
Comparison of PARPi efficacy according to homologous recombination deficiency biomarkers in patients with ovarian cancer: a systematic review and meta-analysis
Highlight box
Key findings
• Patients with BRCA mutations benefit the most from PARPi, followed patients with HRD.
• The benefit of PARPi in patients with HRP tumors is limited.
What is known and what is new?
• There are two HRD tests with different ways of evaluating genomic scars and a similar benefit of PARPi was found for patients with BRCA1/2 wild-type with gLOH-high and myChoice®+.
• The benefit the PARPi in patients with HRP is much less pronounced and just had statistically significant in overall but not in first line setting.
What is the implication, and what should change now?
• Clinical development of further HRD biomarkers may help identify more patients who benefit from PARPi. This could lead to improved patient selection and potentially improve the efficacy of PARPi. Additionally, it could also help to identify patients who are unlikely to respond to PARPi treatment, allowing clinicians to make more informed decisions.
Introduction
Ovarian cancer is the third most common gynecological malignancy encompassing 313,959 new cases and 207,252 deaths worldwide in 2020 (1). High-grade serous ovarian cancer (HGSOC) is the most common (90%) and aggressive subtype, which retains a poor prognosis. However, epithelial ovarian cancer is a heterogeneous disease that includes several histotypes such as non-epithelial ovarian cancer (10%) based on molecular changes, clinical behavior and treatment (2,3). The cost of treatment per patient with ovarian cancer remains the highest among all cancer types, with an average initial cost in the first year of around USD 80,000 and the final year cost potentially increasing to USD 100,000. Despite efforts to develop effective tools for general population screening, patients with these tumors are commonly diagnosed at stages III (51%) or IV (29%) and their 5-year survival rates in the of 42% and 26% in the United States (US), respectively (4,5). Homologous recombination repair (HRR) is a critical mechanism for high-fidelity repair of double-strand DNA breaks (6). Mutations—defined here as pathogenic or likely pathogenic variants—in genes related to this repair pathway may lead to homologous recombination deficiency (HRD) (7).
Poly(ADP-ribose) polymerase (PARP) are enzymes involved in base-excision and are critical for DNA repair of single-strand breaks (8). Hence, PARP inhibitors (PARPi) lead to further chromosomal instability and cell death and are particularly toxic to HRD tumors (9). Over the past decade, PARPi have dramatically changed the treatment landscape of ovarian cancer. They were initially approved for patients with advanced disease as second-line monotherapy maintenance, based on improvement in progression-free survival (PFS) for patients with sustained partial or complete response to platinum-based chemotherapy (10,11). In this context, olaparib was the first PARPi to become available in clinical practice, followed by rucaparib and niraparib (12). Subsequent trials also confirmed the benefit of olaparib, niraparib, veliparib, and rucaparib in the first line setting as maintenance for patients who achieve a complete or partial response to platinum-based chemotherapy (13-17). However, the Food and Drug Administration (FDA) has restricted its approval to olaparib and niraparib in the front-line setting (18), while veliparib and rucaparib remain investigational. Despite differences in patient characteristics, treatment setting, and design, trials consistently show that patients with BRCA mutations have a more favorable response to PARPi, with similar hazard ratios (HRs) across studies. Consequently, BRCA mutations are a robust predictor of positive response. PARPi therapy may benefit some patients with wild-type (wt) BRCA, which raises the question of identifying HRD biomarkers beyond BRCA mutations. In addition, synthetic lethality can be used in tumors with similar molecular characteristics to BRCA-muted tumors, referred to as “BRCAness”. Mutations in genes outside of BRCA in the homologous recombination pathway may expand the indication for the use of a PARP inhibitor, although this is still being studied (19).
There is rationale to believe that patients with mutations in a HRR gene leading to functional HRD will have genomic instability and will therefore be sensitive to PARPi. In fact, PARPi have been approved with a similar indication for patients with prostate cancer who carry a mutation in a HRR gene (20). For example, early events in prostate cancer development, such as CDH1 gene loss or inactivation of the SPOP gene, may also increase sensitivity to PARPi (21). In breast cancer, olaparib and talazoparib are approved for metastatic HER2-negative breast cancer, but these are restricted to patients with germline BRCA mutations (22,23). The TBCRC-048 study investigated olaparib in patients with other HRR genes but responses have been limited to PALB2 or BRCA (24,25).
Alternatively, another approach is to identify patients based on the genomic aberrations thought to be consequence of HRD. The genomic scars commonly found in BRCA carriers include loss of heterozygosity (LOH), telomeric allelic imbalance (TAI), and large-scale transitions (LST) (26). The two main composite HRD tests available in clinical practice apply next-generation sequencing (NGS) or microarray assays to simultaneously search for BRCA mutations and genomic scars. The Foundation Medicine T5 utilizes a genome-wide LOH (gLOH) (27), whereas the Myriad MyChoice employs the genomic instability score (GIS-score), which incorporates LOH, TAI, and LST (28).
In the present study, we aimed to perform an up-to-date meta-analysis of the various HRD biomarkers studied in the first and second line randomized clinical trials and their association with survival in patients with ovarian cancers treated with PARPi. We present this article in accordance with the PRISMA reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-22-114/rc).
Methods
We performed this meta-analysis under the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29,30). A prospective protocol was formulated and uploaded to PROSPERO (CRD42021248112).
Eligibility criteria
Eligible studies were phase II or phase III randomized clinical trials comparing PARPi with placebo or chemotherapy. Retrospective biomarker analyses from the same studies were also eligible. Single-arm studies or studies that had PARPi in both arms were ineligible. In the presence of multiple references for the same study, we chose the most recent and complete publication.
Data sources and extraction
We conducted a comprehensive database search (PubMed, Embase, and Cochrane Central) for entries from inception to June 18, 2022. We further analyzed abstracts from the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) libraries during the equivalent period. The detailed search strategy is available (Table S1).
We uploaded titles and abstracts to Rayyan QCRI, a web-based platform for systematic review management (31). Two authors independently performed the screening. Data extraction from the included studies was performed by two authors, in tandem, and using a pre-piloted spreadsheet containing trial identification, including and exclusion criteria, treatment arms, and HRD definition, including the test manufacturer.
The outcome of interest was the HR for PFS according to different patient subgroups. The BRCA mutations (BRCAmut) subgroup included germline mutations (gBRCAm), somatic mutations (sBRCAm), or tumor mutations (tBRCAm), which refers to a composite of gBRCAm or sBRCAm. Conversely, the BRCAwt group had to be all gBRCAwt, sBRCAwt, and tBRCAwt. The BRCAwt & other HRD included those without BRCA mutation that had either GIS-high or gLOH-high. The BRCAm & other HRD included those with a BRCA mutation plus either GIS-high or gLOH-high. The BRCAm or other HRD included those that had a BRCA mutation or either GIS-high or gLOH-high. The HRP group comprised those without any evidence of HRD.
Risk of bias assessment
Risk of bias in randomized trials was assessed using RoB (version 2.0). This included five domains (randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, and selection of reported results) and resulted in judgments of “low risk of bias”, “some concerns”, or “high risk of bias” (32). Two authors independently applied the tool to each included trial. Inconsistencies were solved through discussions among all authors.
Statistical analysis
Meta-analysis was performed using generic inverse variance and a random-effects model described by Borenstein and Higgins (33). We conducted analyses comparing the effect of the presence of the HRD biomarker on the efficacy of PARPi. Patients were classified into three categories according to their HRD status: (I) BRCAm (patients with BRCA mutation of germline or somatic origin); (II) non-BRCA HRD (patients BRCAwt with another HRD biomarker—gLOH or myChoice®); and (III) homologous recombination proficiency (HRP) (BRCAwt and HRD biomarker negative). From those that were BRCAwt, we compared myChoice®+ with gLOH-high. Sub-analyses included patients treated in the first-line setting, and another with all patients (including first line and recurrence settings). We did not conduct a separate analysis restricted to the recurrence setting as this was recently reported by another group (34). Statistical analysis was executed in RevMan (version 5.4). We generated forest plots for back-transformed effect estimates, expressing PFS as HRs with the respective 95% confidence interval (CI). We assessed heterogeneity between and within designs using Cochran’s Q statistics and quantified using I2 statistics. I2 can be used to describe the proportion of the variability in effect estimates due to heterogeneity within three thresholds 25% (low), 50% (moderate) and, 75% (high) (35,36).
Results
Study selection
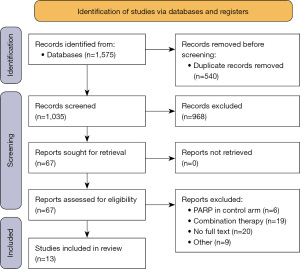
A total of 1,575 unique entries were found, and 968 studies were screened after excluding duplicates. Sixty-seven full-text publications were reviewed. A PRISMA flow diagram is shown on Figure 1. Thirteen trials (n=5,529) were included in the quantitative synthesis: five trials in the first-line setting (n=3,225), and eight trials in the recurrence setting (n=2,304) (Table S2) (37-42). We used a recent meta-analysis to pool previously additional subgroup data that was unavailable in the recurrent-setting publications. All studies were deemed “low risk of bias” (Figure S1). Funnel plots for publication bias in each subgroup evaluated in the main analysis are shown in (Figure S2).
Study characteristics
All first-line studies were phase III and compared the efficacy of PARPi monotherapy maintenance against placebo maintenance. SOLO-1 and PAOLA-1 evaluated olaparib, PRIMA evaluated niraparib, ATHENA-MONO evaluated rucaparib, while VELIA studied two combinations of carboplatin/taxane with either veliparib or placebo (13-17). All studies in the recurrent setting compared PARPi with placebo or chemotherapy or anti-vascular endothelial growth factor (anti-VEGF). The PAOLA-1 study, unlike all other studies, treated both intervention and placebo groups with bevacizumab association (17). Study19 and Stan B. Kaye, 2011, were the only phase II and used olaparib (10,11). The remaining were phase III, SOLO-2 and SOLO-3 with olaparib, ARIEL-3 and ARIEL-4 with rucaparib, and NOVA and NORA with niraparib (37-42). Most of the studies used either the Myriad myChoice genomic instability score (GIS) or the Foundation Medicine T5 NGS loss of heterozygosity (gLOH) as surrogates. The VELIA trial used GIS ≥33 as a cutoff for HRD; the remainder of studies using Myriad myChoice adopted GIS ≥42 as a cutoff.
Statistical analysis
In the comparison of PARPi efficacy according to HRD biomarker, we find that patients with BRCA mutations derived the most pronounced benefit from PARPi with PFS HR 0.37 (95% CI: 0.30–0.48, P<0.00001) (Figure 2), followed by patients BRCAwt and other HRD with PFS HR 0.45 (95% CI: 0.37–0.55, P<0.00001) (Figure 3). HRP patients derived much less benefit from PARPi with PFS HR 0.70 (95% CI: 0.57–0.85, P=0.0004) (Figure 4). The benefit of PARPi according to HRD status in the first and recurrent line settings were comparable, except that the benefit of PARPi were not statistically significant in the first line setting for HRP patients (Figure 5). The benefit is similar across BRCA mutation subtype (Figure S3) and HRD biomarker (Figure 3). Specifically, patients with BRCAwt and GIS ≥42 via Myriad myChoice® had HR 0.43 (95% CI: 0.34–0.56, P<0.00001), similar to patients with BRCAwt and gLOH-high via Foundation Medicine T5 NGS with HR 0.46 (95% CI: 0.33–0.64, P<0.00001) (Figures 6,7). One study used GIS ≥33 as cutoff value and was not included in this subgroup analysis.
Discussion
Our results confirm that both patients with BRCAm or either of the commercially available HRD biomarker derive a clinically meaningful benefit from PARPi, and the benefit in patients that are HRP is much less pronounced overall and was not statistically significant for patients in the first-line setting.
For the efficacy of PARPi in the HRP population, the results were different between the first-line studies: Athena-Mono showed better outcomes than PAOLA-1 and PRIMA. Differences were not only present in the PARPi used but also in the HRD test—Athena-Mono used Foundation whereas PRIMA and PAOLA-1 used Myriad—and patient population. This could be in part attributed to the differences in the patient populations in those studies. Athena-Mono included patients with lower risk disease when compared with other studies. Patients with inoperable disease were excluded, 80% of patients underwent optimal debulking, only 10% had measurable disease after surgery and/or chemotherapy, and approximately, 50% of patients were HRP. PAOLA-1 studied olaparib in combination with bevacizumab, 8% of patients had inoperable disease, only 40% had optimal debulking, and only 35% were HRP. Lastly, PRIMA evaluated niraparib and only included patients at a higher risk, including patients with inoperable disease, stage III with suboptimal cytoreduction, and stage IV. Approximately half of the patients in PRIMA were HRP.
In recurrent setting, reports of detrimental overall survival in patients with BRCAmut or HRD from the ARIEL 4 (rucaparib), SOLO 3 (olaparib), and NOVA (niraparib) have led to treatment recommendation updates. PARPi monotherapy should not routinely be offered to patients who have recurrent platinum-sensitive cancer. It may be offered to patients who have not already received PARPi and who have responded to platinum-based therapy regardless of BRCA mutation status. PARPi monotherapy is not recommended for patients with either BRCAwt or platinum-resistant recurrent (43).
This study summarizes the current evidence from randomized clinical trials in the first and later lines of therapy and stratifies patients according to different types of BRCA mutations and different HRD assays that are already commercially available. The trials in the recurrence setting reported BRCA status as germline or somatic, except for Study19, where patients received a classification of tumor BRCA (tBRCA), which encompassed both gBRCA and sBRCA. All front-line studies used tBRCA, except for SOLO1, that only included patients with gBRCAm. Our analysis confirms that patients with BRCA-related or non-BRCA-related HRD—GIS or gLOH—derive the most benefit from PARPi. We underscore that the magnitude of benefit from patients selected based on GIS and gLOH was similar.
Our analysis also demonstrates that robust detection of HRD is still an unmet need. This is exemplified by the fact that some HRP patients also benefited from PARPi use, although to a significantly lower degree, and mostly restricted to the recurrent setting. A possible explanation is that PARPi can promote anti-tumor activity by mechanisms other than HRD, such as a microenvironment reshaping towards immune activation via macrophage activity (https://doi.org/10.1016/j.celrep.2022.111462). Moreover, preclinical models support a therapeutical synergism between PARPi and immune checkpoint inhibitors, notably anti-programmed cell death 1/PD-1 ligand 1 (anti-PD-1/PD-L1) and anti-cytotoxic T lymphocyte-associated antigen 4 (anti-CTLA-4) which induces a PARPi sensitization and provokes a major antitumor immune response than either drug alone (44,45). Recent advancements in proteomics, including mass spectrometry and protein array analysis, have significantly contributed to a deeper understanding of the molecular signaling events and proteomic characterization of ovarian cancer (46). Novel approaches such as the use of single-base substitutions or rearrangement signatures or their combination resulted in better HRD identification—measured by PARPi efficacy—in patients with ovarian and breast cancer (47). It is also noteworthy that the present techniques detect genomic scars that accumulated over time instead of measuring real-time DNA repair capacity, which would better reflect the dynamic nature of the homologous recombination status. Immunohistochemistry (IHC)-based RAD51 assays showed promising pre-clinical results in small datasets and will require further validation and clinical utility testing (48).
Limitations of our study include indirect comparisons, which is inherent to meta-analyses. The use of trial-level data as opposed to individual patient data can reduce the power of our analysis. Subgroup analyses included patients treated with different drugs, a limitation that is minimized by the fact that they belong to the same class and presented similar results across trials when similar patients are compared. Our study was not designed to compare efficacy among the different PARPi since this was evaluated in a previous meta-analysis (49). Most trials have not yet published overall survival data, and therefore PFS was used as a surrogate endpoint, but the expectation is that the final analyses of these studies will demonstrate the translation of PFS into OS benefit, as seen in the SOLO2/ENGOT-Ov21 trial (37).
Conclusions
Patients with BRCA mutations benefit the most from PARPi. From patients with BRCA1/2wt, a comparable benefit was found between patients with HRD detected via gLOH-high and those via myChoice®. HRP patients derived limited benefit. The clinical development of further HRD biomarkers (i.e., Sig3 and HRDetect) may help identify more patients who may benefit from PARPi.
Acknowledgments
The abstract was already published as a Meeting Abstract in the 2022 ASCO Annual Meeting.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-22-114/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-22-114/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-22-114/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med 2017;14:9-32. [Crossref] [PubMed]
- Cheung A, Shah S, Parker J, et al. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? Int J Environ Res Public Health 2022;19:1106. [Crossref] [PubMed]
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284-96. [Crossref] [PubMed]
- Ghose A, Bolina A, Mahajan I, et al. Hereditary Ovarian Cancer: Towards a Cost-Effective Prevention Strategy. Int J Environ Res Public Health 2022;19:12057. [Crossref] [PubMed]
- Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet 2010;44:113-39. [Crossref] [PubMed]
- Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609-15. [Crossref] [PubMed]
- De Lorenzo SB, Patel AG, Hurley RM, et al. The Elephant and the Blind Men: Making Sense of PARP Inhibitors in Homologous Recombination Deficient Tumor Cells. Front Oncol 2013;3:228. [Crossref] [PubMed]
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917-21. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852-61. [Crossref] [PubMed]
- Kaye SB, Lubinski J, Matulonis U, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol 2012;30:372-9. [Crossref] [PubMed]
- Mirza MR, Pignata S, Ledermann JA. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann Oncol 2018;29:1366-76. [Crossref] [PubMed]
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N Engl J Med 2019;381:2403-15. [Crossref] [PubMed]
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018;379:2495-505. [Crossref] [PubMed]
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019;381:2391-402. [Crossref] [PubMed]
- Monk BJ, Parkinson C, Lim MC, et al. A Randomized, Phase III Trial to Evaluate Rucaparib Monotherapy as Maintenance Treatment in Patients With Newly Diagnosed Ovarian Cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol 2022;40:3952-64. [Crossref] [PubMed]
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med 2019;381:2416-28. [Crossref] [PubMed]
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:191-226. [Crossref] [PubMed]
- Shah S, Cheung A, Kutka M, et al. Epithelial Ovarian Cancer: Providing Evidence of Predisposition Genes. Int J Environ Res Public Health 2022;19:8113. [Crossref] [PubMed]
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2091-102. [Crossref] [PubMed]
- Boussios S, Rassy E, Moschetta M, et al. BRCA Mutations in Ovarian and Prostate Cancer: Bench to Bedside. Cancers (Basel) 2022;14:3888. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- Tung NM, Robson ME, Ventz S, et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J Clin Oncol 2020;38:4274-82. [Crossref] [PubMed]
- Menezes MCS, Raheem F, Mina L, et al. PARP Inhibitors for Breast Cancer: Germline BRCA1/2 and Beyond. Cancers (Basel) 2022;14:4332. [Crossref] [PubMed]
- Miller RE, Leary A, Scott CL, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol 2020;31:1606-22. [Crossref] [PubMed]
- Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer 2012;107:1776-82. [Crossref] [PubMed]
- Telli ML, Timms KM, Reid J, et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin Cancer Res 2016;22:3764-73. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [Crossref] [PubMed]
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [Crossref] [PubMed]
- Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci 2013;14:134-43. [Crossref] [PubMed]
- Lee CK, Friedlander ML, Tjokrowidjaja A, et al. Molecular and clinical predictors of improvement in progression-free survival with maintenance PARP inhibitor therapy in women with platinum-sensitive, recurrent ovarian cancer: A meta-analysis. Cancer 2021;127:2432-41. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Poveda A, Floquet A, Ledermann JA, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2021;22:620-31. [Crossref] [PubMed]
- Penson RT, Valencia RV, Cibula D, et al. Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J Clin Oncol 2020;38:1164-74. [Crossref] [PubMed]
- Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:1949-61. [Crossref] [PubMed]
- Kristeleit R, Lisyanskaya A, Fedenko A, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:465-78. [Crossref] [PubMed]
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375:2154-64. [Crossref] [PubMed]
- Wu XH, Zhu JQ, Yin RT, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial Ann Oncol 2021;32:512-21. [Crossref] [PubMed]
- Tew WP, Lacchetti C, Kohn EC, et al. Poly(ADP-Ribose) Polymerase Inhibitors in the Management of Ovarian Cancer: ASCO Guideline Rapid Recommendation Update. J Clin Oncol 2022;40:3878-81. [Crossref] [PubMed]
- Mehta AK, Cheney EM, Hartl CA, et al. Targeting immunosuppressive macrophages overcomes PARP inhibitor resistance in BRCA1-associated triple-negative breast cancer. Nat Cancer 2021;2:66-82. [Crossref] [PubMed]
- Revythis A, Limbu A, Mikropoulos C, et al. Recent Insights into PARP and Immuno-Checkpoint Inhibitors in Epithelial Ovarian Cancer. Int J Environ Res Public Health 2022;19:8577. [Crossref] [PubMed]
- Ghose A, Gullapalli SVN, Chohan N, et al. Applications of Proteomics in Ovarian Cancer: Dawn of a New Era. Proteomes 2022;10:16. [Crossref] [PubMed]
- Batalini F, Gulhan DC, Mao V, et al. Mutational Signature 3 Detected from Clinical Panel Sequencing is Associated with Responses to Olaparib in Breast and Ovarian Cancers. Clin Cancer Res 2022;28:4714-23. [Crossref] [PubMed]
- Waks AG, Cohen O, Kochupurakkal B, et al. Reversion and non-reversion mechanisms of resistance to PARP inhibitor or platinum chemotherapy in BRCA1/2-mutant metastatic breast cancer. Ann Oncol 2020;31:590-8. [Crossref] [PubMed]
- Xu Y, Ding L, Tian Y, et al. Comparative Efficacy and Safety of PARP Inhibitors as Maintenance Therapy in Platinum Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis. Front Oncol 2021;10:573801. [Crossref] [PubMed]








