Proton therapy for the management of uveal melanoma and other ocular tumors
Introduction
Ocular tumors are overall rare malignancies that can threaten vision, quality of life, and life expectancy. Radiation therapy (RT) with external beam radiation, plaques, stereotactic techniques and charged particles, including proton beam radiation therapy (PBRT), helium and carbon ion therapy, have been used in treatment. Proton beam radiation is well established as the “gold standard” treatment for ocular melanomas (OMs), the most common primary adult tumor of the eye (1-15). PBRT results in high local control (LC) and relatively high eye preservation rates with long follow-up in multiple international studies. PBRT has also been used in the context of other ocular conditions including conjunctival melanomas, choroidal metastases, retinoblastomas, angiomas, circumscribed and diffuse hemangiomas, and macular degeneration (10).
OM is the most common worldwide indication for proton beam therapy for ocular tumors. Also referred to as uveal melanoma, it includes choroidal, ciliary body and iris melanomas. Primary RT has been the standard of care for decades, and the potential benefits of PBRT include improved tumor dose delivery and decreased collateral damage due to its uniform dose distribution throughout the tumor volume, minimal scatter, significant dose rate, linear energy transfer, and sharp dose falloff outside the target region (9-13).
LC is a primary endpoint in evaluating the utility of radiation practices for OM. Retrospective data, prospective randomized studies, and meta-analyses have shown consistently high LC after proton treatment, on the order of 95% or greater (1-15). Furthermore, since the physical quality of a proton beam allows for reduced peripheral doses on critical structures, this can result in improved clinical outcomes in terms of eye retention and functional vision preservation. Proton treatment has developed tremendously over the past decades in terms of treatment planning, dose delivery, and post-RT care to maintain a high level of tumor control while reducing the incidence and/or severity of side effects. Pioneering work by dedicated ocular oncology particle centers worldwide has led to excellent results with PBRT for OM patients (1-15).
PBRT for ocular tumors can be difficult to access due to limited centers with the required capital equipment and clinical and physics/treatment planning expertise. In the future PBRT may be more accessible to patients as centers are being developed and as with all available radiation techniques, appropriate patient selection and education regarding the most effective treatments is crucial.
Epidemiology of OM
Among eye tumors, OM is the most common primary intraocular malignancy of adults and occurs in the uveal tract or vascular support layer of the eye. It includes tumors of the choroid most commonly, as well as the iris and ciliary body. The annual incidence is estimated to be between four and seven cases per million people in the United States, Canada, and Europe. OMs in the U.S. represent approximately 3–5% of all melanomas. Uveal melanoma occurs more frequently in light-skinned people with light eyes. A steady increase in incidence with age is seen, with peak incidence between 60–79 years of age, while less than 2% are younger than 20 years old at the time of diagnosis (16-18).
The etiology of uveal melanoma is largely unknown, and there is mixed evidence regarding genetic susceptibility and host factor patterns along with environmental factors such as exposure to sunlight, welding flashburns, and other factors. More recently, gene expression profiling data shows that a more accurate assessment of prognosis is possible with a transcriptomic classification of uveal melanomas based on RNA analysis of the primary tumor. A “class 1a” signature is associated with an excellent prognosis, whereas a “class 1b” and “class 2” signature portends higher risk of metastatic death respectively (19,20).
Diagnosis and work-up for ocular proton radiation
The evaluation of patients with possible ocular tumors may include clinical examination, fundus/iris photography, ocular ultrasonography, as well as other diagnostic tests such as visual field testing, indocyanine green angiography, optical coherence tomography, and CT/MRI. Fine needle aspiration and gene profiling of tumor samples can be used for diagnostic and prognostic purpose. Metastatic work-up may include laboratory testing such as liver function tests and liver imaging with CT, PET-CT, MRI, or ultrasound and may include chest imaging.
In small, indeterminate, pigmented tumors (<3 mm thick and <10 mm in diameter) without associated risk factors, serial observation of these lesions may be considered unless growth, development of associated risk factors or clinical changes is documented. Approximately two thirds of these lesions may not grow and many such patients are allowed to maintain good vision in an eye that otherwise would have had marked treatment effects (21,22). For medium and large-sized OM tumors, various surgical and radiation-based therapeutic alternatives are reported in the literature. Surgical intervention includes local resection with or without adjuvant radiation, enucleation, or exenteration. Radiation modalities include plaque brachytherapy, charged particles (i.e., protons, helium, or carbon), and radiosurgery. Both prospective Collaborative Ocular Melanoma Study (COMS) and retrospective studies demonstrate comparable survival rates between enucleation and irradiation. Selecting the optimal radiation modality is dependent on tumor and patient parameters as well as accessibility to specialized treatment facilities. Proton beam radiation is considered the “gold standard” of care for OM treatment due to the high level of tumor control and eye preservation achieved (10-15,23-25).
Proton beam radiation technique
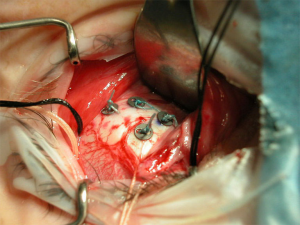
In preparation for proton beam treatment, patients undergo a thorough work-up as described above with their ocular oncology and radiation oncology team. Following diagnostic work-up and confirmation of planned proton treatment, the patient will undergo surgical placement of tantalum marker rings (Figure 1). These rings are placed at the tumor border on the sclera and serve as radiographic markers of the tumor edge for treatment planning and daily image guidance. Following surgery, the patient undergoes a radiation simulation in which an immobilization device is prepared and the markers are imaged on X-ray for confirmation of their three dimensional positioning in the eye. Thereafter the treatment planning can be completed in anticipation of treatment.
The EYEPLAN software used for treatment planning was developed by Goitein and Miller at MGH and has since been modified (26,27). The input data to the planning program includes (I) the spatial coordinates of the rings relative to the axis of the eye, obtained from orthogonal X-ray films; (II) the axial length and tumor height as measured on ultrasound; and (III) the shape of the tumor as drawn manually on the computer screen. The tumor relation to the rings is obtained from the surgeon’s mapping, fundus drawing, fundus photography, as well as three-dimensional (3D) MRI images. The program schematically displays a line drawing of the patient’s eye, including such anatomic structures as the globe, lens, optic nerve and macula.
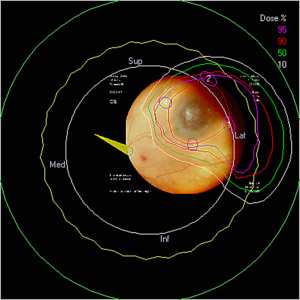
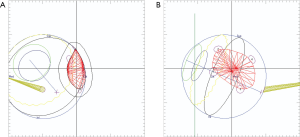
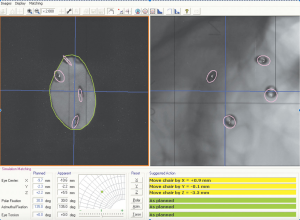
The optimal gaze direction is established which minimizes the dose to critical structures including optic nerve, macula, lens and cornea. An aperture is designed to define the shape of the field, generally with a 2 to 3 mm margin around the projection of the tumor in the beam’s-eye-view, which allows for possible microscopic extension of the tumor, small errors in patient setup, movement of the eye during treatment and beam penumbra (Figure 2A,B). The appropriate depth of beam penetration and width of the spread-out-Bragg peak (SOBP) necessary to encompass the target volume is determined. Dose-volume-histograms are produced for each structure and the dose distribution is assessed in different planes (Figure 3). The relationship of the rings to the beam and collimator are projected to ensure proper planned tumor coverage and daily treatment alignment. On treatment days, desired patient position, gaze angle, and possible eyelid retraction from the field are appropriately set and treatment position is verified usually with flat panel digital images (Figure 4). The radiation is generally delivered over 4 to 5 days and the dose across worlwide institutions for choroidal melanoma is generally 56–60 GyE in 4 daily fractions up to 70 GyE in 5 fractions. Small posterior macular tumors have been given 50 GyE in 5 fractions at one institution (10).
Other ocular conditions are treated with proton radiation for excellent LC and are dosed variably. A recent survey of dedicated ocular centers treating other malignant and benign conditions used the following schemas. Conjunctival melanomas may be treated to equivalent dose as choroidal melanomas, however are usually in the context of multimodality treatment including surgery, chemotherapy and adjuvant radiation. Hemangiomas have been treated with dosing between 15 to 20 GyE in 4–8 fractions with either marker ring delineation or clinical light field set-up. Macular degeneration has been treated to a total dose of 18 to 24 GyE in 2–4 fractions with clinical light field alignment. Angiomas have been given 18 to 35 GyE in 4–8 fractions. Choroidal metastases area treated from 20 to 60 GyE in 2–4 fractions generally depending on the histology and the estimated radiosensitivity. Retinoblastoma has been treated with protons to 31.6 GyE in 6 fractions (10).
The use of MRI technology is potentially of interest to ocular proton RT centers, however there are significant challenges to implementation into the major current model-based eye planning software (EYEPLAN® TPS). MRI offers (I) potential confirmation of surgical tantalum clip placement in relation to tumor and eye, appearing as voids post-operatively on MRI (versus CT in which metal artifact is present). MRI may be particularly significant for tumors that are more difficult to mark, or for newer surgeons as a benchmark for surgical ring assessment; (II) MRI serves as a 3D confirmation of the tumor and critical structure anatomy used in treatment planning model. For certain benign tumors or nonsurgical candidates, MRI assists in planning requiring clinical set up; (III) axial length can be confirmed on MRI, particularly if an eye is post-surgery, post-cataract and an axial length cannot be properly estimated with ultrasound; or as a reconfirmation of ultrasound measurement if a discrepancy is suspected (left to right eye axial length; very large or small axial length); (IV) also MRI can be used for additional visualization of hemorrhage and detachment. Dedicated proton ocular centers have developed an excellent approach over decades for ocular tumor treatment. The common practice uses fundus photography, clinical/surgical assessment, and ultrasound which can be incorporated well into the widely utilized eye planning software EYEPLAN®. As imaging advances and new proton centers arise requiring surgical and planning expertise to develop, MRI incorporation into the current eye TPS may improve the accuracy, reproducibility, and quality of care.
Proton beam results for uveal melanoma
Numerous reports on proton beam therapy describe 5-year LC for uveal melanomas, in the range of 95% or greater. With 15-year follow-up, authors report excellent sustained LC at ~95%. Five-year overall survival is approximately 80% across series (range, 70–88%), with small tumors ranging 95–98%, medium 80–86%, and large 60%. For class 1a tumors, overall survival is generally 95%, class 1b is approximately 80%, and class 2 is 50% or lower. Enucleation rate after proton therapy at 5 years is approximately 10% (range, 0–25%) with small tumors ranging 2–3%, medium 7–8%, and large 22–25%. The overall enucleation rate at 15 years is approximately 15% (1-10,28-32).
More than 3,000 patients with uveal melanoma have been treated with protons at the Harvard cyclotron (28,29). The prescribed dose in their series is 70 cobalt gray equivalent (CGE) in 5 fractions, assuming CGE = proton Gy × RBE 1.1. Reporting on more than 2,000 of their patients, their LC at 5 years was 97% and at 15 years was 95% (4). The 5-year actuarial survival for the patients treated with protons was 78.5% for the entire group with 98%, 86%, and 58% survival for patients with small, intermediate, and large melanomas, respectively. The 5-, 10-, and 15-year tumor-specific survival rates were 86%, 77%, and 73%, respectively. Factors that predict decreased survival include larger tumor diameter, ciliary body involvement, older age, and the presence of extrascleral tumor extension (30).
Egger et al. at the Paul Sherrer Institute confirmed these findings reporting on 2,435 patients treated between 1984 and 1998 with 54.5 Gy in 4 fractions (corresponding to 60 CGE). The LC rate was 96% at 5 years and 95% at 10 years. A higher 5-year local failure rate of 29% was found in patients treated with a reduced safety margin due to proximity of the tumor to the fovea. The 10-year cause-specific survival (CSS) was 73% for patients with controlled tumors versus 48% for those with local recurrence, showing potentially a relationship between LC and CSS, different from other findings such as those of the COMS Group trials (9).
A dose comparison study randomized 188 proton ocular patients to 50 vs. 70 CGE in 5 fractions and found no significant difference between dose regimens in terms of ocular toxicity or LC at 5 years (31). Presently 50 CGE in 5 fractions is used at this one institution for small posterior macular tumors, and otherwise 70 CGE for most tumors. The vast majority of institutions currently treat with dosing of 56–60 GyE in 4 fractions for choroidal melanomas (10).
A comprehensive recent meta-analysis reviewed patients undergoing brachytherapy (n=3,868 patients) and particle (n=7,043 patients) treatment for OM (11). Weighted by study sample size and with an average of 5 years follow-up, brachytherapy studies showed a weighted average local failure rate of 9.5% vs. particle studies 4.2%. Of note, the particle studies had better results overall despite having somewhat larger tumors to treat on average. Likewise, the UCSF-LBNL randomized trial of helium vs. plaque treatment update showed improved results from helium particles with long-term higher LC, eye preservation and disease-free survival rates at 12 years follow-up (23).
In terms of enucleations post proton treatment averaging 10%, approximately half are performed for complications related to neovascular glaucoma (NVG) (1-10). The remainder of enucleations is done for local salvage after recurrence, and treatment of other complications, including retinal detachment, severe inflammation, painful eye, and functional loss. The rates of NVG have lowered recently perhaps due to changes in treatment planning dose-volume parameters, particularly in relation to anterior chamber dosing, as well as changes in early intervention and treatment of NVG with anti-VEGF therapy and other techniques (33). Enucleation rate is shown to vary based on risk factors including tumor height and diameter, volume of lens irradiated, tumor distance from optic disc/macula, retinal detachment, ciliary body involvement, patient gender, intraocular pressure, and baseline visual acuity. The majority of enucleations are done between 0–3 years after treatment, but can be required at longer follow-up as well. Useful vision preservation (>20/200) with proton irradiation is reported at 3-year to be variable in the range of 40–65%. Important factors include tumor height, distance from optic disc or fovea, retinal detachment, and initial visual acuity. A recent comparison of patients treated with stereotactic radiation versus proton beam showed improved visual outcomes in the proton cohort (1-10,34-36).
Recurrent tumor without signs of metastatic disease most often requires surgical salvage, though proton re-irradiation for local relapse has been described (37). A second course of proton therapy between 48–70 CGE (for a total lifetime dose of 118–140 CGE) was delivered to 31 patients with resultant 5-year rates for LC of 69%, overall survival 64%, eye-retention 55%, and useful vision preservation in 27%.
Interestingly, a recent study of OM-specific treatment costs showed the relative cost-effectiveness of proton treatment compared with brachytherapy. Based on national inpatient and Medicare payments, the mean total treatment cost for plaque was found to be higher than proton beam and that both were higher than enucleation (38). Proton beam requires a single surgery and is given over 4–5 fractions, whereas plaque requires two surgeries and additional inpatients costs which may account for some of the difference in cost overall found in the study.
Follow-up
After proton treatment for uveal melanoma is complete, patients should be followed closely for local recurrence as well as distant disease. Patients should undergo a thorough clinical examination, with ocular ultrasound every 3–4 months for the first 2 years and then spaced to every 6 months for an additional 2–3 years and then annually. Liver function tests and imaging are done according to tumor parameters, genetic classification, and patient/physician preferences.
During the first months after treatment, some tumors may continue to enlarge or there may be retinal detachment or bleeding present that cause the appearance of growth, before shrinking. Historically these tumors were often enucleated immediately. These tumors may instead be followed for a finite amount of time (i.e., 3 months) and enucleated if continuous growth is measured or if other high risk features are noted during the observation period (16). Other ocular conditions may vary in terms of follow-up based on benign or malignant condition and potential for disseminated disease.
Conclusions
The treatment of uveal melanomas and other ocular tumors has been extensively evaluated for decades and proton beam therapy is considered the gold standard of care. PBRT approaches require specific expertise and great care in the application so that optimal LC, eye conservation, and long-term visual preservation can be achieved.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Char DH, Phillips T, Daftari I. Proton teletherapy of uveal melanoma. Int Ophthalmol Clin 2006;46:41-9. [Crossref] [PubMed]
- Damato B, Kacperek A, Chopra M, et al. Proton beam radiotherapy of choroidal melanoma: the Liverpool-Clatterbridge experience. Int J Radiat Oncol Biol Phys 2005;62:1405-11. [Crossref] [PubMed]
- Courdi A, Caujolle JP, Grange JD, et al. Results of proton therapy of uveal melanomas treated in Nice. Int J Radiat Oncol Biol Phys 1999;45:5-11. [Crossref] [PubMed]
- Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years. The Weisenfeld Lecture. Invest Ophthalmol Vis Sci 2006;47:4666-73. [Crossref] [PubMed]
- Dendale R, Lumbroso-Le Rouic L, et al. Proton beam radiotherapy for uveal melanoma: results of Curie Institut-Orsay proton therapy center (ICPO). Int J Radiat Oncol Biol Phys 2006;65:780-7. [Crossref] [PubMed]
- Mosci C, Mosci S, Barla A, et al. Proton beam radiotherapy of uveal melanoma: Italian patients treated in Nice, France. Eur J Ophthalmol 2009;19:654-60. [PubMed]
- Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology 1999;106:1579-87. [Crossref] [PubMed]
- Fuss M, Loredo LN, Blacharski PA, et al. Proton radiation therapy for medium and large choroidal melanoma: preservation of the eye and its functionality. Int J Radiat Oncol Biol Phys 2001;49:1053-9. [Crossref] [PubMed]
- Egger E, Schalenbourg A, Zografos L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys 2001;51:138-47. [Crossref] [PubMed]
- Hrbacek J, Mishra KK, Kacperek A, et al. Practice Patterns Analysis of Ocular Proton Therapy Centers: The International OPTIC Survey. Int J Radiat Oncol Biol Phys 2016;95:336-43. [Crossref] [PubMed]
- Chang MY, McCannel TA. Local treatment failure after globe-conserving therapy for choroidal melanoma. Br J Ophthalmol 2013;97:804-11. [Crossref] [PubMed]
- Wang Z, Nabhan M, Schild SE, et al. Charged particle radiation therapy for uveal melanoma: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2013;86:18-26. [Crossref] [PubMed]
- Mishra KK, Chiu-Tsao ST, Orton CG. Point/Counterpoint. Particle therapy is ideal for the treatment of ocular melanomas. Med Phys 2016;43:631-4. [Crossref] [PubMed]
- Weber DC, Bogner J, Verwey J, et al. Proton beam radiotherapy versus fractionated stereotactic radiotherapy for uveal melanomas: A comparative study. Int J Radiat Oncol Biol Phys 2005;63:373-84. [Crossref] [PubMed]
- Allen AM, Pawlicki T, Dong L, et al. An evidence based review of proton beam therapy: the report of ASTRO's emerging technology committee. Radiother Oncol 2012;103:8-11. [Crossref] [PubMed]
- Mishra KK, Quivey JM, Daftari IK, et al. Uveal Melanoma. In: Hoppe R, Phillips TL, Roach M. editors. Leibel and Phillips Textbook of Radiation Oncology. 3rd ed. Philadelphia: Elsevier Inc., 2010:1400-21.
- Egan KM, Seddon JM, Glynn RJ, et al. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol 1988;32:239-51. [Crossref] [PubMed]
- Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology 2003;110:956-61. [Crossref] [PubMed]
- Onken MD, Worley LA, Ehlers JP, et al. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res 2004;64:7205-9. [Crossref] [PubMed]
- Onken MD, Worley LA, Dávila RM, et al. Prognostic testing in uveal melanoma by transcriptomic profiling of fine needle biopsy specimens. J Mol Diagn 2006;8:567-73. [Crossref] [PubMed]
- Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study. COMS report no. 1. Arch Ophthalmol 1990;108:1268-73. [Crossref] [PubMed]
- Char DH. The management of small choroidal melanomas. Surv Ophthalmol 1978;22:377-86. [Crossref] [PubMed]
- Mishra KK, Quivey JM, Daftari IK, et al. Long-term Results of the UCSF-LBNL Randomized Trial: Charged Particle With Helium Ion Versus Iodine-125 Plaque Therapy for Choroidal and Ciliary Body Melanoma. Int J Radiat Oncol Biol Phys 2015;92:376-83. [Crossref] [PubMed]
- Toyama S, Tsuji H, Mizoguchi N, et al. Long-term results of carbon ion radiation therapy for locally advanced or unfavorably located choroidal melanoma: usefulness of CT-based 2-port orthogonal therapy for reducing the incidence of neovascular glaucoma. Int J Radiat Oncol Biol Phys 2013;86:270-6. [Crossref] [PubMed]
- Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology 2002;109:2197-206. [Crossref] [PubMed]
- Goitein M, Miller T. Planning proton therapy of the eye. Med Phys 1983;10:275-83. [Crossref] [PubMed]
- Sheen M. Development of the EYE proton therapy planning program. 20th PTCOG Meeting; Chester, England: 1994:29.
- Constable IJ, Koehler AM, Schmidt RA. Proton irradiation of simulated ocular tumors. Invest Ophthalmol 1975;14:547-555. [PubMed]
- Gragoudas ES. The Bragg peak of proton beams for treatment of uveal melanoma. Int Ophthalmol Clin 1980;20:123-33. [PubMed]
- Gragoudas ES, Seddon JM, Egan KM, et al. Prognostic factors for metastasis following proton beam irradiation of uveal melanomas. Ophthalmology 1986;93:675-80. [Crossref] [PubMed]
- Gragoudas ES, Lane AM, Regan S, et al. A randomized controlled trial of varying radiation doses in the treatment of choroidal melanoma. Arch Ophthalmol 2000;118:773-8. [Crossref] [PubMed]
- Egger E, Zografos L, Schalenbourg A, et al. Eye retention after proton beam radiotherapy for uveal melanoma. Int J Radiat Oncol Biol Phys 2003;55:867-80. [Crossref] [PubMed]
- Mishra KK, Daftari IK, Weinberg V, et al. Risk factors for neovascular glaucoma after proton beam therapy of uveal melanoma: a detailed analysis of tumor and dose-volume parameters. Int J Radiat Oncol Biol Phys 2013;87:330-6. [Crossref] [PubMed]
- Zytkovicz A, Daftari I, Phillips TL, et al. Peripheral dose in ocular treatments with CyberKnife and Gamma Knife radiosurgery compared to proton radiotherapy. Phys Med Biol 2007;52:5957-71. [Crossref] [PubMed]
- Seddon JM, Gragoudas ES, Polivogianis L, et al. Visual outcome after proton beam irradiation of uveal melanoma. Ophthalmology 1986;93:666-74. [Crossref] [PubMed]
- Sikuade MJ, Salvi S, Rundle PA, et al. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye (Lond) 2015;29:1194-8. [Crossref] [PubMed]
- Marucci L, Lane AM, Li W, et al. Conservation treatment of the eye: Conformal proton reirradiation for recurrent uveal melanoma. Int J Radiat Oncol Biol Phys 2006;64:1018-22. [Crossref] [PubMed]
- Moriarty JP, Borah BJ, Foote RL, et al. Cost-effectiveness of proton beam therapy for intraocular melanoma. PLoS One 2015;10:e0127814. [Crossref] [PubMed]