
Radiotherapy, lymphopenia and improving the outcome for glioblastoma: a narrative review
Introduction
Background
Glioblastoma is the most common malignant primary brain tumor in the adult population. Maximal safe surgical resection is considered the gold standard to achieve maximal survival benefit while preserving neurological function whenever possible. Even with maximal therapy including a gross total resection, however, most patients with glioblastoma will develop recurrent tumors. Consequently, standard of care for glioblastoma generally includes maximal safe resection followed by adjuvant radiation and concurrent temozolomide for 6 weeks, followed by 6 months of maintenance temozolomide (1). Even with maximal combination therapy, glioblastoma has a high rate of recurrence and poor median survival at 17 months even for younger patients with excellent performance status (2).
Glioblastoma, remains incurable even though it is a localized tumor and many treatments appear effective in preliminary studies with cell culture or rodent models. A contributing factor to poor control appears to be an in vivo suppression of anti-tumor immune response both as an adaptive response to the tumor itself, and also as a consequence of the therapies used (3-6). Standard radiation treatment for glioblastoma, with or without concurrent temozolomide, causes significant and prolonged lymphopenia which even while being cytotoxic to tumor cells could result in impaired tumor control and reduced survival outcomes (5,6).
Rationale and knowledge gap
In this review, we will explore the evidence that radiotherapy treatment causes lymphopenia with a consequent decrease in tumor control. Conventionally fractionated radiation has a daily cytotoxic effect on circulating lymphocytes cells, among the most radiosensitive tissues in the human body (7,8). Conversely, high-dose hypofractionated radiotherapy given over several days, rather than over weeks, may result in less lymphotoxicity through reduced dose to the circulating blood (6,9-12). In addition, high-dose hypofractionated treatment has potential to boost the anti-tumor immune response when used synergistically with lymphocyte-mediated immune therapies by increasing tumor neo-antigens that may be detectible and then targeted by the immune system (13-15). For this reason, there is increasing attention that is directed towards improving radiotherapy by developing an understanding of both the lymphotoxic effects of standard fractionated radiotherapy and the potential to harness the synergistic effects of high-dose hypofractionated radiotherapy in improving tumor outcomes.
This review is significant in that it provides new updates regarding the recent advances in lymphocyte-mediated immune therapies over the past several years and emerging interest in considering the use of high-dose hypofractionated radiotherapy which may actually increase the effectiveness of treatment in certain scenarios and disease sites, although this remains an area of active research in glioblastoma and other solid tumors (6,16).
Objective
Our objective is to describe the immunosuppressive effects of radiotherapy and its impact on local control and survival outcomes. Further, we aim to explore the systemic effect of radiation on circulating white blood cells in glioblastoma patients. We then aim to review other contributing factors to immunosuppression and describe the potential role of cancer cells in triggering neuroprotective anti-inflammatory mechanisms (generally part of the normal adaptive protective response to prevent injury from excessive neuro-inflammation after ischemic stroke and traumatic brain injuries), and how this is exacerbated by radiotherapy creating an immune environment permissive of tumor growth within the central nervous system. Finally, we provide updates regarding lymphocyte-mediated immune therapies over the past several years and how immunotherapy (combined with possible use of hypofractionated radiotherapy) may provide insights towards limiting the impact of treatment-related lymphopenia and optimizing treatment outcomes in the future for glioblastoma patients. We present this article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-22-94/rc).
Methods
A literature review was performed using PubMed and Google Scholar to identify scientific articles published between 1970 and 2022. The search terms used included “glioblastoma”, “treatment-related lymphopenia”, “radiotherapy”, “tumor microenvironment”, “anti-tumor immunity”, “immunosuppression” and “immunomodulation”. We used a table (Table 1) to present detailed search strategy.
Table 1
| Items | Specification |
|---|---|
| Date of search | 08/01/2022–09/26/2022 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | Glioblastoma; treatment-related lymphopenia; radiotherapy; tumor microenvironment; anti-tumor immunity; immunosuppression; immunomodulation |
| Timeframe | 1970–2022 |
| Inclusion and exclusion criteria | Inclusion criteria: research articles and reviews in English about themes such as treatment related lymphopenia and immunosuppression. Exclusion criteria: some papers which we considered were controversial or with low reliability |
| Selection process | Carmen Kut and Lawrence Kleinberg conducted the selection and discussed the literature and obtained the consensus |
| Any additional considerations, if applicable | Some papers were identified by reviewing reference lists of relevant publications and from a priori knowledge of key publications |
Immunosuppressive tumor microenvironment unique to glioblastoma
Unlike most solid tumors, glioblastoma is characterized by a highly immunosuppressive tumor microenvironment within the brain (17). First of all, the central nervous system is generally protected by the blood-brain-barrier. While the goal of the blood-brain-barrier is to restrict pathogen entry into the brain, it also contributes towards an immunosuppressive microenvironment by preventing circulating immune cells from infiltrating the brain parenchyma (17,18). In addition, glioblastoma cells generate an immunosuppressive environment that are conductive to tumor growth (19,20). For example, glioblastomas are known to render T cells dysfunctional once they have migrated from the circulating blood pool into the tumor microenvironment (21). Glioblastoma cells are also known to trigger decreased T cell production and reduce the availability of T cells for anti-glioblastoma immunity (19,22). All of these mechanisms create an immune environment rendering glioblastoma patients especially vulnerable to the severity and consequences of treatment-induced lymphopenia.
Definition of lymphopenia
Lymphopenia is commonly defined by the Common Terminology Criteria for Adverse Events (CTCAE Version 5.0) (23). Grade 2 lymphopenia is defined by an absolute lymphocyte count (ALC) of <500 cells/mm3; grade 3 lymphopenia by ALC <200 cells/mm3; and grade 4 lymphopenia by ALC <50 cells/mm3 (23).
Glioblastoma treatment
Glioblastoma is the most common and most aggressive adult primary brain cancer, with a median survival of 14 to 15 months (24). The current standard of care is maximal safe resection, followed by radiotherapy with temozolomide chemotherapy. Radiotherapy is given daily for six weeks (30 treatments) with concurrent temozolomide at 75 mg/m2, administered Monday to Friday before each daily radiotherapy session. This was followed by maintenance temozolomide at 150 mg/m2 monthly for 6 additional cycles. The radiation component of the therapy consists of an initial gross target volume (GTV) defined by contrast enhancement plus the post-operative cavity plus surrounding edema treated to 46 Gy in 23 fractions, followed by a boost of 14 Gy in 7 fractions to the area of contrast enhancement plus the post-operative cavity. Wide margins in the brain (generally a 1.5 to 2.5 cm expansion from gross treatment volume to planned treatment volume) are necessary for glioblastoma radiotherapy coverage given the aggressive histology and the tendency for glioblastoma to develop finger-like projections that infiltrate deep into the surrounding tissue. Additionally, corticosteroids are often used to reduce intracranial pressure and limit inflammatory side effects secondary to radiotherapy. It is generally administered from diagnosis at a maximum dose of 4 mg every 6 hours for a period of >8–12 weeks, and gradually tapered as tolerated following the completion of radiotherapy. This combination of cytotoxic therapies (radiotherapy, temozolomide and corticosteroids) for glioblastoma treatment often places our patients at high risk for severe treatment-related lymphopenia that can persist for months (25).
Treatment-related lymphopenia in glioblastoma
Lymphopenia was first noted as a potentially important complication in 2003 by an unexpected increase in cases of Pneumocystis Jiroveci pneumonia cases in patients with primary brain tumors who were treated primarily with post-operative radiotherapy and corticosteroids (26). This led to a retrospective study which found that radiotherapy and high-dose corticosteroids (without chemotherapy) were sufficient to trigger continuous decline in CD4 lymphocytes throughout the weeks of treatment, with 24% of patients resulting in CD4 counts <200/mm3 (27). After temozolomide was established as part of standard of care, a second prospective study evaluated 96 patients and found that that those with CD4 counts <200/mm3 at 2-month follow up had significantly worse survival (median survival at 13.1 vs. 19.7 months, P=0.002) (28). Most importantly, 88% of these deaths resulted from disease progression, whereas only 2.5% were due to infection. Rahman et al. (29) published an abstract in 2016 and provided retrospective data with 196 glioblastoma patients which supported this observed association of treatment-related lymphopenia with reduced survival outcomes. In this study, Rahman et al. found that 47% of the patients developed grade 3–4 lymphopenia (defined by post-treatment ALC <500 mm3), and that lymphopenic patients had reduced overall survival (13.1 vs. 18.2 months, P=0.023). Similarly, Mendez et al. observed shorter survival patterns in elderly (age ≥65) glioblastoma patients with ALC <500 cells/mm3 two months after initiating radiotherapy and temozolomide (4.6 vs. 11.6 months, P=0.008) (30). This observation is supported by more recent studies highlighting the association of severe treatment-related lymphopenia with inferior overall survival (HR 1.08, P=0.009) (31).
Treatment-related lymphopenia in other solid tumors
Radiation-induced lymphopenia has since been observed in patients diagnosed with other solid tumors and is similarly associated with worse survival outcomes. Since chemotherapy and radiotherapy often provide synergistic mechanisms in targeting tumor cells, they are often used concurrently for their cytotoxic and anti-neoplastic effects in many disease sites. However, myelosuppressive systemic therapies can also significantly impact circulating blood cells and bone marrow reserves. While irradiation of the bone marrow can result in myelosuppression and pancytopenia, radiotherapy generally has a more selective role in inducing lymphopenia through its effect on circulating blood cells rather than the bone marrow progenitor cells. Mammalian lymphocytes are exquisitely radiosensitive and rapidly undergo apoptotic death within hours of radiation exposure within the treatment field. Consequently, treatment-related lymphopenia occurs in 40% to 70% of patients diagnosed with solid tumors and treated with radiotherapy, regardless of the type of corticosteroid and/or chemotherapy regimens that were administered concurrently (30,32-44).
Treatment-related lymphopenia is associated with significantly worse overall and progression-free survival in many other solid tumor types outside the central nervous system (CNS) (32,33,35,37,40,44-47). Table 2 summarizes both prospective and retrospective data highlighting the impact of lymphopenia on survival outcomes in including nasopharyngeal cancer, malignant glioma, gastrointestinal malignancies, pancreatic cancer, esophageal cancer, lung cancer, cervical cancer and rectal cancer (30,32-40,43,44,48,49). For example, Liu et al. (32) conducted a prospective study with 413 patients diagnosed with nasopharyngeal cancer, and found that those with on-treatment ALC nadir of <390 cells/mm3 had significantly lower 5-year overall survival (79% vs. 90%, P=0.002) and 5-year progression free survival (72.4% vs. 79.8%, P=0.005). Similarly, several retrospective studies have demonstrated significantly reduced median survival for lung and pancreatic cancer patients treated with chemoradiation with ALC <500 cells/mm3 and 2-month follow-up status post treatment. A recent study showed that patients with decreased vs. increased T-cell receptor repertoire post-radiotherapy had a 3-year overall survival of 22% vs. 75% (P=0.04) in gastrointestinal malignancies (48).
Table 2
| Ref | Disease site | Study design | n | Timing | Tx criteria | Lymphocyte categories | Survival data | Survival type | P value | Additional Adjusted covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| (32) | Nasopharyngeal cancer (II–IVB) | P | 413 | TRL | CRT | ALC nadir <390 vs. ≥390 cells/mm3 during treatment | 79.0% vs. 90.0% | 5-year OS | 0.002* | Age, sex, stage, family history, preTx EBV DNA, chemo, VCA-IgA, EA-IgA, chemo and radiation dose, ALC 3 months after Tx |
| 72.4% vs. 79.8% | 5-year PFS | 0.005* | ||||||||
| (28,33,34) | Malignant glioma | P | 96 | TRL | CRT | ALC <500 cells/mm3vs. ALC >500 cells/mm3, 2 months following Tx | 16 vs. 18 mo | Median OS | 0.02* | Age, surgery, histology, KPS, infection |
| (30) | Glioblastoma in elderly | R | 72 | TRL | CRT | ALC <500 cells/mm3vs. ALC ≥500 cells/mm3, 2 months following Tx | 4.6 vs. 11.6 mo | Median OS | 0.008* | MGMT status, surgical extent, steroid use, radiation dose |
| (33,35) | Resected pancreatic cancer | R | 53 | TRL | CRT | ALC <500 cells/mm3vs. ALC >500 cells/mm3, 2 months following Tx | 14 vs. 20 mo | Median OS | 0.05* | Baseline ALC, node-positive |
| (33,36) | Unresectable pancreatic cancer | R | 101 | TRL | CRT | ALC <500 cells/mm3vs. ALC > 500 cells/mm3, 2 months following Tx | 9 vs. 13 mo | Median OS | 0.03* | Baseline albumin, baseline BUN, baseline platelets, PTV |
| (37) | Stage I–III Esophageal Cancer | R | 504 | TRL | CRT | ALC nadir <50 cells/mm3vs. >200 cells/mm3 | 2.8 vs. 5.0 years | Median OS | 0.027* | Age, CAD, DM, tumor characteristics, stage, chemo regimen, surgery, radiation technique |
| (33,38) | Stage III NSCLC | R | 47 | TRL | CRT | ALC <500 cells/mm3vs. ALC >500 cells/mm3, 2 months following Tx | 22 vs. 27 mo | Median OS | 0.17 | Age, radiation dose, neoadjuvant chemotherapy |
| (39) | Limited stage NSCLC | R | 73 | TRL | CRT | ALC nadir ≤297 vs. >297 cells/mm3 during treatment | 12.2 vs. 35.3 mo | Median OS | <0.001* | – |
| (39) | Limited Stage NSCLC | R | 73 | Post-Tx | CRT | ALC ≤698 cells/mm3vs. ALC >698 cells/mm3, 1 month following Tx | 19.3 vs. 46.9 mo | Median OS | 0.0001* | – |
| (40) | FIGO stage I–III cervical cancer | R | 124 | TRL | CRT and Brachy | ALC <200 cells/mm3vs. ALC ≥200 cells/mm3 | 50.4% vs. 84.8% | 5-year DSS | <0.001* | Age, FIGO stage, baseline ALC, radiation fractionation & technique, overall treatment time |
| 50.0% vs. 80.7% | 3-year PFS | 0.002* | ||||||||
| (48) | Gastrointestinal malignancies | R | 109 | TRL | During RT | TCR repertoire diversity low vs. high as defined by Gini diversity index | 22% vs. 75% | 3-year OS | 0.026* | ECOG, stage, radiation dose, chemotherapy use, pathologic complete response, R0 resection, baseline WBC |
| (49) | Locally advanced rectal cancer | R | 102 | TRL | Neoadj. CRT | ALC nadir ≤440 vs. >440 cells/mm3 during treatment | 41.3 vs. 69.2 mo | Median DFS | 0.01* | Tumor size, stage, tumor differentiation |
| 46.1 vs. 80.1 mo | Median OS | 0.004* |
*P<0.05. P, prospective; R, retrospective. ALC, absolute lymphocyte count; Brachy, brachytherapy; BUN, blood urea nitrogen; CAD, coronary artery disease; CRT, chemo-radiation treatment; DM, diabetes mellitus; DSS, disease-specific survival; EA-IgA, immunoglobulin A against early antigen; EBV, Epstein Barr Virus; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecollogy and Obstetrics; KPS, Karnofsky Performance Status; MGMT, O6-Methylguanine-DNA Methyltransferase; NSCLC, non-small cell lung cancer; OS, overall survival; P, prospective; PFS, progression-free survival; postTx, post-treatment; preTx, pre-treatment; PTV, planned treatment volume; R, retrospective; RT, radiation treatment; R0 resection, microscopically margin-negative resection; TCR, T-cell receptor; TRL, treatment-related lymphopenia; Tx, treatment; VCA-IgA, immunoglobulin A against viral capsid antigen.
Radiotherapy is cytotoxic to circulating lymphocytes
Lymphopenia in patients treated with cranial radiotherapy is predominantly the result of radiation exposure of circulating blood cells (50). Bone marrow or lymphatic tissue are less likely contributing factors since only a limited volume of these tissues were exposed to cranial radiotherapy. For example, localized cranial radiotherapy when added to marrow depleting chemotherapy resulted in increased leukopenia and thrombocytopenia (51). Importantly, extracorporeal irradiation of the circulating blood for dialysis patients (for sterilization purposes) has been shown to cause severe lymphopenia in the absence of any radiation exposure to the patient’s marrow or other tissues (50).
As irradiation of circulating blood is a cause of lymphopenia, the fractionation regimen and size of radiation target can have a significant impact. In 1978, MacLennan et al. explored this issue and found that the extent of lymphopenia in patients treated with whole brain radiotherapy is dependent upon the number of radiotherapy fractions (with a constant total combined dose of 24 Gy) (52). Importantly, this study found that irradiation of the brain in patients with childhood leukemia can result in a 65% greater reduction in the averaged lymphocyte counts after 20 daily fractions when compared with five daily fractions. This suggests that repeated daily radiotherapy sessions exposed more of the circulating lymphocytes to the radiotherapy treatment field, which results in more severe immunosuppression. We note that the effect may be particularly important for cranial radiotherapy given that the brain is a highly perfused organ.
This observation of the immunosuppressive effect based upon radiation fractionation is supported by a modeling study which generated a typical glioblastoma plan with an 8 cm tumor planned for 60 Gy in 30 fractions to assess the percent of circulating cells receiving ≥0.5 Gy (53). This study showed that while a single radiation fraction would expose only 5% of the circulating blood cells to a dose of ≥0.5 Gy, thirty radiotherapy fractions would have exposed 99% of the circulating blood cells (53) (see Figures 1,2). Similarly, the target irradiation volume also plays a significant role; for a 60 Gy plan administered in 30 fractions, the average dose to the circulating blood was 2.2 Gy for an 8-cm diameter planned target volume (PTV), but only 0.3 Gy for a 2-cm diameter PTV (53). This finding also highlights the importance of addressing treatment-related lymphopenia in glioblastoma populations, since large volume irradiation of the brain (with 1.5 to 2.5 cm margins) are often necessary for local control given the aggressive histology and the tendency for glioblastoma cells to generate finger-like projections and infiltrate surrounding normal brain tissues. Byun et al. presented clinical data in 2019 which supported the hypothesis that large PTV is indeed significantly associated with increased incidence of acute severe lymphopenia after chemoradiation in glioblastoma patients (47).
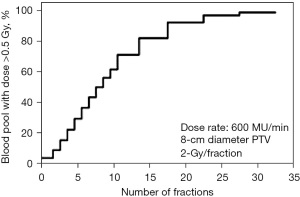
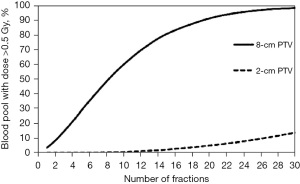
Although reported studies primarily focused on knowledge gained from endpoints measured in routine clinical care, the immunosuppressive effects of radiotherapy and temozolomide extend beyond overall lymphocyte and CD4 counts (6). Campian et al. collected blood sample for patients with high-grade astrocytoma (54) and found that 75% patients developed lymphopenia with significant decline in all tested lymphocyte subsets included NK cells, B cells and all T lymphocyte subsets after concurrent radiotherapy and temozolomide. Furthermore, all lymphocyte populations remained suppressed with little evidence for recovery through follow-up testing for a period of up to a year. Similar results were demonstrated with medulloblastoma patients who experienced reduced overall lymphocyte counts during post-operative chemoradiation (55).
Tumor-related immunosuppression likely contributes to persistence of radiation-induced lymphopenia
The severity and duration of radiation-induced lymphopenia are likely exacerbated by the direct and indirect actions of glioblastoma in generating an immunosuppressive environment that is conducive to tumor growth. In the brain, there are built-in immunosuppressive mechanisms in response to injury for protection of normal brain tissues during catastrophic events such as strokes and traumatic brain injury (56-58). These mechanisms are highly controlled and regulated, and are meant to protect neurons from the fluctuations of nutrients, hormones, metabolites, electrolytes and other endogenous and exogenous compounds.
In the glioblastoma patient, however, these neuroprotective anti-inflammatory responses may actually be taken over by the cancer cells to evade the host immune system (3,6,59). As they adapt to escape immune surveillance, glioma cells release a number of soluble mediators which increase the percentage of T-regulatory (Treg) cells, induce activation of immunosuppressive M2 macrophages, stimulate proliferation of myeloid-derived suppressor cells (MDSCs), and hamper anti-tumor immune activity within the tumor microenvironment (59). This results in an immunosuppressive environment at baseline which is then exacerbated by radiotherapy, and allows glioblastoma to infiltrate and divide and grow relatively unchecked. An international prospective trial demonstrated pretreatment lymphopenia in 24.4% of patients that was associated with worse survival outcome (60). These tumor-mediated mechanisms likely explain why post-radiotherapy immunosuppression can persist for close to one year for glioblastoma patients, even though the radiotherapy treatment field includes only the brain with no exposure to the progenitor cells in bone marrow and/or lymphatic tissues (6,54).
Other contributing factors to lymphopenia
Lymphopenia in glioblastoma is a complex phenomenon with contributions from multiple biological mechanisms in addition to radiotherapy treatment. Temozolomide is a myelosuppressive systemic therapy and clearly contributes to the incidence of lymphopenia (6,61); Perry et al. used short-course radiotherapy for elderly glioblastoma patients and found lymphopenia <500 cells/mm3 in 27.3% of patients treated with both radiation and temozolomide (compared with 10.3% of patients treated with radiotherapy alone) (61). Similarly, glucocorticoids are known to suppress the immune system by triggering apoptotic death for lymphocytes, and also by upregulating the CTLA-4 which limits the production of naïve T cells and enables tumor to escape immune surveillance (62,63).
In the context of tumor-related immune suppression, regulatory T (Treg) cells are key players in maintaining an immunosuppressive environment in glioblastoma patients (64-66). In normal tissues, Treg cells have an important role in dampening potentially damaging excessive inflammatory responses and to protect host tissues by limiting autoimmunity (6). In cancer tissues, however, Treg cells appear to be significantly upregulated. Fecci et al. presented evidence for significantly diminished CD4 counts in patients with malignant glioma prior to therapy; however, the percentage of Treg cells appear to be 2.6 times higher in glioma patients when compared with control (15.9% vs. 6.1%, P=0.004) (67). Similarly, circulating myeloid cells also contribute to the inhibition of lymphocyte function and proliferation. Myeloid-derived suppressor cells, for example, are theorized to produce arginase that depletes L-arginine, curtails T cell receptor expression, and ultimately results in a blunted anti-tumor immunological response (68-71). Myeloid cells are one of the predominant cell types within a glioblastoma and are essential in the development of an immunosuppressive environment that promotes tumor growth. In fact, up to 30-50% of the glioma tissue is made up of tumor-associated macrophages and microglia rather than tumor cells (72).
Radiotherapy and immunotherapy: opportunity for a beneficial liaison?
Limiting treatment-related lymphopenia is especially important with the introduction of promising lymphocyte-medicated immune therapies in recent years. In the past several years, immunotherapy and checkpoint inhibitors have revolutionized the oncologic field with remarkable clinical efficacy in many solid tumors (73-77), and we are hopeful to see similar advances in primary brain tumors as well. Initial preclinical data highlights the potential of checkpoint inhibitor use in glioblastoma patients. For example, Guan et al. enrolled 471 glioblastoma cases and found that the expression of CTLA4 in the tumor specimen was positively correlated with the infiltration level and macrophage function in the glioblastoma tumor microenvironment (62), which points towards CTLA4 as a prospective target for glioblastoma treatment. Mathios et al. found that anti-PD-1 treatments are efficacious in facilitating an anti-tumor response and improving survival in glioblastoma mice models (78). Nevertheless, a recent phase III trial in human patients did not show a benefit (79). However, a robust lymphocyte count is essential to optimize the efficacy of any immune-directed therapies which may be impaired in glioblastoma as a result of the radiotherapy and systemic therapy. Anti-PD-1 treatment, for example, is enhanced by locally delivered chemotherapy, but abrogated by systemic chemotherapy considering its myelosuppressive side effects (78).
Radiotherapy and checkpoint inhibitors: synergistic or adversarial relationship?
Since radiotherapy is cytotoxic and a key driver for lymphodepletion, logically we would expect a dampened treatment efficacy when radiotherapy is used in conjunction with immune checkpoint inhibitors. However, the answer is not so simple, as radiation has been linked with both immuno-potentiating and immunosuppressive effects (10,36,53,79,80). In the 1950s, the term “abscopal effect” was coined to describe the regression of distant tumors that were outside of the radiation fields (81). While this term remains controversial, there is preclinical evidence that support the observation for abscopal effect especially when radiation is used in conjunction with immunotherapies. Zeng et al. in 2012 evaluated the use of a single-fraction stereotactic radiotherapy to enhance anti-tumor immune responses in mice implanted with an orthotopic brain tumor, and found that long-term survival was only observed in the combined treatment arm with both PD-1 blockade and stereotactic radiotherapy (9). Similar results were observed in other disease sites (10,79,80,82,83). Short-course radiotherapy (12 Gy in 1 fraction, or 10 Gy in 5 fractions), when used in combination with anti-PD-L1 treatments, were found to have a synergistic effect in potentiating the immune response and triggering an abscopal response in murine tumor models (84,85). In clinical studies, the combination of focal radiotherapy (6 Gy × 5 or 9.5 Gy × 3) and CTLA-4 blockade was found to augment anti-tumor responses in patients with chemo-refractory metastatic non-small cell lung cancer (10,79,80).
Investigational strategies to improve immune response to glioma
There is increasing evidence that the fractionation scheme and target volume are essential in determining whether radiotherapy has an immuno-suppressive vs. immuno-potentiating effect. The standard fractionation regimen (60 Gy in 30 fractions) is effective in killing glioblastoma cells and takes advantage of the better therapeutic ratio with a small daily fraction size i.e., a more favorable tradeoff with high tumor control and less potential injury of normal tissues. However, the treatment field covers large intracranial volumes and irradiates >99% of the circulating lymphocytic population over the course of 30 daily fractions (53). As a result, this fractionation regimen promotes an immunosuppressive regimen which could impede local tumor control and reduce survival outcomes even while effectively killing tumor cells.
On the other hand, there is emerging evidence that stereotactic and/or hypofractionated radiotherapy regimens (with higher daily doses) are amenable to boosting anti-tumor immune activity through upregulation of tumor antigens, activation of inflammatory pathways, and reduction of Treg and myeloid-derived suppressor cells in the tumor microenvironment, amongst other changes (6,9-11). Stereotactic approaches allows for tighter treatment margins which translates to a smaller irradiated volume and lowered lymphopenia risks during the course of radiotherapy. While this remains an area of active research, there is preliminary evidence that support the use of stereotactic and/or hypofractionated approaches in glioblastoma. For example, investigators at Memorial Sloan Kettering Cancer Center used a “dose painting” integrated boost to administer 600 cGy × 6 (plus 1 cm margin) to the contrast enhanced tumor cavity and 400 cGy × 5 to the larger T2/FLAIR volume at risk for subclinical disease (12) along with temozolomide with favorable 1-year overall survival at 93%, and favorable median survival at 19 months. Hypofractionated regimens are already accepted in routine therapy for elderly glioblastoma patients (62). Similar regimens were attempted with other hypofractionated regimens with mixed outcomes (86-89). There is evidence from a randomized phase II study that proton radiotherapy, which results in less exposure of normal tissues and less exposure of circulating blood outside the target, may reduce the likelihood of severe treatment-related lymphopenia for glioblastoma patients when compared with conventional photon radiation therapies (90). Similarly, limiting radiation dose to lymphocyte-rich structures may help to curb the extent of treatment-related lymphopenia and possibly improve cancer-related outcomes (91).
Clinical trials are ongoing to investigate the optimal administration of radiotherapy along with immunomodulatory therapies in glioblastoma. For example, our group initiated a pilot study to assess the safety and feasibility of 5-fraction hypofractionated stereotactic radiosurgery with concurrent combined anti-PD-1 and anti-TIM3 checkpoint inhibitors for recurrent glioblastoma (92). We are also testing the use of locally administered non-myelosuppressive chemotherapy (Carmustine wafers) along with anti-PD-1 treatment prior to initiation of lymphotoxic treatment with radiotherapy (93). The role of hypofractionated stereotactic radiation (as an alternative to conventionally fractionated radiation) in the initial combined modality therapy of newly diagnosed glioblastoma remains to be assessed as well with regard to the extent of lymphopenia and tumor outcomes. Other potential investigative strategies include the depletion of regulatory T cells within the tumor microenvironment, targeting of the myeloid-derived suppressor cells, and considerations to administer locally delivered chemotherapy (rather than systemic myelosuppressive therapy). Finally, alternative therapies including interleukin-7 have also been explored with the goals to restore and maintain total lymphocyte counts while salvageable systemic therapies are administered for recurrent glioblastoma patients (94).
Strengths and limitations
In this narrative review, we have provided a broad overview related to treatment-related lymphopenia in glioblastoma patients, explored mechanisms behind the unique immunosuppressive environments in the brain, and summarized recent research in understanding how we may be able to adjust our current treatment regimens to maximize treatment impact and limiting treatment-related lymphopenia in this era of immunotherapeutics. Data from this review is provided primarily by a combination of literature search and research experience from the authors. There are some limitations of this narrative review. First of all, this review is subject to publication bias as positive results are more likely to be accepted for publication and cited during the literature search process. Furthermore, the glioblastoma tumor microenvironment is highly complex and heterogeneous in nature. Despite recent research attempts, the cellular and molecular complexity of the glioblastoma microenvironment remains poorly understood and recently published papers may be subject to biased interpretations in the setting of limited data available. Future research is necessary to further understand the tumor microenvironment and how we can maximize treatment impact especially in this era of immunotherapeutics. Finally, since the review is narrative in nature, it can be subject to the authors’ unconscious biases during the literature search process and the interpretation of study findings.
Conclusions
Glioblastoma is an aggressive cancer type with median survival of approximately 14-15 months with rare 5-year survivorship after maximal safe resection followed by the combination of radiation, temozolomide and often corticosteroids when indicated. While these adjuvant treatments (including radiotherapy, temozolomide, and corticosteroids) are effective, they are also a cause of significant lymphopenia which can impair tumor outcome. At the same time, the tumor often “hijacks” neuroprotective anti-inflammatory mechanisms to perpetuate an immunosuppressive environment that promotes tumor growth. Radiotherapy, in particular, has been identified as a treatment modality with both immuno-modulatory and immuno-suppressive effects adding to the complexity of designing an approach that best enhances the anti-neoplastic immune response when combined with the use of checkpoint inhibitor therapy for this disease. Future research is necessary to further understand this dynamic and how we can adjust radiation fractionation scheme and target volumes to maximize treatment impact in this era of immunotherapeutics. Prospective studies will be required to determine whether improving lymphopenia will lead to better tumor outcome, especially as there are many other immunosuppressive effects within the tumor microenvironment. For this reason, it is equally important to better understand the complex interplay of immune-mediated factors within the tumor microenvironment, and how we can devise innovative investigational approaches in the future to limit lymphopenia risks, maximize anti-tumor immune responses, and improve treatment outcomes in glioblastoma. The decreased survival associated with treatment-related lymphopenia suggests the potential of improved anti-neoplastic immune response in improving the outcome of this disease.
Acknowledgments
Funding: This work was supported by the Nicholl Family Foundation.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Joshua Palmer, Iyad Alnahhas and Wenyin Shi) for the series “Recent Advances in Neuro-Oncology” published in Chinese Clinical Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-22-94/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-94/coif). The series “Recent Advances in Neuro-Oncology” was commissioned by the editorial office without any funding or sponsorship. CK received AACR Scholar in Training Award and has a pending provisional patent application related to lymphopenia research and survival outcomes in head and neck cancer. LK received funding from Nicholl Family Foundation for this manuscript; received grants or contracts from BMS, Incyte, Novartis and Novocure; consulting fees from Novocure; and receipt of drugs for clinical trial from Incyte. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang H, Wang R, Yu Y, et al. Glioblastoma Treatment Modalities besides Surgery. J Cancer 2019;10:4793-806. [Crossref] [PubMed]
- Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys 2011;81:623-30. [Crossref] [PubMed]
- Razavi SM, Lee KE, Jin BE, et al. Immune Evasion Strategies of Glioblastoma. Front Surg 2016;3:11. [Crossref] [PubMed]
- Pillay J, Tak T, Kamp VM, et al. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci 2013;70:3813-27. [Crossref] [PubMed]
- Kim WJ, Dho YS, Ock CY, et al. Clinical observation of lymphopenia in patients with newly diagnosed glioblastoma. J Neurooncol 2019;143:321-8. [Crossref] [PubMed]
- Kleinberg L, Sloan L, Grossman S, et al. Radiotherapy, Lymphopenia, and Host Immune Capacity in Glioblastoma: A Potentially Actionable Toxicity Associated With Reduced Efficacy of Radiotherapy. Neurosurgery 2019;85:441-53. [Crossref] [PubMed]
- Swanson GP, Jhavar SG, Hammonds K. The effect of pelvic radiation alone on lymphocyte subgroups. Clin Transl Radiat Oncol 2020;23:100-2. [Crossref] [PubMed]
- Swanson GP, Hammonds K, Jhavar S. Lymphocyte response and recovery to radiation therapy alone. Ann Blood 2022;10.
- Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 2013;86:343-9. [Crossref] [PubMed]
- Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845-51. [Crossref] [PubMed]
- Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 2015;3:345-55. [Crossref] [PubMed]
- Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res 2014;20:5023-31. [Crossref] [PubMed]
- Wang Y. Advances in Hypofractionated Irradiation-Induced Immunosuppression of Tumor Microenvironment. Front Immunol 2020;11:612072. [Crossref] [PubMed]
- Popp I, Grosu AL, Niedermann G, et al. Immune modulation by hypofractionated stereotactic radiation therapy: Therapeutic implications. Radiother Oncol 2016;120:185-94. [Crossref] [PubMed]
- Nesseler JP, Lee MH, Nguyen C, et al. Tumor size matters—understanding concomitant tumor immunity in the context of hypofractionated radiotherapy with immunotherapy. Cancers 2020;12:714. [Crossref] [PubMed]
- Ahluwalia M, Mehta MP, Kotecha R. The End-of-the-Road for Immunotherapies in GBM or New Opportunities for More Nuanced/aggressive Approaches? CheckMate 498. 2022. Available online: https://www.practiceupdate.com/content/the-end-of-the-road-for-immunotherapies-in-gbm-or-new-opportunities-for-more-nuancedaggressive-approaches-checkmate-498/135377
- DeCordova S, Shastri A, Tsolaki AG, et al. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front Immunol 2020;11:1402. [Crossref] [PubMed]
- Kwok D, Okada H. T-Cell based therapies for overcoming neuroanatomical and immunosuppressive challenges within the glioma microenvironment. J Neurooncol 2020;147:281-95. [Crossref] [PubMed]
- Pombo Antunes AR, Scheyltjens I, Duerinck J, et al. Understanding the glioblastoma immune microenvironment as basis for the development of new immunotherapeutic strategies. Elife 2020;9:e52176. [Crossref] [PubMed]
- De Leo A, Ugolini A, Veglia F. Myeloid Cells in Glioblastoma Microenvironment. Cells 2020;10:18. [Crossref] [PubMed]
- Woroniecka KI, Rhodin KE, Chongsathidkiet P, et al. T-cell Dysfunction in Glioblastoma: Applying a New Framework. Clin Cancer Res 2018;24:3792-802. [Crossref] [PubMed]
- Andaloussi AE, Han Y, Lesniak MS. Progression of intracranial glioma disrupts thymic homeostasis and induces T-cell apoptosis in vivo. Cancer Immunol Immunother 2008;57:1807-16. [Crossref] [PubMed]
- Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr 2021;112:90-2. (Engl Ed). [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Ellsworth S, Balmanoukian A, Kos F, et al. Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology 2014;3:e27357. [Crossref] [PubMed]
- Mahindra AK, Grossman SA. Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors. J Neurooncol 2003;63:263-70. [Crossref] [PubMed]
- Hughes MA, Parisi M, Grossman S, et al. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys 2005;62:1423-6. [Crossref] [PubMed]
- Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 2011;17:5473-80. [Crossref] [PubMed]
- Rahman R, Catalano P, Arvold N, et al. Chemoradiation-related lymphopenia is common among glioblastoma patients and is associated with worse progression-free and overall survival. International Journal of Radiation Oncology, Biology, Physics 2016;96:E123. [Crossref]
- Mendez JS, Govindan A, Leong J, et al. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol 2016;127:329-35. [Crossref] [PubMed]
- Le Rhun E, Oppong FB, Vanlancker M, et al. Prognostic significance of therapy-induced myelosuppression in newly diagnosed glioblastoma. Neuro Oncol 2022;24:1533-45. [Crossref] [PubMed]
- Liu LT, Chen QY, Tang LQ, et al. The Prognostic Value of Treatment-Related Lymphopenia in Nasopharyngeal Carcinoma Patients. Cancer Res Treat 2018;50:19-29. [Crossref] [PubMed]
- Grossman SA, Ellsworth S, Campian J, et al. Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J Natl Compr Canc Netw 2015;13:1225-31. [Crossref] [PubMed]
- Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 2010;16:2443-9. [Crossref] [PubMed]
- Balmanoukian A, Ye X, Herman J, et al. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest 2012;30:571-6. [Crossref] [PubMed]
- Wild AT, Ye X, Ellsworth SG, et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol 2015;38:259-65. [Crossref] [PubMed]
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:128-35. [Crossref] [PubMed]
- Campian JL, Ye X, Brock M, et al. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest 2013;31:183-8. [Crossref] [PubMed]
- Cho O, Oh YT, Chun M, et al. Radiation-related lymphopenia as a new prognostic factor in limited-stage small cell lung cancer. Tumour Biol 2016;37:971-8. [Crossref] [PubMed]
- Cho O, Chun M, Chang SJ, et al. Prognostic Value of Severe Lymphopenia During Pelvic Concurrent Chemoradiotherapy in Cervical Cancer. Anticancer Res 2016;36:3541-7. [PubMed]
- Rudra S, Hui C, Rao YJ, et al. Effect of Radiation Treatment Volume Reduction on Lymphopenia in Patients Receiving Chemoradiotherapy for Glioblastoma. Int J Radiat Oncol Biol Phys 2018;101:217-25. [Crossref] [PubMed]
- Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89:1084-91. [Crossref] [PubMed]
- Chadha AS, Liu G, Chen HC, et al. Does Unintentional Splenic Radiation Predict Outcomes After Pancreatic Cancer Radiation Therapy? Int J Radiat Oncol Biol Phys 2017;97:323-32. [Crossref] [PubMed]
- Ellsworth SG. Field size effects on the risk and severity of treatment-induced lymphopenia in patients undergoing radiation therapy for solid tumors. Adv Radiat Oncol 2018;3:512-9. [Crossref] [PubMed]
- Deng W, Xu C, Liu A, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol 2019;133:9-15. [Crossref] [PubMed]
- Zhang M, You G, Xu H, et al. Severe lymphopenia as a prognostic factor in rectal cancer patients receiving adjuvant chemoradiotherapy: a retrospective study. 2019. Available online: https://assets.researchsquare.com/files/rs-2294/v1/2cca92c4-f0a7-4e45-b936-736b47eb631b.pdf?c=1631826226.
- Byun HK, Kim N, Yoon HI, et al. Clinical predictors of radiation-induced lymphopenia in patients receiving chemoradiation for glioblastoma: clinical usefulness of intensity-modulated radiotherapy in the immuno-oncology era. Radiat Oncol 2019;14:51. [Crossref] [PubMed]
- Kim D, Heather J, Lee G, et al. Serial T-cell Receptor (TCR) Repertoire Sequencing to Predict Outcomes in Gastrointestinal (GI) Malignancies. International Journal of Radiation Oncology, Biology, Physics 2020;108:S110. [Crossref]
- Liu H, Wang H, Wu J, et al. Lymphocyte nadir predicts tumor response and survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: Immunologic relevance. Radiother Oncol 2019;131:52-9. [Crossref] [PubMed]
- Weeke E. EXTRACORPOREAL IRRADIATION OF THE BLOOD: Further Investigations on the Effect of Varying Transit Dose, Blood Flow Rate and Frequency of Treatment on the Development of Lymphopenia in Uremic Patients. Acta Medica Scandinavica 1974;195:149-54. [Crossref] [PubMed]
- Kleinberg L, Grossman SA, Piantadosi S, et al. The effects of sequential versus concurrent chemotherapy and radiotherapy on survival and toxicity in patients with newly diagnosed high-grade astrocytoma. Int J Radiat Oncol Biol Phys 1999;44:535-43. [Crossref] [PubMed]
- MacLennan IC, Kay HE. Analysis of treatment in childhood leukemia. IV. The critical association between dose fractionation and immunosuppression induced by cranial irradiation. Cancer 1978;41:108-11. [Crossref] [PubMed]
- Yovino S, Kleinberg L, Grossman SA, et al. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 2013;31:140-4. [Crossref] [PubMed]
- Campian JL, Piotrowski AF, Ye X, et al. Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol 2017;135:343-51. [Crossref] [PubMed]
- Gururangan S, Reap E, Schmittling R, et al. Regulatory T cell subsets in patients with medulloblastoma at diagnosis and during standard irradiation and chemotherapy (PBTC N-11). Cancer Immunol Immunother 2017;66:1589-95. [Crossref] [PubMed]
- Faura J, Bustamante A, Miró-Mur F, et al. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation 2021;18:127. [Crossref] [PubMed]
- Plata-Salamán CR. Brain injury and immunosuppression. Nat Med 1998;4:768-9. [Crossref] [PubMed]
- Sribnick EA, Warner T, Hall MW. Traumatic brain injury and hemorrhage in a juvenile rat model of polytrauma leads to immunosuppression and splenic alterations. J Neuroimmunol 2021;361:577723. [Crossref] [PubMed]
- Mangani D, Weller M, Roth P. The network of immunosuppressive pathways in glioblastoma. Biochem Pharmacol 2017;130:1-9. [Crossref] [PubMed]
- Song AJ, Ding K, Alnahhas I, et al. Impact of lymphopenia on survival for elderly patients with glioblastoma: A secondary analysis of the CCTG CE. 6 (EORTC 26062-22061, TROG08. 02) randomized clinical trial. Neurooncol Adv 2021;3:vdab153. [Crossref] [PubMed]
- Perry JR, Laperriere N, O'Callaghan CJ, et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med 2017;376:1027-37. [Crossref] [PubMed]
- Guan X, Wang Y, Sun Y, et al. CTLA4-Mediated Immunosuppression in Glioblastoma is Associated with the Infiltration of Macrophages in the Tumor Microenvironment. J Inflamm Res 2021;14:7315-29. [Crossref] [PubMed]
- Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ 2002;9:6-19. [Crossref] [PubMed]
- Miska J, Lee-Chang C, Rashidi A, et al. HIF-1α Is a Metabolic Switch between Glycolytic-Driven Migration and Oxidative Phosphorylation-Driven Immunosuppression of Tregs in Glioblastoma. Cell Rep 2019;27:226-237.e4. [Crossref] [PubMed]
- Moreno Ayala MA, Li Z, DuPage M. Treg programming and therapeutic reprogramming in cancer. Immunology 2019;157:198-209. [Crossref] [PubMed]
- Zhang R, Xu K, Shao Y, et al. Tissue Treg Secretomes and Transcription Factors Shared With Stem Cells Contribute to a Treg Niche to Maintain Treg-Ness With 80% Innate Immune Pathways, and Functions of Immunosuppression and Tissue Repair. Front Immunol 2020;11:632239. [Crossref] [PubMed]
- Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 2006;66:3294-302. [Crossref] [PubMed]
- Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 2005;65:3044-8. [Crossref] [PubMed]
- Kramer ED, Abrams SI. Granulocytic Myeloid-Derived Suppressor Cells as Negative Regulators of Anticancer Immunity. Front Immunol 2020;11:1963. [Crossref] [PubMed]
- Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009;69:1553-60. [Crossref] [PubMed]
- Brandau S, Trellakis S, Bruderek K, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol 2011;89:311-7. [Crossref] [PubMed]
- Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 2016;19:20-7. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Zhou X, Yao Z, Yang H, et al. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med 2020;18:87. [Crossref] [PubMed]
- Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11:3801. [Crossref] [PubMed]
- Esfahani K, Roudaia L, Buhlaiga N, et al. A review of cancer immunotherapy: from the past, to the present, to the future. Curr Oncol 2020;27:S87-97. [Crossref] [PubMed]
- Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 2020;30:507-19. [Crossref] [PubMed]
- Mathios D, Kim JE, Mangraviti A, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med 2016;8:370ra180. [Crossref] [PubMed]
- Omuro A, Brandes AA, Carpentier AF, et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase 3 trial. Neuro Oncol 2023;25:123-34. [Crossref] [PubMed]
- Weller M, Lim M, Idbaih A, et al., editors. A randomized phase 3 study of nivolumab or placebo combined with radiotherapy plus temozolomide in patients with newly diagnosed glioblastoma with methylated MGMT promoter: CheckMate 548. Neuro-Oncology; 2021: OXFORD UNIV PRESS INC JOURNALS DEPT, 2001 EVANS RD, CARY, NC 27513 USA.
- Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and Immunotherapy for Cancer: From "Systemic" to "Multisite". Clin Cancer Res 2020;26:2777-82. [Crossref] [PubMed]
- Li D, Zhu W, Zhou J, et al. Hypofractionated Low-Dose Radiotherapy Combined with Immune Checkpoint Inhibition in Metastatic Solid Tumors. Onco Targets Ther 2021;14:773-83. [Crossref] [PubMed]
- van Hattum JW, de Ruiter BM, Oddens JR, et al. Bladder-Sparing Chemoradiotherapy Combined with Immune Checkpoint Inhibition for Locally Advanced Urothelial Bladder Cancer-A Review. Cancers (Basel) 2021;14:38. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [Crossref] [PubMed]
- Reddy K, Gaspar LE, Kavanagh BD, et al. Hypofractionated intensity-modulated radiotherapy with temozolomide chemotherapy may alter the patterns of failure in patients with glioblastoma multiforme. J Med Imaging Radiat Oncol. 2014;58:714-21. [Crossref] [PubMed]
- Iuchi T, Hatano K, Kodama T, et al. Phase 2 trial of hypofractionated high-dose intensity modulated radiation therapy with concurrent and adjuvant temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys 2014;88:793-800. [Crossref] [PubMed]
- Floyd NS, Woo SY, Teh BS, et al. Hypofractionated intensity-modulated radiotherapy for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2004;58:721-6. [Crossref] [PubMed]
- Cardinale R, Won M, Choucair A, et al. A phase II trial of accelerated radiotherapy using weekly stereotactic conformal boost for supratentorial glioblastoma multiforme: RTOG 0023. Int J Radiat Oncol Biol Phys 2006;65:1422-8. [Crossref] [PubMed]
- Mohan R, Liu AY, Brown PD, et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: phase II randomized study of protons vs photons. Neuro Oncol 2021;23:284-94. [Crossref] [PubMed]
- Venkatesulu B, Giridhar P, Pujari L, et al. Lymphocyte sparing normal tissue effects in the clinic (LymphoTEC): A systematic review of dose constraint considerations to mitigate radiation-related lymphopenia in the era of immunotherapy. Radiother Oncol 2022;177:81-94. [Crossref] [PubMed]
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Novartis Pharmaceuticals. Trial of Anti-Tim-3 in Combination With Anti-PD-1 and SRS in Recurrent GBM. Available online: https://ClinicalTrials.gov/show/NCT03961971; 2020.
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Incyte Corporation. Carmustine Wafer in Combination With Retifanlimab and Radiation With/Without Temozolomide in Subjects With Glioblastoma. Available online: https://ClinicalTrials.gov/show/NCT05083754; 2022.
- Ahn S, Park JS, Kim H, et al. Compassionate use of recombinant human IL-7-hyFc as a salvage treatment for restoring lymphopenia in patients with recurrent glioblastoma. Cancer Med 2022; Epub ahead of print. [Crossref] [PubMed]


