIS is hormonally sensitive (estrogen and progesterone receptor positive) and generally does not overexpress Ki67 and human epidermal growth factor (Her-2) (82,99). The United Kingdom National Health Service Breast Screening Program (NHSBSP) has provided guidelines for this nomenclature, and currently recommends using the all-encompassing term LN instead of differentiating the subtypes as ALH or CLCIS, arguing that the distinction between CLCIS and ALH are arbitrary and subjective (6,100).
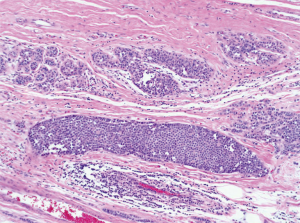
PLCIS is characterized by enlarged discohesive epithelial cells, irregular shaped nuclei and abundant eosinophilic cytoplasm (101) (Figure 6). Additional features such as comedo necrosis and calcifications found in PLCIS make it histopathologically similar to DCIS. Such similarities to DCIS have resulted in some authors arguing that PLCIS has an increased probability of progression to invasive cancer (82,84,102). In their retrospective review on the genetic and phenotypic characteristics of PLCIS, Chen et al. reported PLCIS was not hormonally sensitive (estrogen and progesterone receptor negative), exhibited a higher proliferation rate and overexpression of epidermal growth factor Her-2 (82). These finding further confirmed the similarities between PLCIS and DCIS. Immunohistochemistry staining shows both PLCIS and CLCIS lack staining for E-cadherin. Conversely, DCIS stains positive for E-cadherin enabling differentiation of PLCIS from DCIS with immunohistochemistry (9,82).
Treatment and management
The 2016 National Comprehensive Cancer Network (NCCN) guidelines for the management of LCIS recommend surgical excision for LCIS diagnosed on CNB (103). However, some have recently argued that this recommendation be revised based on new data. Multiple studies, including one prospective study, have recently reported low upgrade rates ranging from 1–5% upon exclusion of specimens with high risk characteristics such as non-classic morphology, discordant imaging and pathology, and extensive LCIS (>4 foci) (90,104-106). Moreover, Ciocca et al. showed that the presence of LCIS at the margin of breast conservation therapy specimens (BCT) did not affect local recurrence (107). These low upgrade rates indicate that surgical excision provides little benefit for patients who present with concordant imaging and pathology with pure LN on CNB.
Active surveillance
As indicated above, LCIS is considered both a high-risk lesion and a non-obligate precursor which confers a 10–20% risk for development of breast cancer (9,103). Due to this designation, women with LCIS are classified as high risk and have different screening recommendations compared to average risk women. In particular, the NCCN guidelines recommend clinical breast exam (CBE) every 6–12 months in conjunction with an annual mammogram (103). In King et al.’s 29-year single institutional review of their experience with LCIS, surveillance was reported as the most frequently selected management modality. In this study, women who underwent surveillance without chemoprevention had a cancer rate (invasive ductal, invasive lobular or DCIS) of 7% and 21% at 5 and 10 years respectively. This was significantly higher than the 5-year 3% and 10-year 12% cancer (invasive ductal, invasive lobular or DCIS) rates reported for women in the cohort undergoing surveillance combined with chemoprevention (108).
Surgery
Historically, surgery has been at the cornerstone for the management of LN. At this time, recommendations from many of the major cancer organizations endorse excision of LN associated with any invasive cancer, DCIS or discordant radiologic and pathologic findings (6,81,103). However, the surgical management of pure LN has proved controversial. Based on Foote and Stewart’s initial description of LCIS as a precursor to invasive lobular carcinoma (ILC), mastectomy was recommended to prevent the progression to ILC (81). Nonetheless, with new genetic and molecular information, in conjunction with better insight into the natural history of LN, the recommendation of surgery to prevent progression is no longer the prevailing paradigm (6).
Current surgical recommendations differ between PLCIS and CLCIS. Due to differences in the histological and molecular features between the subtypes surgical recommendations are different depending on the subtype. As mentioned previously, imaging-concordant pure CLCIS has a low risk of a concomitant invasive lesion; therefore, surgical excision is not warranted and it can be effectively managed with surveillance and chemoprevention (6,84,90). Due to concerns that PLCIS is more aggressive and exhibits histologic and molecular characteristics similar to DCIS, the NCCN, European Society of Medical Oncology (ESMO) and NHSBSP all recommend excision with negative margins (102). Flannagan et al. in their retrospective analysis on patients with PLCIS noted a high rate of upgrades to invasive cancer or DCIS (84). Pieri et al. in their systematic review reported a high rate of concomitant invasive disease with PLCIS and as a result, recommended surgical excision similarly to how DICS would be treated. However, they mentioned there is currently no evidence on the effectiveness of adjuvant therapy, such as radiation (102).
For patients with LN and other risk factors such as family history of breast cancer, genetic abnormalities, or extremely dense breasts bilateral prophylactic mastectomy (BPM) can be offered as a risk reduction strategy (6). Studies report a 90–95% risk reduction among patients who undergo BPM (109,110). However, there is general consensus that this represents a more invasive treatment than is warranted for most patients, particularly for those patients who lack genetic predisposition for breast cancer.
Chemoprevention
The American Society of Clinical Oncology (ASCO) and the NCCN recommend placing high-risk (Gail model risk ≥1.7% or history of LCIS) premenopausal women on selective estrogen receptor modulators (SERM) and post-menopausal women on AIs. Specifically, tamoxifen for premenopausal women and raloxifene or exemestane for post-menopausal women (6,103).
Multiple studies have established the risk reduction to invasive cancer conferred by chemoprevention in high risk populations. The National Surgical Adjuvant Breast and Bowel Project (NASBP) P-1 study was one of the early transformative studies in the management of patients with high risk lesions such as LCIS. Inclusion criteria for the study was age ≥60 years; age 35–59 years with at least a 1.66% 5-year predicted risk of breast cancer; or any age with a history of LCIS. Participants were randomized to receive a placebo or 20 mg/day of tamoxifen. Results showed a 44%, 51%, and 55% risk reduction in women ≤49 years, between 50–59 and ≥60 years respectively. Moreover, there was a 56% risk reduction among women with a history of LCIS (111). These results were confirmed in the NSABP (STAR, P-2) trial which reported tamoxifen and raloxifene were equivalent in reducing the risk of invasive cancer. In addition, they noted raloxifene had a reduced risk of thromboembolic events and cataracts compared to tamoxifen (112). In the MAP.3 trial, exemestane use resulted in a 65% risk reduction in invasive cancer (113). These studies show chemoprevention as an acceptable alternative to surgery or active surveillance in appropriately selected individuals with LCIS.
Conclusions
The increased detection of in situ lesions on screening mammography has made both DCIS and LCIS important clinical entities. Currently, DCIS is treated as a precursor to invasive cancer, and LCIS as a risk factor for invasive cancer. However, evidence supports that both confer risk of invasive progression, including increased breast cancer risk to the contralateral breast. Emerging studies in the biology of DCIS and LCIS have revealed tremendous heterogeneity among in situ lesions. Such discoveries have the potential to provide better risk stratification of in situ disease, and will provide future opportunities to individualize treatment recommendations based upon extent of future cancer risk.
Acknowledgements
We would like to thank Dr. Lars Grimm for supplying the radiologic images and Dr. Edgardo Parilla for the pathology slides.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ward EM, DeSantis CE, Lin CC, et al. Cancer statistics: Breast cancer in situ. CA Cancer J Clin 2015;65:481-95. [Crossref] [PubMed]
- Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, et al. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology 2010;57:171-92. [Crossref] [PubMed]
- Bailie K, Dobie I, Kirk S, et al. Survival after breast cancer treatment: the impact of provider volume. J Eval Clin Pract 2007;13:749-57. [Crossref] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Harris RJ, Lippman EM, Morrow M, et al, editors. Diseases of The Breast. 5 ed. Philadelphia: Wolters Kluwer Tealth, 2014.
- Virnig BA, Tuttle TM, Shamliyan T, et al. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst 2010;102:170-8. [Crossref] [PubMed]
- Lui CY, Lam HS, Chan LK, et al. Opportunistic breast cancer screening in Hong Kong; a revisit of the Kwong Wah Hospital experience. Hong Kong Med J 2007;13:106-13. [PubMed]
- Oliveira TM, Elias J Jr, Melo AF, et al. Evolving concepts in breast lobular neoplasia and invasive lobular carcinoma, and their impact on imaging methods. Insights Imaging 2014;5:183-94. [Crossref] [PubMed]
- Hussain M, Cunnick GH. Management of lobular carcinoma in-situ and atypical lobular hyperplasia of the breast--a review. Eur J Surg Oncol 2011;37:279-89. [Crossref] [PubMed]
- Esserman LE, Lamea L, Tanev S, et al. Should the extent of lobular neoplasia on core biopsy influence the decision for excision? Breast J 2007;13:55-61. [Crossref] [PubMed]
- Philpotts LE, Shaheen NA, Jain KS, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology 2000;216:831-7. [Crossref] [PubMed]
- Luke C, Priest K, Roder D. Changes in incidence of in situ and invasive breast cancer by histology type following mammography screening. Asian Pac J Cancer Prev 2006;7:69-74. [PubMed]
- Breast Cancer Surveillance Consortium. Cancers for 2,061,691 Screening Mammography Examinations from 2004 - 2008 -- based on BCSC data through 2009. Cited 2016 1/30/2016. Available online: http://breastscreening.cancer.gov/statistics/benchmarks/screening/2009/table4.html
- Kayhan A, Gurdal SO, Ozaydin N, et al. Successful first round results of a Turkish breast cancer screening program with mammography in Bahcesehir, Istanbul. Asian Pac J Cancer Prev 2014;15:1693-7. [Crossref] [PubMed]
- Jara-Lazaro AR, Thilagaratnam S, Tan PH. Breast cancer in Singapore: some perspectives. Breast Cancer 2010;17:23-8. [Crossref] [PubMed]
- Virnig BA, Shamliyan T, Tuttle TM, et al. Diagnosis and management of ductal carcinoma in situ (DCIS). Evid Rep Technol Assess (Full Rep) 2009.1-549. [PubMed]
- Si W, Li Y, Han Y, et al. Epidemiological and Clinicopathological Trends of Breast Cancer in Chinese Patients During 1993 to 2013: A Retrospective Study. Medicine (Baltimore) 2015;94:e820. [Crossref] [PubMed]
- Claus EB, Stowe M, Carter D. Breast carcinoma in situ: risk factors and screening patterns. J Natl Cancer Inst 2001;93:1811-7. [Crossref] [PubMed]
- American Cancer Society. Breast Cancer Facts & Figures 2015-2016 Atlanta2015 [updated 2015; cited 2016 2/11/2016]. Available online: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdf
- Ikeda DM, Andersson I. Ductal carcinoma in situ: atypical mammographic appearances. Radiology 1989;172:661-6. [Crossref] [PubMed]
- Stomper PC, Connolly JL, Meyer JE, et al. Clinically occult ductal carcinoma in situ detected with mammography: analysis of 100 cases with radiologic-pathologic correlation. Radiology 1989;172:235-41. [Crossref] [PubMed]
- Bent CK, Bassett LW, D'Orsi CJ, et al. The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. AJR Am J Roentgenol 2010;194:1378-83. [Crossref] [PubMed]
- Rominger M, Wisgickl C, Timmesfeld N. Breast microcalcifications as type descriptors to stratify risk of malignancy: a systematic review and meta-analysis of 10665 cases with special focus on round/punctate microcalcifications. Rofo 2012;184:1144-52. [Crossref] [PubMed]
- Del Turco MR, Mantellini P, Ciatto S, et al. Full-field digital versus screen-film mammography: comparative accuracy in concurrent screening cohorts. AJR Am J Roentgenol 2007;189:860-6. [Crossref] [PubMed]
- Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005;353:1773-83. [Crossref] [PubMed]
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014;311:2499-507. [Crossref] [PubMed]
- Hwang ES, Kinkel K, Esserman LJ, et al. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol 2003;10:381-8. [Crossref] [PubMed]
- Menell JH, Morris EA, Dershaw DD, et al. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J 2005;11:382-90. [Crossref] [PubMed]
- Santamaría G, Velasco M, Farrus B, et al. Preoperative MRI of pure intraductal breast carcinoma--a valuable adjunct to mammography in assessing cancer extent. Breast (Edinburgh, Scotland) 2008;17:186-94. [Crossref] [PubMed]
- Schouten van der Velden AP, Boetes C, Bult P, et al. The value of magnetic resonance imaging in diagnosis and size assessment of in situ and small invasive breast carcinoma. Am J Surg 2006;192:172-8. [Crossref] [PubMed]
- Allen LR, Lago-Toro CE, Hughes JH, et al. Is there a role for MRI in the preoperative assessment of patients with DCIS? Ann Surg Oncol 2010;17:2395-400. [Crossref] [PubMed]
- Itakura K, Lessing J, Sakata T, et al. The impact of preoperative magnetic resonance imaging on surgical treatment and outcomes for ductal carcinoma in situ. Clin Breast Cancer 2011;11:33-8. [Crossref] [PubMed]
- Davis KL, Barth RJ Jr, Gui J, et al. Use of MRI in preoperative planning for women with newly diagnosed DCIS: risk or benefit? Ann Surg Oncol 2012;19:3270-4. [Crossref] [PubMed]
- Pilewskie M, Olcese C, Eaton A, et al. Perioperative breast MRI is not associated with lower locoregional recurrence rates in DCIS patients treated with or without radiation. Ann Surg Oncol 2014;21:1552-60. [Crossref] [PubMed]
- Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007;356:1295-303. [Crossref] [PubMed]
- Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol 2009;27:1362-7. [Crossref] [PubMed]
- Frank GA, Danilova NV, Andreeva I, et al. WHO classification of tumors of the breast, 2012. Arkh Patol 2013;75:53-63. [PubMed]
- Lester SC, Bose S, Chen YY, et al. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch Pathol Lab Med 2009;133:15-25. [PubMed]
- Schnitt SJ, Connolly JL, Tavassoli FA, et al. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol 1992;16:1133-43. [Crossref] [PubMed]
- Elmore JG, Longton GM, Carney PA, et al. Diagnostic concordance among pathologists interpreting breast biopsy specimens. JAMA 2015;313:1122-32. [Crossref] [PubMed]
- Holland R, Peterse JL, Millis RR, et al. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 1994;11:167-80. [PubMed]
- Faverly DR, Burgers L, Bult P, et al. Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol 1994;11:193-8. [PubMed]
- Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer. Radiology 2011;260:119-28. [Crossref] [PubMed]
- Kettritz U, Rotter K, Schreer I, et al. Stereotactic vacuum-assisted breast biopsy in 2874 patients: a multicenter study. Cancer 2004;100:245-51. [Crossref] [PubMed]
- Houssami N, Ciatto S, Ellis I, et al. Underestimation of malignancy of breast core-needle biopsy: concepts and precise overall and category-specific estimates. Cancer 2007;109:487-95. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer: Version 1, 2015. Available online: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. Accessed December 3, 2015.
- Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst 2015;107:djv263. [Crossref] [PubMed]
- Hwang ES. The impact of surgery on ductal carcinoma in situ outcomes: the use of mastectomy. J Natl Cancer Inst Monogr 2010;2010:197-9.
- Crowe JP, Patrick RJ, Yetman RJ, et al. Nipple-sparing mastectomy update: one hundred forty-nine procedures and clinical outcomes. Arch Surg 2008;143:1106-10; discussion 1110. [Crossref] [PubMed]
- Sakurai T, Zhang N, Suzuma T, et al. Long-term follow-up of nipple-sparing mastectomy without radiotherapy: a single center study at a Japanese institution. Med Oncol 2013;30:481. [Crossref] [PubMed]
- Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol 2012;19:3402-9. [Crossref] [PubMed]
- Wagner JL, Fearmonti R, Hunt KK, et al. Prospective evaluation of the nipple-areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137-44. [Crossref] [PubMed]
- McGhan LJ, McKeever SC, Pockaj BA, et al. Radioactive seed localization for nonpalpable breast lesions: review of 1,000 consecutive procedures at a single institution. Ann Surg Oncol 2011;18:3096-101. [Crossref] [PubMed]
- Chagpar AB, Killelea BK, Tsangaris TN, et al. A Randomized, Controlled Trial of Cavity Shave Margins in Breast Cancer. N Engl J Med 2015;373:503-10. [Crossref] [PubMed]
- Lyman GH, Temin S, Edge SB, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2014;32:1365-83. [Crossref] [PubMed]
- Francis AM, Haugen CE, Grimes LM, et al. Is Sentinel Lymph Node Dissection Warranted for Patients with a Diagnosis of Ductal Carcinoma In Situ? Ann Surg Oncol 2015;22:4270-9. [Crossref] [PubMed]
- Tunon-de-Lara C, Chauvet MP, Baranzelli MC, et al. The Role of Sentinel Lymph Node Biopsy and Factors Associated with Invasion in Extensive DCIS of the Breast Treated by Mastectomy: The Cinnamome Prospective Multicenter Study. Ann Surg Oncol 2015;22:3853-60. [Crossref] [PubMed]
- Dominguez FJ, Golshan M, Black DM, et al. Sentinel node biopsy is important in mastectomy for ductal carcinoma in situ. Ann Surg Oncol 2008;15:268-73. [Crossref] [PubMed]
- Moore KH, Sweeney KJ, Wilson ME, et al. Outcomes for women with ductal carcinoma-in-situ and a positive sentinel node: a multi-institutional audit. Ann Surg Oncol 2007;14:2911-7. [Crossref] [PubMed]
- Fraile M, Gubern JM, Rull M, et al. Is it possible to refine the indication for sentinel node biopsy in high-risk ductal carcinoma in situ? Nucl Med Commun 2006;27:785-9. [Crossref] [PubMed]
- McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol 2015;33:709-15. [Crossref] [PubMed]
- Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol 2008;26:1247-52. [Crossref] [PubMed]
- Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol 2006;24:3381-7. [Crossref] [PubMed]
- Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet 2003;362:95-102. [Crossref] [PubMed]
- Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 1998;16:441-52. [PubMed]
- McCormick B, Winter K, Hudis C, et al. RTOG 9804: A prospective randomized trial for “good risk” ductal carcinoma in situ (DCIS), comparing radiation (RT) to observation (OBS). J Clin Oncol 2012;30:abstr 1004.
- Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2009;27:5319-24. [Crossref] [PubMed]
- Solin LJ, Gray R, Hughes LL, et al. Surgical Excision Without Radiation for Ductal Carcinoma in Situ of the Breast: 12-Year Results From the ECOG-ACRIN E5194 Study. J Clin Oncol 2015;33:3938-44. [Crossref] [PubMed]
- Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS). Breast Cancer Res Treat 2014;143:343-50. [Crossref] [PubMed]
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [Crossref] [PubMed]
- Vicini F, Shah C, Ben Wilkinson J, et al. Should ductal carcinoma-in-situ (DCIS) be removed from the ASTRO consensus panel cautionary group for off-protocol use of accelerated partial breast irradiation (APBI)? A pooled analysis of outcomes for 300 patients with DCIS treated with APBI. Ann Surg Oncol 2013;20:1275-81. [Crossref] [PubMed]
- Benitez PR, Streeter O, Vicini F, et al. Preliminary results and evaluation of MammoSite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ: a phase II clinical study. Am J Surg 2006;192:427-33. [Crossref] [PubMed]
- Abbott AM, Portschy PR, Lee C, et al. Prospective multicenter trial evaluating balloon-catheter partial-breast irradiation for ductal carcinoma in situ. Int J Radiat Oncol Biol Phys 2013;87:494-8. [Crossref] [PubMed]
- Shah C, Badiyan S, Ben Wilkinson J, et al. Treatment efficacy with accelerated partial breast irradiation (APBI): final analysis of the American Society of Breast Surgeons MammoSite((R)) breast brachytherapy registry trial. Ann Surg Oncol 2013;20:3279-85. [Crossref] [PubMed]
- Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol 2012;30:1268-73. [Crossref] [PubMed]
- Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 2011;12:21-9. [Crossref] [PubMed]
- Margolese R. Primary results, NRG Oncology/NSABP B-35: A clinical trial of anastrozole (A) versus tamoxifen (tam) in postmenopausal patients with DCIS undergoing lumpectomy plus radiotherapy. J Clin Oncol 2015;33:abstr LBA500.
- Francis A, Thomas J, Fallowfield L, et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer 2015;51:2296-303. [Crossref] [PubMed]
- Sinn HP, Kreipe H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care (Basel) 2013;8:149-54.
- Kuerer HM, editor. Breast Surgical Oncology. 1 ed. China: Mcgraw Hill, 2010.
- Chen YY, Hwang ES, Roy R, et al. Genetic and phenotypic characteristics of pleomorphic lobular carcinoma in situ of the breast. Am J Surg Pathol 2009;33:1683-94. [Crossref] [PubMed]
- Hwang H, Sullivan ME, Susnik B. Lobular neoplasia. Diagn Histopathol 2010;16:337-44. [Crossref]
- Flanagan MR, Rendi MH, Calhoun KE, et al. Pleomorphic Lobular Carcinoma In Situ: Radiologic-Pathologic Features and Clinical Management. Ann Surg Oncol 2015;22:4263-9. [Crossref] [PubMed]
- Andrade VP, Ostrovnaya I, Seshan VE, et al. Clonal relatedness between lobular carcinoma in situ and synchronous malignant lesions. Breast Cancer Res 2012;14:R103. [Crossref] [PubMed]
- Hwang ES, Nyante SJ, Yi Chen Y, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer 2004;100:2562-72. [Crossref] [PubMed]
- Logan GJ, Dabbs DJ, Lucas PC, et al. Molecular drivers of lobular carcinoma in situ. Breast Cancer Res 2015;17:76. [Crossref] [PubMed]
- Wheeler JE, Enterline HT, Roseman JM, et al. Lobular carcinoma in situ of the breast. Long-term followup. Cancer 1974;34:554-63. [Crossref] [PubMed]
- Neal CH, Coletti MC, Joe A, et al. Does digital mammography increase detection of high-risk breast lesions presenting as calcifications? AJR Am J Roentgenol 2013;201:1148-54. [Crossref] [PubMed]
- Rendi MH, Dintzis SM, Lehman CD, et al. Lobular in-situ neoplasia on breast core needle biopsy: imaging indication and pathologic extent can identify which patients require excisional biopsy. Ann Surg Oncol 2012;19:914-21. [Crossref] [PubMed]
- Scoggins M, Krishnamurthy S, Santiago L, et al. Lobular carcinoma in situ of the breast: clinical, radiological, and pathological correlation. Acad Radiol 2013;20:463-70. [Crossref] [PubMed]
- Friedlander LC, Roth SO, Gavenonis SC. Results of MR imaging screening for breast cancer in high-risk patients with lobular carcinoma in situ. Radiology 2011;261:421-7. [Crossref] [PubMed]
- American Cancer Society. American Cancer Society recommendations for early breast cancer detection in women without breast symptoms 2015 [updated 10/20/2015; cited 2016 2/4/2016]. Available online: http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis [cited 2015 2/4/2016]. Available online: http://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf
- Port ER, Park A, Borgen PI, et al. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol 2007;14:1051-7. [Crossref] [PubMed]
- Lehman CD, Smith RA. The role of MRI in breast cancer screening. J Natl Compr Canc Netw 2009;7:1109-15. [PubMed]
- King TA, Muhsen S, Patil S, et al. Is there a role for routine screening MRI in women with LCIS? Breast Cancer Res Treat 2013;142:445-53. [Crossref] [PubMed]
- Silverstein MJ, Recht A, Lagios MD, et al. Special report: Consensus conference III. Image-detected breast cancer: state-of-the-art diagnosis and treatment. J Am Coll Surg 2009;209:504-20. [Crossref] [PubMed]
- Mohsin SK, O'Connell P, Allred DC, et al. Biomarker profile and genetic abnormalities in lobular carcinoma in situ. Breast Cancer Res Treat 2005;90:249-56. [Crossref] [PubMed]
- Walker RA, Hanby A, Pinder SE, et al. Current issues in diagnostic breast pathology. J Clin Pathol 2012;65:771-85. [Crossref] [PubMed]
- Frost AR, Tsangaris TN, Silverberg SG. Pleomorphic lobular carcinoma in situ. Pathol Case Rev 1996;1:27-30. [Crossref]
- Pieri A, Harvey J, Bundred N. Pleomorphic lobular carcinoma in situ of the breast: Can the evidence guide practice? World J Clin Oncol 2014;5:546-53. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Guidelines in Oncology: Breast Cancer 2016 [cited 2016 1/17/2016]. Available online: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Hwang H, Barke LD, Mendelson EB, et al. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol 2008;21:1208-16. [Crossref] [PubMed]
- Murray MP, Luedtke C, Liberman L, et al. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer 2013;119:1073-9. [Crossref] [PubMed]
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of Adjacent Synchronous Invasive Carcinoma and/or Ductal Carcinoma In-situ in Patients with Lobular Neoplasia on Core Biopsy: Results from a Prospective Multi-Institutional Registry (TBCRC 020). Ann Surg Oncol 2016;23:722-8. [Crossref] [PubMed]
- Ciocca RM, Li T, Freedman GM, et al. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann Surg Oncol 2008;15:2263-71. [Crossref] [PubMed]
- King TA, Pilewskie M, Muhsen S, et al. Lobular Carcinoma in Situ: A 29-Year Longitudinal Experience Evaluating Clinicopathologic Features and Breast Cancer Risk. J Clin Oncol 2015;33:3945-52. [Crossref] [PubMed]
- Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med 1999;340:77-84. [Crossref] [PubMed]
- Geiger AM, Yu O, Herrinton LJ, et al. A population-based study of bilateral prophylactic mastectomy efficacy in women at elevated risk for breast cancer in community practices. Arch Intern Med 2005;165:516-20. [Crossref] [PubMed]
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-88. [Crossref] [PubMed]
- Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006;295:2727-41. [Crossref] [PubMed]
- Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011;364:2381-91. [Crossref] [PubMed]


