
A narrative review of intrahepatic cholangiocarcinoma: a surgical curative option
Introduction
Intrahepatic cholangiocarcinoma (iCCA) is the second commonest hepatic malignancy, with a reported mortality increasing in several areas of the world, likely due to increased prevalence of risk factors and improved cancer diagnosis and classification. The peak age of incidence for iCCA is the seventh decade and the disease affects both genders, with a slight male preponderance (1).
The pathogenesis of iCCA is linked to a multifactorial process characterized by genetics and environmental factors. Several risk factors are associated with the development of iCCA, including cirrhosis, hepatitis B and C, diabetes, metabolic syndrome, and alcohol excess.
In Eastern countries, parasitic infections (i.e., Opisthorchis viverrini and Clonorchis sinensis), congenital biliary cystic diseases disease and hepatolithiasis are involved in the disease.
Recently, novel risk factors are detected, such as asbestosis, nitrosamine-contaminated food, dioxins, and vinyl chlorides (2).
In 10–15% of cases jaundice is the presentation symptom of iCCA; biliary obstruction is often related to compression of the liver hilum by lymph nodes (LNs) or large mass compressing biliary tree.
Other frequent initial symptoms are abdominal pain, asthenia, and weight loss, acholia and/or pruritus (3).
When localized, potentially-resectable disease is diagnosed, surgery represent the treatment of choice. Nevertheless, in only 35% of patients, surgical resection is feasible at the time of diagnoses (4,5).
Five years survival rate is 30–40% in patients with a R0 surgical resection. Disease recurrence is the main factor affecting survival and was reported from 43% to 66% of patients (6-8).
Resectability of iCCA depends on several factors including tumour size, number of lesions, localization, vascular involvement, and LNs status. Assessment of surgical treatment requires specialized hepato-biliary multidisciplinary team (i.e., oncologists, surgeons, radiologists, and pathologists) to select the best treatment strategy.
Patient performance status and risk factors evaluation are pivotal and mandatory to select patients with adequate liver function reserve and to minimize peri-operative mortality (9).
In this review, we discuss the outcomes of surgery for iCCA and explore the treatment procedures that may improve the prognosis. We present this article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-22-85/rc).
Methods
A literature review of the published literature focused on the surgical aspects of iCCA was carried out on the 14th of February 2023.
A search of the PubMed, Scopus and Cochrane Database was conducted using the following terms: (“intrahepatic cholangiocarcinoma”) AND (“liver transplantation” or “surgery” or “resection” or “laparoscopic” or “robotic” or “neoadjuvant” or “epidemiology” or “diagnosis”).
The qualitative review included a priori search criteria of journal articles among adult (age ≥18 years) human patients; studies were limited to the English language. Published reports were excluded in the following cases: (I) data on animal models; (II) overlapping data; (III) lacked sufficient clinical details.
Studies originating from the same centres were analysed and overlapping of clinical cases was taken into account.
Following the review of the full text from eligible studies, two independent authors (FM and RAN) performed the data extraction and cross-checked all outcomes. During the selection of articles and extraction of the data, potential discrepancies were resolved with the consensus of a third reviewer (MG).
Epidemiology
Cholangiocarcinoma (CCA) are a group of malignancies arising from the biliary epithelium and represent at least 3% of all gastrointestinal malignancies.
CCA is classified on the anatomical site of origin: intrahepatic; perihilar and distal CCA. This review will focus on iCCA. iCCA origin from the second order bile ducts (10), and comprises approximately 10% of primitive liver cancers, the second disease after hepatocellular carcinoma (HCC) (1).
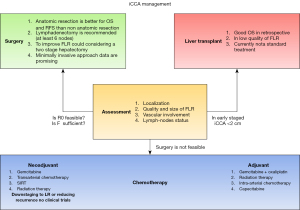
In this review the authors will focus on the management of iCCA and in Figure 1 we summarized the treatment algorithm.
Diagnostic assessment
Carefully preoperative staging, CT-scan based, should be aimed with the aim of facilitating clinical detection of distant metastasis and assessment of tumour local extension.
In the evaluation of patients presented with signs and symptoms of biliary obstruction a resonance cholangiopancreatography (MRCP) is the imaging modality of choice.
Fluorodeoxyglucose positron emission tomography (FDG-PET) imaging should be considered when evaluating newly diagnosed biliary malignancy, to identify distant metastasis and to obtain more information about primitive disease.
Serum tumour markers have not high specificity and sensibility in iCCA: carcinoma embryonic antigen (CEA) is altered only in one third of patients (11). Carbohydrate antigen 19-9 (CA19-9), is marker associated with several types of adenocarcinomas as well as in conditions causing cholestasis or cholangitis. Moreover, CA19-9 is not measurable in Lewis A antigen negative population (12).
Histological confirmation with biopsy (percutaneous or surgical) of malignancy is not mandatory. With no suggestive past medical history. such as prior biliary tract operation; primary sclerosing cholangitis (PSC); hepatolithiasis, the finding of focal stenotic lesion combined with the appropriate clinical presentation is sufficient for a presumptive diagnosis in iCCA.
Multiples attempts are moving to obtain an earlier diagnosis. New tumoral features and genetic material are pivotal for the diagnosis, management, and selection of addressed therapies: but sampling tumour tissue could be unsafe (13).
Liquid biopsy is considered a less invasive, attractive, new tool to investigate the tumour genetic profile and open new opportunity to treat disease and understanding the response to therapy (14).
Curative surgery
In the last years the outcomes, after hepatic resection, are improving through technical advances in the hepatobiliary surgery, improvement of peri-operative management, and combination of locoregional and systemic therapies.
R0 is the goal of the surgical treatment. Free surgical margin is a well described factor influencing recurrence-free survival (RFS) and overall survival (OS). Indeed, positive margin status was identified as strong factors for unfavourable prognosis in patients underwent resection (15,16). Moreover, surgical margins <1 cm is linked to worse prognosis compared to margin more than 1 cm (17).
Kosaka et al. (18) proposed a specific therapeutic strategy based on tumour localization. The liver was divided into three areas based on the distance from the first and second portal vein branches, so we can define a central, intermediate, or peripheral area. Vascular invasion and regional LNs metastasis show a significant difference between tumour location groups. Surgical treatment differs by tumour location. Peripheral iCCA was treated by anatomical sectionectomy. Intermediate iCCA deserved bisectionectomy, regional lymphadenectomy and adjuvant chemotherapy; in the case of central tumor was suggested bisectionectomy or major liver resection, en bloc extrahepatic bile duct resection, caudatectomy, regional lymphadenectomy, and adjuvant chemotherapy.
Treatment at high volume centres has also been associated with a lower incidence of a positive surgical margin, as well as decreased 90-day mortality, and improved OS in surgically treated patients (19).
The role of tumour burden score (TBS), a new score incorporating tumour size and number, was recently investigated in large multicentre cohort. High TBS affected 5-year OS of patients underwent resection. In the same study, high CA19-9 is another factor that influence the prognosis, showing that biological and morphological factors affect long term prognosis in these patients (20).
Moreover, in patients with high TBS, unfavourable prognosis is described irrespective to margin status (21).
Multifocal presentation has been independently associated with poor outcome in iCCA patients and often is considered as a contraindication for the curative surgery.
A meta-analysis (22), described the harmful role of multiple and satellite lesions in tumour recurrence and patient death rate.
Spolverato et al. (23) showed negative impact on disease free survival (DFS) and OS of two or more nodes in patient underwent surgical resection. Moreover, multiple lesions are also associated with early recurrence (less than 12 months) after resection.
Conci et al. (24), observed the association between clinical and pathological features and long-term outcomes in resected iCCA and different LNs status. Two hundred and fifty-one patients were classified in 3 groups: (I) patients with single lesion; (II) single lesion with satellites; (III) multifocal lesions. Five-year OS rate were 49.4%, 34.2%, and 9.9%, in group I, II and III respectively (P<0.001). Moreover, at multivariate analysis, groups II and III as the strongest independent prognostic factors for poor survival. Worse prognosis has showed in group III patients with LNs metastases and R1 resections.
Vascular and lymphatic involvement are associated with a better outcome, in terms of OS, compared to perineural invasion. The OS at 1-, 3-, and 5-year were 80%, 35%, and 23% vs. 75%, 23% and 0%, respectively (P=0.027) (25).
Vascular invasion is even associated with better survival compared to multiple lesions in patients with stage II iCCA [1-, 3-, and 5-year OS of 60.8%, 32.1%, and 22.0%, respectively vs. 41.4%, 13.4%, and 10.0% respectively, P<0.001; hazard ratio (HR), 1.61] (26).
The role of explorative laparoscopy is not worldwide accepted. American Hepato-Pancreato-Biliary Association (27) statement recommended performing laparoscopy only in high-risk candidates (i.e., high CA19-9, multifocal disease, suspected vascular invasion).
Since surgery represent the only curative option in iCCA management, combined vascular and/or biliary reconstruction with or without resection of surrounding organs seems justified to achieve complete tumour removal.
When major hepatic veins or inferior cava vein are involved, it may be required a complete vascular exclusion such as hypothermic perfusion, ex situ surgery or autologous, heterologous, or synthetic grafts reconstruction. However, this aggressive surgery doesn’t decrease tumour recurrence rate and the long-term disease-free survival remain worse (28).
Anatomical vs. non-anatomical liver resection
Si et al. (29), demonstrated the importance of anatomic liver resection (ALR): in a study on 671 patients, ALR were associated with improved OS and DFS compared to non-anatomic liver resection (NALR) (1-, 3-, and 5-year OS 58.1%, 35.7% and 28.1% vs. 44.1%, 23.9% and 18.0%; P=0.002; DFS 72.9%, 45.7% and 36.0% vs. 62.0%, 30.8% and 25.3%; P=0.002). Moreover, NALR was an independent factor of poor OS and DFS at multivariate analysis.
The oncological advantage of ALR is confirmed in another recent, large, Chinese single-centre experience on 3,880 patients (30). The results demonstrate that AR improved long-term survival in terms of 1-, 3- and 5-year OS (70%, 46% and 34%, respectively) and a DFS (61%; 21%and 10%, respectively) with a statistical significance after PSM analysis. Postoperative complications are comparable between AR and NAR with similar recurrence rate.
On the other hand, Li et al. (31), in a study of 150 iCCA, showed no significant differences in OS and DFS between NLAR and ALR 1-, 3-, and 5-year (OS: 70.2%, 22.9% and 22.9% vs. 71.1%, 51.7% and 51.7%, P=0.229; DFS 53.2%, 19.2% and 19.2% vs. 58.6%, 41.0% and 41.0%, P=0.370); furthermore, at multivariate analysis, surgical approach was shown to not be predictors of survival.
Role of lymphadenectomy
LNs status is a determinant prognostic factor in iCCA management. Forty-five to 65% of patients are affected by LNs metastases at the time of surgery. Five years survival in pN1 stage is 0–20% (32,33).
CEA, CA19-9, and lymphadenopathy on imaging are prognostic factors for lymph node metastasis (34).
According to “8th American Joint Committee on Cancer Staging Manual (AJCC)” (35), standard lymphadenectomy is defined as surgical step including porta hepatis nodes, along common hepatic artery (station 8) and hepato-duodenal ligament nodes (station 12), regardless of tumour location (36). In left-sided tumour, inferior phrenic, hilar, and gastro-hepatic nodes should be considered as regional LNs. In right-sided hilar, peri-duodenal, and peripancreatic are considered regional LNs. Positive celiac, periaortic, and pericaval nodes should be considered as metastatic disease.
Current guidelines recommend retrieval at least 6 LNs for a correct staging (37). The presence of at least one positive LN constitutes N1 and consequently stage IIIB disease (37,38).
Zhang et al. (39) showed comparable survival in patients with no LNs involvement vs. 1–2 lymph nodes metastasis (LNM) whereas a detection of three positive LNs was related to worse survival
The role of adequate lymphadenectomy (≥6 retrieved LNs) in survival is still debating in literature.
No advantages in overall and disease-free survival for patient underwent lymph nodes dissection (LND) were found in recent meta-analysis published by Zhou et al. (40).
Conversely, a French/Japanese study that considers 192 patients with clinical node-negative iCCA of a cohort of 258 suggest that LND seems to be associated with more favourable outcome in patients with clinical absence of LNM (41).
Recently, Sposito et al. (42), using Italian multicentre retrospective database, concluded that adequate lymphadenectomy improves staging and survival in patients with N1 status, but no difference in recurrence is demonstrated.
Zhang et al. (39), found that, in patient with an adequate lymphadenectomy the localization changes the OS: patient with LNs metastasis within the hepatoduodenal ligament had a better OS than patients with LNs metastasis beyond the hepatoduodenal ligament.
Analysis carried out in 1,138 Korean and Japanese resected patients, demonstrated that removal of more than four positive lymph-nodes had a beneficial effect in terms of median survival compared to less than four retrieval lymph-nodes (30 vs. 13 months, P=0.001) (43).
Lymphadenectomy could be affected by intra and perioperative complications. In this setting, Vitale et al. (44), analysed a cohort of 826 patients underwent to surgical resection. After a propensity score (PS) matching, the authors concluded that LND survival benefit is positive in patients aged less than 60 years and in those with tumour size more than 5 cm.
Another retrospective experience by Kim et al. (36), obtained similar conclusions on 34 patients who underwent LND (stations 7, 12a-p-b and 13) with retrieval of more than 6 LNs, compared to 34 patients who did not receive LND. The authors found better OS (90 vs. 44 months) and DFS (64 vs. 20 months) in LND group. Notably, this is the only study in which a systematic LND is defined by both anatomical and numeric criteria.
Staged hepatectomies
In the setting of curative surgery. patient undergoing to extend hepatectomy could develop a post-operative liver failure. The preoperative study of future liver remnant (FLR) is pivotal to avoid this occurrence. Several strategies could improve FLR including portal vein embolization (PVE), venous deprivation of the liver (LVD), and recent assessed technique named associating liver partition and portal vein ligation (ALPPS).
The more recently published multi-centre study about ALPPS for iCCA including 102 patients reported higher rate of R0 resection (87.85%) and an improved OS when compared with palliative care (1-, 2-, and 3-year survival of 82.4%, 70.5%, and 39.6% vs. 51.2%, 21.4%, and 11.3%, respectively, P<0.01). On the other hand, 90-day mortality and morbidity after second stage were reported of 77% (45).
Bednarsch et al. (46), in a retrospective single-centre experience confirmed an advantageous outcome in patients undergoing ALPPS without lymph-nodes involvement (median OS of 4.2 years and a 3-year survival of 64%). No patients with lymph node metastases (n=5) were alive 1 year after surgery.
The importance of patient selection and ratio between FRL and body weigh play a pivotal role in achieving acceptable outcomes in this procedure.
Liver transplantation
Nowadays hepatocellular carcinoma (HCC) has become a common indication for liver transplant (LT) for malignancy, iCCA was historically considered a contraindication due to its aggressive behaviour.
Some experiences published in the early era of LT showed a poor outcome reporting a 5-year survival ranged between 10% and 18% (47,48).
In 2014, a Spanish multicentre study (49), evaluated the outcome of cirrhotic patients with mixed hepatocellular carcinoma—CCA or iCCA on pathological finding after LT for HCC.
No significant differences in the survival rates between patients with a single iCCA ≤2 cm and patient with HCC was observed (5-year OS of 73%).
Afterwards, Facciuto et al. (50), in a series of 32 patients with cirrhosis and iCCA on explant specimens, showed a 10% recurrence rate and 78% of survival rate after five years of follow-up in patients with iCCA fulfilling Milan Criteria, comparable with patients with HCC selected by Milan Criteria.
In 2018, Lunsford et al. (51), reported a series of 6 iCCA patients receiving gemcitabine-based neoadjuvant chemotherapy before LT, with an OS of 100% at 1 year and 83.3% after 3 and 5 years; Three patients experienced recurrence after a median of 7.6 months from LT.
Gruttadauria et al. (52), recently reported an Italian experience with 14 LT performed for iCCA, 12 detected after transplantation based on histologic findings and two cases of unresectable iCCA transplanted after neoadjuvant selective internal radiation therapy (SIRT) and a period of clinical observation. The two patients were alive after 19 and 2 months of follow-up, respectively.
These results suggest that in patients where liver resection is not feasible (e.g., due to cirrhosis), LT might be an option in very early iCCA.
Minimally invasive surgery
Minimally invasive surgery guarantees similar results in terms of OS, DFS, RFS compared to open approach, and improve the short-term outcomes.
As reported in a meta-analysis conducted by Guerrini et al. (53), and in three more, Western and Eastern, recent experiences, laparoscopic surgery was consistently associated with better outcome compared with open surgery in terms of blood loss, transfusions, numbers of Pringle manoeuvres, hospital stay, and postoperative morbidity (54-56).
Regarding oncological outcomes, recent experience from three large international databases comparing laparoscopic and open surgery, showed an excellent OS in laparoscopic group [1-, 3-, 5-year survival 92%, 75%, and 63% vs. 92%, 58%, and 49% in open group (P=0.0043)]. Transfusions, major postoperative complications, and liver steatosis were statistically related to patient death and recurrence (57).
Ratti et al. (58), in a series collected in a high volume-centre with several laparoscopic procedures, reported 446 liver resections performed for iCCA, 179 were performed with laparoscopic approach and 267 with the open approach. No differences were shown in terms of median OS and disease-free survival between laparoscopic and the open group.
Robotic resection for iCCA is limited to few small experiences. Recently Magistri et al. (59), reported two robotic right hepatectomies for this indication with acceptable perioperative outcome.
An US national cohort on 77 robotic resection showed similar oncological results compared to open procedures and less length of hospital stay (robotic approach: 5.8±4.6 days vs. open approach: 8.9±10.2 days; P=0.012) (60).
Neoadjuvant therapy (NT)
The role of NT is debated in clinical practice; to date, currently guidelines do not recommend NT for resectable iCCA. However, the NT usage in patients with clinical nodal involvement (cN) and advanced clinical T stage increased over the time.
Patients underwent NT showed significant higher 5-year OS compared to upfront surgery (37.2 vs. 29.9 months; log rank =0.001). NT had a decreased risk of death in overall cohort, cN−, cN+, cT2 and cT3 patients [HR 0.79 (0.69–0.89), HR 0.76 (0.66–0.89), HR 0.75 (0.57–1.00), HR 0.63 (0.51–0.79) and HR 0.71 (0.53–0.95), respectively]. Stratified by NT protocol, risk of death decreases significantly both in chemotherapy or chemotherapy and radiation [HR 0.81 (0.69–0.95) and HR 0.69 (0.54–0.88), respectively] (61).
In a single-centre study by Le Roy et al. (62), on unresectable disease, no differences in terms of median survival (24.1 vs. 25.7 months, P=0.391) and post-operative complications were found between 39 patients underwent surgery following chemotherapy or locoregional treatments and 35 patients managed by upfront resection.
NT can be an effective approach in patients with locally advanced iCCA. Ongoing clinical trials based on the combination of gemcitabine-cisplatin are trying to explore the role of systemic chemotherapy as neoadjuvant setting.
Repeated resection for treatment of intrahepatic recurrence
Postoperative recurrence, which is reported at 50–70%, influences the long-term survival of patients underwent surgery. When the site of recurrence is the liver, repeated resection could play a role for the control of disease. Spolverato et al. (63), in a multicentre study, reported a 26.1 months median survival in 41 repeated resections performed on 400 cases of recurrence, statistically better than 9.6 and 16.8 months median survival in patients treated by intra-arterial or systemic chemotherapy. Bartsch et al. (64), recently reported 113 re-resections in patient with iCCA resulting in a 1-, 3- and 5-year OS of 86%, 51% and 34% respectively. Factors related to repeated resectability were CA19-9 and R status at the time of first resection, and median time to recurrence.
Patients with recurrent iCCA may benefit from repeated surgical resection. In this setting, further studies involving systemic therapies are required.
Strengths and limitations
The idea of this paper was to provide a comprehensive and concise overview of the surgical treatment and neo-adjuvant therapies, reporting relevant literature in terms of original article and meta-analysis in the field of iCCA. Despite this, we are aware that most articles included in this narrative review are retrospective studies and most studies reported single centre data; most of them are limited to small samples and there is a lack of confirmation of large samples and high-quality prospective randomized trial. Moreover, the narrative nature of this review could be limited by selection bias of the included studies.
Conclusions
iCCA management remains challenging due to low rate of resectability at time of diagnosis. To date, in well selected patients, vascular resection, neoadjuvant chemotherapy and liver transplantation could offer an option to provide survival benefit in locally advanced stage. A comprehensive evaluation of patient’s liver functional status and tumour burden are pivotal for optimal patient and oncologic outcomes. Genetic profile combined with tumour response could drive in selection of resectable patients with favourable outcome.
Local or systemic neoadjuvant protocols potentially improves resectability rate (65).
Management of iCCA has evolved resulting in improved outcomes. However overall prognosis is still poor. To improve our results, we need to upgrade in accuracy and timing of diagnosis, optimizing surgical approaches and a more effective neoadjuvant and adjuvant therapies. So, multidisciplinary approach is pivotal to select the best strategy in each step (diagnosis, chemotherapy, and surgery) for patients with iCCA.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Levi Sandri) for the series “Hepatobiliary and Pancreatic Cancers: an Update of Surgical Treatments” published in Chinese Clinical Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-22-85/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-85/coif). The series “Hepatobiliary and Pancreatic Cancers: an Update of Surgical Treatments” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int 2019;39:19-31. [Crossref] [PubMed]
- Vicent S, Lieshout R, Saborowski A, et al. Experimental models to unravel the molecular pathogenesis, cell of origin and stem cell properties of cholangiocarcinoma. Liver Int 2019;39:79-97. [Crossref] [PubMed]
- Forner A, Vidili G, Rengo M, et al. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int 2019;39:98-107. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol 2008;15:600-8. [Crossref] [PubMed]
- Doussot A, Gonen M, Wiggers JK, et al. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J Am Coll Surg 2016;223:493-505.e2. [Crossref] [PubMed]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. [Crossref] [PubMed]
- Hu LS, Zhang XF, Weiss M, et al. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2019;26:2549-57. [Crossref] [PubMed]
- Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol 2019;71:104-14. [Crossref] [PubMed]
- Di Matteo S, Nevi L, Overi D, et al. Metformin exerts anti-cancerogenic effects and reverses epithelial-to-mesenchymal transition trait in primary human intrahepatic cholangiocarcinoma cells. Sci Rep 2021;11:2557. [Crossref] [PubMed]
- Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis 2008;12:131-50. ix. [Crossref] [PubMed]
- Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol 2009;25:279-84. [Crossref] [PubMed]
- Wetter LA, Ring EJ, Pellegrini CA, et al. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg 1991;161:57-62; discussion 62-3. [Crossref] [PubMed]
- Rompianesi G, Di Martino M, Gordon-Weeks A, et al. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J Gastrointest Oncol 2021;13:332-50. [Crossref] [PubMed]
- Tsilimigras DI, Sahara K, Wu L, et al. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg 2020;155:823-31. [Crossref] [PubMed]
- Dai YS, Hu HJ, Lv TR, et al. The influence of resection margin width in patients with intrahepatic cholangiocarcinoma: a meta-analysis. World J Surg Oncol 2023;21:16. [Crossref] [PubMed]
- Spolverato G, Yakoob MY, Kim Y, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:4020-8. [Crossref] [PubMed]
- Kosaka H, Kaibori M, Matsui K, et al. Investigation of a Tumor Location-Specific Therapeutic Strategy for Intrahepatic Cholangiocarcinoma. Asian Pac J Cancer Prev 2021;22:1485-93. [Crossref] [PubMed]
- Lee GC, Gamblin TC, Fong ZV, et al. Facility Type is Associated with Margin Status and Overall Survival of Patients with Resected Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2019;26:4091-9. [Crossref] [PubMed]
- Moazzam Z, Alaimo L, Endo Y, et al. Combined Tumor Burden Score and Carbohydrate Antigen 19-9 Grading System to Predict Outcomes Among Patients with Intrahepatic Cholangiocarcinoma. J Am Coll Surg 2023;236:804-13. [Crossref] [PubMed]
- Endo Y, Sasaki K, Moazzam Z, et al. Higher Tumor Burden Status Dictates the Impact of Surgical Margin Status on Overall Survival in Patients Undergoing Resection of Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Is Hepatic Resection for Large or Multifocal Intrahepatic Cholangiocarcinoma Justified? Results from a Multi-Institutional Collaboration. Ann Surg Oncol 2015;22:2218-25. [Crossref] [PubMed]
- Conci S, Ruzzenente A, Viganò L, et al. Patterns of Distribution of Hepatic Nodules (Single, Satellites or Multifocal) in Intrahepatic Cholangiocarcinoma: Prognostic Impact After Surgery. Ann Surg Oncol 2018;25:3719-27. [Crossref] [PubMed]
- Bartsch F, Heuft LK, Baumgart J, et al. Influence of Lymphangio (L), Vascular (V), and Perineural (Pn) Invasion on Recurrence and Survival of Resected Intrahepatic Cholangiocarcinoma. J Clin Med 2021;10:2426. [Crossref] [PubMed]
- Luo S, Wu L, Li M, et al. Validation of the Prognostic Role for Surgical Treatment in Stage II Intrahepatic Cholangiocarcinoma: A SEER Population-Based Study. J Clin Med 2023;12:675. [Crossref] [PubMed]
- Fábrega-Foster K, Ghasabeh MA, Pawlik TM, et al. Multimodality imaging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:67-78. [Crossref] [PubMed]
- Angelico R, Sensi B, Parente A, et al. Vascular Involvements in Cholangiocarcinoma: Tips and Tricks. Cancers (Basel) 2021;13:3735. [Crossref] [PubMed]
- Si A, Li J, Yang Z, et al. Impact of Anatomical Versus Non-anatomical Liver Resection on Short- and Long-Term Outcomes for Patients with Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2019;26:1841-50. [Crossref] [PubMed]
- Wang C, Ciren P, Danzeng A, et al. Anatomical Resection Improved the Outcome of Intrahepatic Cholangiocarcinoma: A Propensity Score Matching Analysis of a Retrospective Cohort. J Oncol 2022;2022:4446243. [Crossref] [PubMed]
- Li B, Song JL, Aierken Y, et al. Nonanatomic resection is not inferior to anatomic resection for primary intrahepatic cholangiocarcinoma: A propensity score analysis. Sci Rep 2018;8:17799. [Crossref] [PubMed]
- Sposito C, Droz Dit Busset M, Virdis M, et al. The role of lymphadenectomy in the surgical treatment of intrahepatic cholangiocarcinoma: A review. Eur J Surg Oncol 2022;48:150-9. [Crossref] [PubMed]
- Navarro JG, Lee JH, Kang I, et al. Prognostic significance of and risk prediction model for lymph node metastasis in resectable intrahepatic cholangiocarcinoma: do all require lymph node dissection? HPB (Oxford) 2020;22:1411-9. [Crossref] [PubMed]
- Huang T, Liu H, Lin Z, et al. Preoperative prediction of intrahepatic cholangiocarcinoma lymph node metastasis by means of machine learning: a multicenter study in China. BMC Cancer 2022;22:931. [Crossref] [PubMed]
- Benson AB 3rd, D'Angelica MI, Abbott DE, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw 2017;15:563-73. [Crossref] [PubMed]
- Kim SH, Han DH, Choi GH, et al. Oncologic Impact of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma: a Propensity Score-Matched Study. J Gastrointest Surg 2019;23:538-44. [Crossref] [PubMed]
- Moazzam Z, Alaimo L, Endo Y, et al. Predictors, Patterns, and Impact of Adequate Lymphadenectomy in Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2023; Epub ahead of print. [Crossref]
- Zhu J, Liu C, Li H, et al. Adequate lymph node dissection is essential for accurate nodal staging in intrahepatic cholangiocarcinoma: A population-based study. Cancer Med 2023; Epub ahead of print. [Crossref] [PubMed]
- Zhang XF, Xue F, Dong DH, et al. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann Surg 2021;274:e1187-95. [Crossref] [PubMed]
- Zhou R, Lu D, Li W, et al. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB (Oxford) 2019;21:784-92. [Crossref] [PubMed]
- Yoh T, Cauchy F, Le Roy B, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery 2019;166:975-82. [Crossref] [PubMed]
- Sposito C, Ratti F, Cucchetti A, et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol 2023;78:356-63. [Crossref] [PubMed]
- Kang CM, Suh KS, Yi NJ, et al. Should Lymph Nodes Be Retrieved in Patients with Intrahepatic Cholangiocarcinoma? A Collaborative Korea-Japan Study. Cancers (Basel) 2021;13:445. [Crossref] [PubMed]
- Vitale A, Moustafa M, Spolverato G, et al. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2016;113:685-91. [Crossref] [PubMed]
- Li J, Moustafa M, Linecker M, et al. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-center Study. Ann Surg Oncol 2020;27:1372-84. [Crossref] [PubMed]
- Bednarsch J, Czigany Z, Lurje I, et al. The role of ALPPS in intrahepatic cholangiocarcinoma. Langenbecks Arch Surg 2019;404:885-94. [Crossref] [PubMed]
- O'Grady JG, Polson RJ, Rolles K, et al. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg 1988;207:373-9. [Crossref] [PubMed]
- Pichlmayr R, Weimann A, Oldhafer KJ, et al. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg 1995;19:807-13. [Crossref] [PubMed]
- Sapisochin G, Rodríguez de Lope C, Gastaca M, et al. "Very early" intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660-7. [Crossref] [PubMed]
- Facciuto ME, Singh MK, Lubezky N, et al. Tumors with intrahepatic bile duct differentiation in cirrhosis: implications on outcomes after liver transplantation. Transplantation 2015;99:151-7. [Crossref] [PubMed]
- Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018;3:337-48. [Crossref] [PubMed]
- Gruttadauria S, Barbara M, Liotta R. Liver transplantation for unresectable intrahepatic cholangiocarcinoma: an Italian experience. Updates Surg 2021;73:1587-8. [Crossref] [PubMed]
- Guerrini GP, Esposito G, Tarantino G, et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: the first meta-analysis. Langenbecks Arch Surg 2020;405:265-75. [Crossref] [PubMed]
- Pery R, Gudmundsdottir H, Nagorney DM, et al. Laparoscopic versus open liver resections for intrahepatic cholangiocarcinoma and gallbladder cancer: the Mayo clinic experience. HPB (Oxford) 2023;25:339-46. [Crossref] [PubMed]
- Wang J, Wang W, Chen X, et al. Laparoscopic versus open hepatectomy for intrahepatic cholangiocarcinoma in patients aged 60 and older: a retrospective cohort study. World J Surg Oncol 2022;20:396. [Crossref] [PubMed]
- Wang J, Ma D, Du G, et al. Laparoscopic vs. open anatomical hepatectomy for intrahepatic cholangiocarcinoma: A retrospective cohort study. Front Surg 2022;9:1003948. [Crossref] [PubMed]
- Brustia R, Laurent A, Goumard C, et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: Report of an international multicenter cohort study with propensity score matching. Surgery 2022;171:1290-302. [Crossref] [PubMed]
- Ratti F, Casadei-Gardini A, Cipriani F, et al. Laparoscopic Surgery for Intrahepatic Cholangiocarcinoma: A Focus on Oncological Outcomes. J Clin Med 2021;10:2828. [Crossref] [PubMed]
- Magistri P, Assirati G, Ballarin R, et al. Major robotic hepatectomies: technical considerations. Updates Surg 2021;73:989-97. [Crossref] [PubMed]
- Hamad A, Ansari A, Li Y, et al. Short- and long-term outcomes following robotic and open resection for intrahepatic cholangiocarcinoma: A national cohort study. Surg Oncol 2022;43:101790. [Crossref] [PubMed]
- Mason MC, Massarweh NN, Tzeng CD, et al. Time to Rethink Upfront Surgery for Resectable Intrahepatic Cholangiocarcinoma? Implications from the Neoadjuvant Experience. Ann Surg Oncol 2021;28:6725-35. [Crossref] [PubMed]
- Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2018;105:839-47. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol 2016;23:235-43. [Crossref] [PubMed]
- Bartsch F, Eberhard J, Rückert F, et al. Repeated resection for recurrent intrahepatic cholangiocarcinoma: A retrospective German multicentre study. Liver Int 2021;41:180-91. [Crossref] [PubMed]
- Gravely AK, Vibert E, Sapisochin G. Surgical treatment of intrahepatic cholangiocarcinoma. J Hepatol 2022;77:865-7. [Crossref] [PubMed]


