An overview of modern proton therapy
Introduction to proton therapy
It is widely believed that Robert Wilson at the Harvard Physics Research Laboratory was the first to mention utilization of proton beam therapy in a 1946 series titled Radiological Use of Fast Protons (1). His work was being disseminated at a time when high-energy proton beams capable of clinically relevant ranges were being constructed mostly within the scope of high-energy charged particle physics research efforts. As alluded to by Dr. Eric Hall, the first relevant clinical proton therapy use in the United States occurred at these high-energy research centers through medical collaborations with the University of California, Berkeley and the Harvard Cyclotron Facility (Massachusetts General Hospital) (2). This interest was in large part due to the physical characteristics of the proton beam and its discrete dose deposition properties, devoid of significant exit dose. The first hospital-based facility became operational at the Loma Linda Medical Center in 1990. These early efforts all utilized passive-scattered spread-out Bragg peak proton beams, and it was only in 1996, that the Paul Scherer Institute in Switzerland became the first to introduce pencil-beam scanning technology, which forms the basis of modern proton beam therapy.
Physics of proton beam
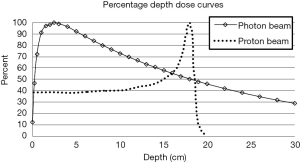
Protons are positively charged particles, with a large rest mass, that continuously lose energy through interactions with surrounding atomic electrons and nuclei in the materials that they traverse (3). For protons the rate of energy loss per unit path length, defined as stopping power increases as the proton slows down. This phenomenon causes a proton to lose a significant amount of its energy very rapidly just prior to it stopping. Therefore, proton dose deposition peaks at the end of the proton range, resulting in the creation of the Bragg peak. A unique characteristic of protons is the negligible dose that is deposited in the regions distal to the Bragg peak once the protons stop.
The depth at which protons stop and create their respective Bragg peak is related to the initial energy of the proton. For clinical applications of proton radiation therapy, one can continuously adjust the proton energies as they enter the patient to allow the protons to deposit their dose within the tumor volume, while sparing tissues distal to that tumor. This is in stark contrast with photon beams, which attenuate in an exponential fashion and therefore always deposit dose distal to the tumor. Figure 1 illustrates the distinct depth-dose deposition characteristics for photon and proton beams.
Proton delivery modes
Two common methods are available for the delivery of proton therapy: passive scattering and active scanning.
In passive scattering, a physical material is placed in the beam path to scatter and hence broaden the beam; of note, as protons exit the cyclotron they travel in a narrow pencil beam. Subsequent collimation allows the lateral extent of the beam to be shaped to the target width. To cover the target’s extent in depth a spread out Bragg peak (SOBP) is created with the help of a range modulating wheel. A range modulator allows generation of different proton energies and resultant Bragg peaks at varying depths such that in combination a SOBP is created. Subsequently, a field specific compensator of varying thickness is used to conform the shape of the dose to the shape of the distal edge of the tumor to prevent irradiation of uninvolved tissue.
For active spot scanning, the pencil beam is moved perpendicular to its path with magnets. Protons of a given energy are deposited at a given position or spot. The beam is moved to the next spot where protons can again be deposited. A unique number of protons can be delivered to each spot therefore allowing “dose-painting” of individual spots in the tumor volume. Modulation of the beam’s energy allows the delivery of different ranges, such that different energy layers can be treated in a sequential fashion. With active scanning, the tumor volume becomes filled with spots of proton dose deposition.
Spot scanning can be further optimized via two different approaches: single field optimization (SFO) or multi-field optimization (MFO). As described by Quan et al., in SFO planning, individual fields are optimized independently to treat the tumor volume (4). As such, the dose distribution for each field in SFO planning fulfills the plans objectives. In MFO, spots are optimized such that the final dose delivery, incorporating dose from all fields, fulfills the plan objectives; all spots from all fields are optimized in unison. While this provides the ability to have tighter dose conformity than SFO, it is also potentially more sensitive to uncertainties of setup, motion and range.
Range uncertainty represents a critical parameter to account for in proton planning. Uncertainty can arise in part due to differential in the tissue composition that the beam may pass through, as a result of interfraction and intrafraction patient motion or set up variances. Additionally, there is inherent uncertainty present in the conversion of CT Hounsfeld units to proton stopping power that can result in different dose distributions to target and normal organs at risk. Accounting for these uncertainties is critical in understanding the robustness of a plan.
Radiobiology of proton therapy
As Paganetti et al. note, proton therapy prescription doses are determined assuming an RBE value of 1.1Jeny (5). This value was based on an average of early results of in vivo studies comparing photon and proton therapy in a multitude of different biological systems, all with various endpoints. Recently, the dogma of a fixed RBE of 1.1 for protons has been questioned. Paganetti et al. highlight in their review the potential for an RBE variance with changing alpha/beta ratio, LET and depth within the SOBP as well as on the clinical endpoint of interest. These elements will likely change the manner in which proton therapy prescriptions are planned in the future. Of particular concern is the distal edge of the beam just beyond the Bragg peak, where the RBE variation can be substantial (as high as 1.4–1.6 in some studies), and therefore “ranging” into a distal critical structure beyond the distal edge of the tumor is avoided in proton therapy planning. This biological uncertainty in the region of the range represents one of the limitations of proton therapy.
Additionally, Girdhani et al. have highlighted the differential biological effects of proton irradiation at the cellular level (6). For instance, they highlight that in various mouse and cell models treated with proton and photon radiation, proton therapy has been shown to have a potential anti-angiogenic effect not readily seen with photon therapy; this leads to potential rebound up-regulation of pro-angiogenic factors, underscoring the need to consider investigating proton therapy in combination with anti-angiogenic agents. Additional studies have also revealed the ability of proton therapy to reduce cell migration of tumor cells, highlighting the potential of local proton delivery in reducing cancer metastases.
Proton therapy eliminates the radiation low dose “bath” associated with photon therapy, which theoretically could diminish the lymphocytopenia associated with radiation therapy thereby potentially resulting in superior outcomes if used in combination with immune check-point inhibitors.
These elements raise the possibility of proton therapy having not only improved physical properties in comparison to photon therapy, but also enhanced biological effects.
Current status
Given the ever-changing landscape of our knowledge of proton therapy with respect to not only its physical properties, but also its clinical and biological effects, we are beginning to learn the true potential of this treatment modality. In this issue, we hope to provide a comprehensive review of the many clinical scenarios and individual patient cases in which proton therapy could be superior to photon therapy. Additionally, given the increasing recognition of the economics of medical care in today’s society, we will discuss the cost comparativeness of photon and proton therapies. We hope that the articles in this issue provide our readers a thorough and highly educational opportunity to increase their understanding of proton radiation therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wilson RR. Radiological use of fast protons. Radiology 1946;47:487-91. [Crossref] [PubMed]
- Hall E. Protons for radiotherapy: a 1946 proposal. Lancet Oncol 2009;10:196. [Crossref] [PubMed]
- Paganetti H. Nuclear interactions in proton therapy: dose and relative biological effect distributions originating from primary and secondary particles. Phys Med Biol 2002;47:747-64. [Crossref] [PubMed]
- Quan EM, Liu W, Wu R, et al. Preliminary evaluation of multifield and single-field optimization for the treatment planning of spot-scanning proton therapy of head and neck cancer. Med Phys 2013;40:081709. [Crossref] [PubMed]
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014;59:R419-72. [Crossref] [PubMed]
- Girdhani S, Sachs R, Hlatky L. Biological effects of proton radiation: an update. Radiat Prot Dosimetry 2015;166:334-8. [Crossref] [PubMed]