
CDH1 and hereditary diffuse gastric cancer: a narrative review
Introduction
Gastric cancer remains the fifth most common and fourth most lethal cancer worldwide in 2020, despite improvements in medical technology, food preservation, and Helicobacter pylori treatment (1). Majority of gastric cancers are sporadic with approximately 10% of gastric cancers having familial predilection. However, 1–3% of gastric cancers are the result of a hereditary cancer syndrome (2). Hereditary diffuse gastric cancer (HDGC) is a rare and autosomal dominant inherited syndrome that is characterized by early onset of diffuse gastric cancer (DGC) in affected individuals.
HDGC was first described by Jones et al. in 1964 when three separate Māori families from New Zealand were discovered to have multigenerational early onset familial gastric cancer (3). Thirty years later, Guilford et al. published his work detailing the families originally described by Jones. While the general population of New Zealand had 80% of gastric cancers occur in people over the age of 60, Guilford noted a stark contrast in the Māori families, with majority of gastric cancers occurring before the age of 40 (4) and the earliest arising in a 14-year-old who succumbed to the disease.
Today, germline CDH1 variants have an estimated population frequency of approximately 5–10/100,000 births (5). Inactivating germline variants in the CDH1 tumor suppressor gene, which codes for a cell adhesion glycoprotein E-cadherin, are the most common cause of HDGC, with CTNNA1 gene mutations a more recently identified but less frequent cause (4,6,7). CDH1 gene variants result in elevated lifetime risk of DGC, lobular breast cancer (LBC), and non-syndromic cleft lip and palate (6,8). Overall, CDH1 loss of function variants are associated with up to a 70% lifetime risk of gastric cancer in males, and 56% lifetime risk in females who also have a 42% lifetime risk of developing LBC (6). HDGC is considered a highly aggressive form of gastric cancer occurring typically in young patients with a median age of diagnosis in the 30s (6,7). Early identification of CDH1 mutations in HDGC patients has important implications for genetic counseling and management. However, HDGC has a peculiar and indolent clinical behavior with occult, microscopic foci of gastric signet ring cells (SRC) that are frequently detected in asymptomatic individuals before clinical diagnosis of advanced cancer (6,9). Symptomatic patients often present with American Joint Committee on Cancer (AJCC) stage III and IV disease, for which 5-year survival is particularly poor at approximately 3–19% (10,11). Due to limited survival associated with advanced stage gastric carcinoma, international consensus guidelines support early screening and prophylactic surgery to prevent advanced cancer and improve survival (5).
Recent advances in genetic testing and risk assessment have facilitated the identification of CDH1 mutation carriers and improved the management of HDGC. However, there is still much to be learned about the pathogenesis of HDGC and the specific mechanisms by which CDH1 mutations contribute to tumorigenesis. Basic science research has shed light on the functional consequences of CDH1 mutations on E-cadherin expression and cell adhesion, as well as the downstream signaling pathways that may be disrupted in the presence of CDH1 mutations. Animal models and organoid cultures of diffuse-type gastric cancer have been developed to study pathogenesis and test potential preventive and therapeutic interventions.
This literature review provides an overview of the current understanding of the role of CDH1 in HDGC, including the genetic and clinical features of the disease, the functional consequences of CDH1 mutations, and the current approaches to prevention and treatment. This review also highlights ongoing research efforts aimed at elucidating the specific mechanisms of CDH1-mediated tumorigenesis and developing targeted therapies and clinical trials for HDGC. This review covers critical aspects of HDGC that include the molecular biology of CDH1 loss and the prospect of secondary (extra-intestinal) cancers. We present the most up-to-date knowledge on the subject that is presented with clinical context for practical clinical applicability. We present this article in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-36/rc).
Methods
A literature review was completed by searching PubMed and ClinicalTrials.gov from the first literature description of HDGC in 1964 up to March 31, 2023. Only articles published in English and with full text were considered. PubMed was searched using the terms “‘CDH1’ AND ‘Hereditary Diffuse Gastric Cancer’”. A total of 142 articles were captured (Figure S1). Types of studies included were case reports, clinical trials, comparative studies, meta-analyses, evaluation studies, guidelines, multicenter studies, observational studies, review articles, systemic reviews, and validation studies. Of the 142 articles, 62 articles were review papers, resulting in a total of 80 non-review papers. All articles were reviewed and verified by both authors (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 3/31/2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “CDH1” AND “Hereditary Diffuse Gastric Cancer” |
| Timeframe | 01/01/1964–3/31/2023 |
| Inclusion and exclusion criteria | Included |
| English | |
| Full text articles | |
| Species: human & animal | |
| Types: case reports, clinical trials, comparative studies, meta-analyses, evaluation studies, guidelines, multicenter studies, observational studies, review articles, systemic reviews, and validation studies | |
| Excluded | |
| Not English | |
| Abstracts | |
| Selection process | Both authors conducted the selection and review of material together |
CDH1-induced gastric carcinogenesis
HDGC is most often the result of a truncating mutation in the CDH1 gene located on chromosome 16q22.1, which encodes E-cadherin (4,12). E-cadherin is an important transmembrane glycoprotein involved in cell adhesion, signal transduction, and maintaining normal tissue architecture (4,6,7). E-cadherin consists of three domains: an extracellular domain with 5 cadherin repeats, a transmembrane domain, and a highly conserved intracellular cytoplasmic tail (13). The extracellular domain is important for cell-cell adhesion (14,15). The intracellular domain interacts with several catenins (α, β, and p120) to perform important cell functions such as autophagy, endo- and exocytosis, and receptor and transmembrane channel recycling (16,17) (Figure 1). E-cadherin also participates in cell differentiation, epithelium maintenance, and alteration of gene expressions in the nucleus through transduction of signals that originate from its extracellular domain (17-19). Loss of E-cadherin leads to cell detachment and disruption of tissue organization. Majority of mutations consist of small insertions and deletions; however, loss of CDH1 has resulted from nonsense mutations, missense mutations, exon/intro splice site mutations, as well as frameshift mutations (6,20-22). CDH1 germline variants have been discovered throughout the entire gene length, including entire gene deletions, with no apparent correlation of genotype with phenotype (23).
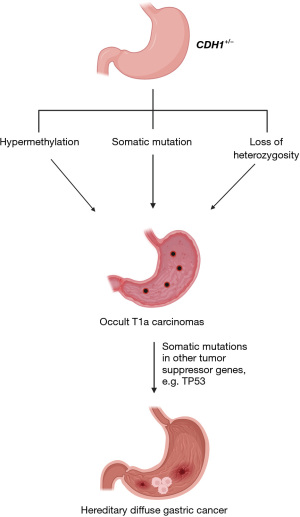
Germline CDH1 variants in one allele are inherited in an autosomal-dominant manner. In order to initiate the neoplastic process, the second copy of CDH1 gene must become inactivated (21). Several mechanisms have demonstrated inactivation of the second CDH1 allele, including promoter hypermethylation, somatic mutation, and loss of heterozygosity (21,24) (Figure 2). CDH1 acts as a tumor suppressor gene, and the loss of function of the second allele promotes tumor initiation through uninhibited cell adhesion and cell proliferation (13,25,26). Despite these established mechanisms, an explanation for why some patients with CDH1 variants will develop HDGC whereas others will not is unknown. Interestingly, both in situ and invasive HDGC have either reduced or absent E-cadherin expression, signifying that E-cadherin inactivation is an early event in the disease process (27). In addition, multifocal lesions appear to arise as independent events given tumor heterogeneity and complex somatic inactivating mechanisms (24).
This loss of E-cadherin function also leads to the activation of oncogenic signaling pathways, such as the Wnt/beta-catenin pathway which contributes to the uncontrolled growth and survival of cancer cells. The Wnt/beta-catenin pathway is a vital signaling cascade involved in cell proliferation, differentiation, migration, and homeostasis of intestinal stem cell function (28). In normal conditions, E-cadherin sequesters Beta-catenin at the cell membrane, preventing its translocation to the nucleus and thus maintaining the pathway’s regulation. However, the loss of E-cadherin liberates beta-catenin, allowing it to translocate to the nucleus and activate downstream target genes involved in cellular proliferation, survival, and invasion (28,29). Consequently, the aberrant activation of the Wnt/beta-catenin pathway promotes tumorigenesis in HDGC. These interconnected pathways contribute to the uncontrolled growth, survival, and invasive properties of cancer cells, ultimately driving the development and progression of HDGC. A sophisticated understanding of these pathways and their interplay is crucial for the development of targeted therapeutic strategies aimed at combating this aggressive malignancy.
To further elucidate these pathways and mechanisms of HDGC, animal models have been utilized. One study by Humar et al. used N-methyl-N-nitrosourea (MNU) to induce gastric carcinogenesis in cdh1+/− mice (30). These mice were shown to have an 11× higher incidence in developing intramucosal signet-ring cell carcinoma (SRCC) compared to their wild-type counterparts, which supports the known importance of E-cadherin loss in the development of malignancy. Similar to humans, this mouse model demonstrated early detection of SRCC indicating that E-cadherin is an early mechanism, and the tumors also revealed a transition to poorly differentiated tumors once invading beyond the mucosa. Additionally, CDH1 knockout mice have demonstrated a significant increase in SRCs and the development of more advanced tumors with the additional loss of TP53, further promoting the two-hit hypothesis (31). While convenient, mice are genetically different from humans and the SRCC found in mice are restricted to only the gastric antrum compared to the diffuse and unpredictable gastric distribution of SRCC in humans (30). To counteract the differences in microenvironments seen in animal models, organoids have emerged as a promising tool for the study of CDH1 and HDGC. Organoids are three-dimensional multi-cellular in vitro models that recapitulate the architecture and functionality of native tissues, such as the stomach. Both murine and human organoids have been utilized to further understand the mechanisms of HDGC to develop treatment and screening modalities. Using the stomachs from CD44-Cre/CDH1loxP/loxP mice that underwent subsequent CDH1 deletion to create organoids, Dixon et al. discovered that cytokeratin 7 (CK7) and its respective differentiation gene Krt7 were markers for early neoplastic lesions in CDH1 carriers (32). The authors recommend the use of CK7 immunohistochemistry analysis on suspicious endoscopic biopsies to aid in the detection of early malignancy. Caution should be noted as published data using human derived organoids is not available to validate these findings. Similar to CDH1 knockout mice, patient-derived organoids that have combined loss of both TP53 and CDH1 have been shown to form highly invasive tumors and grown independently of their required growth factor, R-spondin (33,34). This emphasizes the accumulation of additional mutations in helping the early stages of SRC progress into a more aggressive form. Additionally, emerging evidence has revealed that DGC organoids differentiate into SRC-like cells when Wnt pathway factors are absent (35). However, additional factors are needed to enable the early-stage lesion to grow and progress. In conclusion, the use of organoids and animal models to study CDH1 and HDGC has opened new avenues for understanding the intricate molecular mechanisms underlying this aggressive malignancy. By leveraging these advanced in vitro models, researchers can elucidate the consequences of E-cadherin loss and identify novel therapeutic targets, ultimately paving the way for improved clinical management of HDGC.
Guidelines and diagnosis
Individuals with pathogenic or likely pathogenic germline CDH1 variants are at increased risk of developing DGC and LBC (6,8). For early detection and diagnosis, the International Gastric Cancer Linkage Consortium (IGCLC) established guidelines for germline genetic testing for individuals who meet specific criteria (5). These are separated into individual and family criteria. Individual criteria include DGC in an individual aged <50 years old, DGC at any age in individuals with a personal or family history of cleft lip/palate, history of DGC and LBC in an individual <70 years old, bilateral LBC/lobular carcinoma in situ (LCIS) in individuals <70 years old, or gastric biopsy with in-situ SRCs and/or pagetoid spread of SRCs in individuals <50 years old. Family criteria include ≥2 cases of gastric cancer in family (any age) with a least 1 confirmed DGC, ≥2 cases of family members aged <50 years old, or ≥1 case of DGC any age and ≥1 case of LBC in different family members aged <70 years old. Of note, to meet criteria all diagnoses of DGC and LBC must be histologically confirmed and family members must be first- or second-degree blood relatives of each other.
It is important that individuals who meet testing criteria are provided with both genetic counseling and genetic testing at certified molecular diagnostic laboratories (5). Positive tests from direct-to-consumer testing may be used only if validated by a certified molecular diagnostic laboratory (5). Genetic testing should include CDH1 variants as well as CTNNA1 and other gene variants that have been linked not only to DGC but increased gastric carcinoma risk in general (6,36,37).
Endoscopic cancer surveillance
The most recent clinical practice guidelines for HDGC advocate PTG for carriers of pathogenic CDH1 variants with a family history of DGC. For patients meeting these criteria who either decline PTG or present with contraindications to surgery, annual surveillance endoscopy is recommended (5). The Cambridge method, a consensus approach, is advised to guide endoscopic surveillance in HDGC, primarily aiming to assess early cancer signs through meticulous inspection of the gastric mucosa and evaluation of distensibility. Subsequently, targeted biopsies of visible abnormalities are performed, followed by non-targeted (random) sampling of the gastric mucosa (32-35). Using the Cambridge method, a minimum of 30 random gastric mucosal biopsies are collected, partly due to the presence of intramucosal, occult SRC carcinoma foci in nearly all CDH1 variant carriers (5,38). Historically, detection of SRC lesions in gastric biopsies during endoscopy has reported to be between 9–24% (9,39-41); however, more recent surveillance endoscopies in expert HDGC centers have detected SRC lesions in up to 40–61% (5,38,42-45). While endoscopic detection of SRCs has varied, it remains an insensitive method for occult SRC carcinoma detection when compared to PTG (39,46,47). In fact, gastrectomy explants from CDH1 variant carriers are reported to harbor SRC in 80–100% of specimens, even those with negative endoscopic surveillance biopsies (48-53). The high false-negative rate of SRC detection with Cambridge method of surveillance was addressed using the Bethesda protocol developed by Curtin et al. (46). This protocol utilizes 88 non-targeted biopsies total, which are obtained from twenty-two individual anatomic sites (46). While the cohort reported initially was small, the Bethesda method resulted in a 38% false-negative SRC detection rate compared to 80% with the Cambridge method (46,54). The Cambridge group recently reported their 16-year experience with surveillance endoscopy and reported a sensitivity of 67.3% and a specificity of 90.2% for detecting occult gastric SRC carcinoma (38). Another study by Benesch et al revealed no difference between cancer rates in biopsy positive and biopsy negative groups, although the median number of biopsies was greater in the biopsy positive group (41). Adjuncts to white light endoscopy have been explored but have not proved useful to date. A single-institution phase II clinical trial evaluated the use of a probe-based confocal endomicroscopy (pCLE) compared to the Cambridge method with the aim of improving detection of occult SRC. This study demonstrated an improvement of false-negative SRC detection rate of 67% using pCLE compared to 87% when using the original Cambridge method, however the study was small and did not show clinically meaningful differences in detection (53).
The only comparatively large study of endoscopic surveillance to date demonstrated that endoscopic surveillance can be safe for patients who decline PTG (55). This prospective study challenges the urgency of upfront gastrectomy in patients with CDH1 variants and SRC detected on random biopsy. However, despite this recent evidence supporting the potential efficacy of endoscopic surveillance, PTG remains the recommendation for CDH1 pathogenic variant carriers with a family history of DGC.
PTG
Before undertaking PTG, patients deserve comprehensive consultation and evaluation of operative risks, long-term consequences, and thorough examination of psychosocial and medical comorbidities. The prevailing consensus advises patients with CDH1 pathologic variants to undergo prophylactic gastrectomy as early as age 20, with attention given to social and psychological factors potentially influencing the surgical decision. Total gastrectomy constitutes a life-altering intervention, as it imposes considerable dietary restrictions and necessitates lifelong nutritional supplementation. Consequently, it is paramount that patients receive dietary counseling, maintain a family or peer support network, and comprehend the surgery’s repercussions fully.
Acute perioperative risks of total gastrectomy include anastomotic leaks and intra-abdominal abscesses, among others, whereas mortality remains a rare occurrence. Chronic sequelae encompass weight loss, micronutrient deficiencies, bile acid reflux, dumping syndrome, osteopenia/osteoporosis, and esophageal dysmotility. One cohort study disclosed that approximately 60% of patients reported bile reflux within two years post-operatively: with more patients experiencing bile reflux at 1 year than 3 months post-operatively (56). Weight loss typically reaches a plateau at six months post-gastrectomy; however, patients typically weigh 20% less than their preoperative weight one-year post-gastrectomy (43,57-59). One study demonstrated a supplementary 4% weight gain for patients with extended follow-up, resulting in an overall median weight loss of 15% from preoperative weight (59). Consequently, it is crucial to screen patients for eating disorders before undergoing gastrectomy.
Laszkowska et al. investigated the optimal timing of total gastrectomy to prevent DGC, estimating the optimal age for PTG at 39 years for men and 30 years for women when comparing quality-adjusted life-years, cancer mortality, and life expectancy (60). However, caution is advised when interpreting this study, as it employed mathematical modeling based on cancer penetrance estimates affected by ascertainment bias. The onset of advanced DGC in individuals with CDH1 variants can happen at almost any age, the causes of which are still unknown.
Once the decision to pursue PTG is solidified, CDH1 variant carriers should be directed to high-volume gastrectomy centers under the guidance of a surgical oncologist. Total gastrectomy may be executed laparoscopically or via open surgery, depending on the surgeon’s preference. Laparoscopic surgery may afford patients a shorter initial hospital stay, but no approach has demonstrated long-term outcome superiority (61). For complete gastric cancer risk reduction, total stomach removal is essential. Intraoperative biopsies of the esophageal and duodenal margins must be confirmed free of gastric mucosa before concluding the procedure to verify complete resection of gastric mucosa. It is important to note that while the gastric explant may appear grossly normal, 89–95% of patients are found to harbor multi-focal superficial diffuse invasive SRCC (62,63).
Post-operatively, patients adhere to stringent dietary practices with specific micronutrient supplementation and should have access to a knowledgeable dietitian following hospital discharge. Close clinical follow-up is imperative, with the author’s recommending clinical assessments at 3-month intervals post-gastrectomy for the first year, biannually the subsequent year, and annually thereafter. However, no official follow-up guidelines exist. Patients should be evaluated for common functional complaints, such as bile reflux, dumping, and signs of pancreatic insufficiency, which may necessitate pancreatic enzyme supplementation (56). Despite post-operative symptom burden, a quality-of-life study in British Columbia and Newfoundland uncovered a cohort of CDH1 patients largely satisfied with their quality of life (QOL) following prophylactic gastrectomy (64). In fact, the mean QOL was 70.6, which was comparable to the QOL of the general population of Sweden and Norway (71.2), and remarkably better than patients with gastric cancer (65,66). A detailed pre-operative assessment of individual risks is essential due to the risks associated with surgery. Nevertheless, prophylactic gastrectomy remains the recommendation for individuals with pathologic CDH1 variants and a family history of DGC.
Advanced-stage HDGC
Advanced stages of HDGC carry poor prognosis due to the relative resistance of diffuse-type gastric cancers to existing systemic therapies. Although the overall 5-year survival rate for patients with advanced HDGC is reported at 4%, compared to 13% in patients with sporadic disease, the overall prognosis and treatment options remain the same (10,67). Just as with sporadic cases of diffuse-type gastric cancer, advanced stages of HGDC manifest as linitis plastica, characterized by diffuse infiltration of the stomach with poorly cohesive cancer cells with occasional SRC morphology (66). Interestingly, mutations in RHOA are predominantly found in advanced DGC (33,68). RHOA mutations are responsible for dysregulation of the actomyosin cytoskeleton during early DGC development and have a cumulative malignant effect when occurring with a CDH1 mutation (33,69). For patients without metastases, surgical resection with perioperative or adjuvant chemotherapy is recommended. Metastatic HDGC should be treated according to current treatment guidelines as for sporadic gastric carcinoma (21,70). Notably, HDGC, like many sporadic diffuse-type gastric cancers, rarely overexpresses human epidermal growth factor receptor 2 (HER2) and is considered genomically stable with a low tumor mutational burden (71). Furthermore, these cancers often lack markers indicative of response to current immunotherapy regimens. Clinical trials investigating treatments for isolated peritoneal metastases such as normothermic intraperitoneal chemotherapy, hyperthermic intraperitoneal chemotherapy, and pressurized intraperitoneal aerosolized chemotherapy have been employed for sporadic DGC and HDGC (72-75). Overall, the prognosis for advanced HDGC is dismal, emphasizing the importance of early recognition and genetic testing, enhanced surveillance, and risk-reducing surgery.
Secondary cancers
Germline CDH1 variants also carry an estimated 42% lifetime risk of invasive LBC in women (6). Hereditary lobular breast cancer (HLBC) is defined in patients with a CDH1 variant and LBC, and/or a positive family history of LBC, but without a family history of DGC. The most recent clinical management guidelines for HDGC included specific guidance for women with CDH1 variants at risk for LBC (6). Breast cancer surveillance is advised to commence at age 30 with an annual breast magnetic resonance imaging (MRI). E-cadherin deficient invasive lobular carcinoma does not form well-defined masses or reliable microcalcifications that can be easily picked up on mammography, but rather infiltrates the tissue in single file sheets or cords (8,76). When patients reach 35 years of age, a breast MRI should be continued alongside standard mammography (77). Although bilateral risk-reducing mastectomy can be considered for patients with CDH1, recent guidelines suggest breast-conserving therapy may be sufficient for this patient population (5). However, the appropriateness of breast-conserving therapy for women at risk for multicentric and bilateral breast cancers remains to be determined. For women with CDH1 germline variants, the average age onset for LBC is 53 years of age (78). Interestingly, two studies by Benusiglio et al. and Silva et al. suggest that early-onset LBC might be the first manifestation of HDGC, making early surveillance crucial (79,80). Another report by Benesch et al. examined cases of sporadic gastric SRC in the Surveillance, Epidemiology, and End Results (SEER) data set to estimate the secondary cancer risk in CDH1 variant carriers and described an increased rate of LBC. Nonetheless, it is important to note that HDGC accounts for approximately 1–3% of all gastric cancers, therefore patients with SRC gastric cancers, regardless of CDH1 mutation status, may be at increased risk for LBC due to as yet unknown causes (41).
In addition to HLBC, other cancers associated with germline CDH1 variants have been investigated. One case report by Hamilton et al. described a synchronous SRC carcinoma of the appendix in a patient with CDH1 and gastric cancer (81). Another study reported signet ring colon cancer in 3 out of 79 patients with CDH1 pathogenic variants (23). However, using SEER data, no difference in colon cancer risk for CDH1 carriers was identified (82). Although there is no direct evidence linking colorectal cancer with an increased risk in CDH1 variant carriers, families should receive individualized counseling. In families with CDH1 pathogenic variants and a clustering of colon cancer cases, screening colonoscopy at a younger age may be advised.
Strengths and limitations
This review presents several strengths and limitations in its synthesis of the literature surrounding CDH1 and HDGC. A key limitation is that most existing literature is based on small case series, many of which have been updated more than once. Many reviews exist and likely outnumber original research articles. A strength of this review is the examination of the diverse aspects of HDGC, encompassing genetic factors, surveillance methods, surgical interventions, secondary malignancies, and clinical management guidelines. However, this review does not offer a systematic evaluation of the quality of the included studies because high-quality research is lacking in for this rare disease. Despite these limitations, this review provides a valuable overview of the role of CDH1 in HDGC, emphasizing the importance of early recognition, genetic testing, and tailored clinical management for affected individuals.
Conclusions
In conclusion, germline CDH1 variants are the primary cause of elevated lifetime risk of DGC and LBC. The aggressive biology and poor prognosis of HDGC necessitate accurate diagnosis with genetic testing and appropriate clinical intervention. PTG for CDH1 variant carriers who have a family history of DGC is recommended. While endoscopic surveillance offers a viable alternative for those who are unable or unwilling to undergo surgery, further studies are needed to apply this strategy more broadly. Furthermore, breast cancer risk is significantly elevated in women with CDH1 variants, therefore enhanced breast cancer surveillance and risk-reducing mastectomy should be discussed with affected individuals. As our understanding of CDH1-associated malignancies evolves, it is vital to continue refining clinical management guidelines to optimize outcomes for individuals carrying pathogenic variants. Advances in research methodologies, such as the use of organoids to model HDGC, have expanded our knowledge of the disease and provided invaluable insights into the underlying cancer biology.
Acknowledgments
Funding: This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Adam Yopp and Matthew Porembka) for the series “Multimodal Management of Gastric and Gastroesophageal Cancers” published in Chinese Clinical Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-36/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-36/coif). The series “Multimodal Management of Gastric and Gastroesophageal Cancers” was commissioned by the editorial office without any funding or sponsorship. SNG and JLD report that this research was supported in part by the Intramural Research Program of the NIH. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine 2022;47:101404. [Crossref] [PubMed]
- Oliveira C, Pinheiro H, Figueiredo J, et al. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol 2015;16:e60-70. [Crossref] [PubMed]
- Jones EG. Familial gastric cancer. N Z Med J 1964;63:287-96. [PubMed]
- Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998;392:402-5. [Crossref] [PubMed]
- Blair VR, McLeod M, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 2020;21:e386-97. [Crossref] [PubMed]
- Hansford S, Kaurah P, Li-Chang H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol 2015;1:23-32. [Crossref] [PubMed]
- Cisco RM, Ford JM, Norton JA. Hereditary diffuse gastric cancer: implications of genetic testing for screening and prophylactic surgery. Cancer 2008;113:1850-6. [Crossref] [PubMed]
- Gamble LA, Heller T, Davis JL. Hereditary Diffuse Gastric Cancer Syndrome and the Role of CDH1: A Review. JAMA Surg 2021;156:387-92. [Crossref] [PubMed]
- Rocha JP, Gullo I, Wen X, et al. Pathological features of total gastrectomy specimens from asymptomatic hereditary diffuse gastric cancer patients and implications for clinical management. Histopathology 2018;73:878-86. [Crossref] [PubMed]
- van der Post RS, Vogelaar IP, Manders P, et al. Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1. Gastroenterology 2015;149:897-906.e19. [Crossref] [PubMed]
- Liu K, Wan J, Bei Y, et al. Prognostic Impact of Different Histological Types on Gastric Adenocarcinoma: a Surveillance, Epidemiology, and End Results Database Analysis. Pathol Oncol Res 2017;23:881-7. [Crossref] [PubMed]
- Berx G, Becker KF, Höfler H, et al. Mutations of the human E-cadherin (CDH1) gene. Hum Mutat 1998;12:226-37. [Crossref] [PubMed]
- Norton JA, Ham CM, Van Dam J, et al. CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann Surg 2007;245:873-9. [Crossref] [PubMed]
- Shapiro L, Fannon AM, Kwong PD, et al. Structural basis of cell-cell adhesion by cadherins. Nature 1995;374:327-37. [Crossref] [PubMed]
- Nagar B, Overduin M, Ikura M, et al. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature 1996;380:360-4. [Crossref] [PubMed]
- Godwin TD, Kelly ST, Brew TP, et al. E-cadherin-deficient cells have synthetic lethal vulnerabilities in plasma membrane organisation, dynamics and function. Gastric Cancer 2019;22:273-86. [Crossref] [PubMed]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996;84:345-57. [Crossref] [PubMed]
- Fleming TP, Javed Q, Hay M. Epithelial differentiation and intercellular junction formation in the mouse early embryo. Dev Suppl 1992;105-12. [Crossref] [PubMed]
- Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol 2004;14:589-93. [Crossref] [PubMed]
- Luo W, Fedda F, Lynch P, et al. CDH1 Gene and Hereditary Diffuse Gastric Cancer Syndrome: Molecular and Histological Alterations and Implications for Diagnosis And Treatment. Front Pharmacol 2018;9:1421. [Crossref] [PubMed]
- Cosma LS, Schlosser S, Tews HC, et al. Hereditary Diffuse Gastric Cancer: Molecular Genetics, Biological Mechanisms and Current Therapeutic Approaches. Int J Mol Sci 2022;23:7821. [Crossref] [PubMed]
- Melo S, Figueiredo J, Fernandes MS, et al. Predicting the Functional Impact of CDH1 Missense Mutations in Hereditary Diffuse Gastric Cancer. Int J Mol Sci 2017;18:2687. [Crossref] [PubMed]
- Adib E, El Zarif T, Nassar AH, et al. CDH1 germline variants are enriched in patients with colorectal cancer, gastric cancer, and breast cancer. Br J Cancer 2022;126:797-803. [Crossref] [PubMed]
- Oliveira C, Sousa S, Pinheiro H, et al. Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression. Gastroenterology 2009;136:2137-48. [Crossref] [PubMed]
- Becker KF, Atkinson MJ, Reich U, et al. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 1994;54:3845-52. [PubMed]
- Birchmeier W, Hülsken J, Behrens J. E-cadherin as an invasion suppressor. Ciba Found Symp 1995;189:124-36; discussion 136-41, 174-6.
- Carneiro F, Huntsman DG, Smyrk TC, et al. Model of the early development of diffuse gastric cancer in E-cadherin mutation carriers and its implications for patient screening. J Pathol 2004;203:681-7. [Crossref] [PubMed]
- Flanagan DJ, Austin CR, Vincan E, et al. Wnt Signalling in Gastrointestinal Epithelial Stem Cells. Genes (Basel) 2018;9:178. [Crossref] [PubMed]
- Cheng XX, Wang ZC, Chen XY, et al. Correlation of Wnt-2 expression and beta-catenin intracellular accumulation in Chinese gastric cancers: relevance with tumour dissemination. Cancer Lett 2005;223:339-47. [Crossref] [PubMed]
- Humar B, Blair V, Charlton A, et al. E-cadherin deficiency initiates gastric signet-ring cell carcinoma in mice and man. Cancer Res 2009;69:2050-6. [Crossref] [PubMed]
- Shimada S, Mimata A, Sekine M, et al. Synergistic tumour suppressor activity of E-cadherin and p53 in a conditional mouse model for metastatic diffuse-type gastric cancer. Gut 2012;61:344-53. [Crossref] [PubMed]
- Dixon K, Brew T, Farnell D, et al. Modelling hereditary diffuse gastric cancer initiation using transgenic mouse-derived gastric organoids and single-cell sequencing. J Pathol 2021;254:254-64. [Crossref] [PubMed]
- Monster JL, Kemp LJS, Gloerich M, et al. Diffuse gastric cancer: Emerging mechanisms of tumor initiation and progression. Biochim Biophys Acta Rev Cancer 2022;1877:188719. [Crossref] [PubMed]
- Nanki K, Toshimitsu K, Takano A, et al. Divergent Routes toward Wnt and R-spondin Niche Independency during Human Gastric Carcinogenesis. Cell 2018;174:856-869.e17. [Crossref] [PubMed]
- Togasaki K, Sugimoto S, Ohta Y, et al. Wnt Signaling Shapes the Histologic Variation in Diffuse Gastric Cancer. Gastroenterology 2021;160:823-30. [Crossref] [PubMed]
- Benusiglio PR, Colas C, Guillerm E, et al. Clinical implications of CTNNA1 germline mutations in asymptomatic carriers. Gastric Cancer 2019;22:899-903. [Crossref] [PubMed]
- Fewings E, Larionov A, Redman J, et al. Germline pathogenic variants in PALB2 and other cancer-predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: a whole-exome sequencing study. Lancet Gastroenterol Hepatol 2018;3:489-98. [Crossref] [PubMed]
- Lee CYC, Olivier A, Honing J, et al. Endoscopic surveillance with systematic random biopsy for the early diagnosis of hereditary diffuse gastric cancer: a prospective 16-year longitudinal cohort study. Lancet Oncol 2023;24:107-16. [Crossref] [PubMed]
- Jacobs MF, Dust H, Koeppe E, et al. Outcomes of Endoscopic Surveillance in Individuals With Genetic Predisposition to Hereditary Diffuse Gastric Cancer. Gastroenterology 2019;157:87-96. [Crossref] [PubMed]
- Mi EZ, Mi EZ, di Pietro M, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc 2018;87:408-18. [Crossref] [PubMed]
- Benesch MGK, Bursey SR, O'Connell AC, et al. CDH1 Gene Mutation Hereditary Diffuse Gastric Cancer Outcomes: Analysis of a Large Cohort, Systematic Review of Endoscopic Surveillance, and Secondary Cancer Risk Postulation. Cancers (Basel) 2021;13:2622. [Crossref] [PubMed]
- Hebbard PC, Macmillan A, Huntsman D, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol 2009;16:1890-5. [Crossref] [PubMed]
- Chen Y, Kingham K, Ford JM, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol 2011;18:2594-8. [Crossref] [PubMed]
- Pandalai PK, Lauwers GY, Chung DC, et al. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery 2011;149:347-55. [Crossref] [PubMed]
- Hüneburg R, Marwitz T, van Heteren P, et al. Chromoendoscopy in combination with random biopsies does not improve detection of gastric cancer foci in CDH1 mutation positive patients. Endosc Int Open 2016;4:E1305-10. [Crossref] [PubMed]
- Curtin BF, Gamble LA, Schueler SA, et al. Enhanced endoscopic detection of occult gastric cancer in carriers of pathogenic CDH1 variants. J Gastroenterol 2021;56:139-46. [Crossref] [PubMed]
- Corso G, Montagna G, Figueiredo J, et al. Hereditary Gastric and Breast Cancer Syndromes Related to CDH1 Germline Mutation: A Multidisciplinary Clinical Review. Cancers (Basel) 2020;12:1598. [Crossref] [PubMed]
- Kumar S, Long JM, Ginsberg GG, et al. The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome. World J Gastroenterol 2019;25:2878-86. [Crossref] [PubMed]
- Lim YC, di Pietro M, O'Donovan M, et al. Prospective cohort study assessing outcomes of patients from families fulfilling criteria for hereditary diffuse gastric cancer undergoing endoscopic surveillance. Gastrointest Endosc 2014;80:78-87. [Crossref] [PubMed]
- Shaw D, Blair V, Framp A, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut 2005;54:461-8. [Crossref] [PubMed]
- Friedman M, Adar T, Patel D, et al. Surveillance Endoscopy in the Management of Hereditary Diffuse Gastric Cancer Syndrome. Clin Gastroenterol Hepatol 2021;19:189-91. [Crossref] [PubMed]
- DiBrito SR, Blair AB, Prasath V, et al. Total Gastrectomy for CDH-1 Mutation Carriers: An Institutional Experience. J Surg Res 2020;247:438-44. [Crossref] [PubMed]
- Schueler SA, Gamble LA, Curtin BF, et al. Evaluation of confocal laser endomicroscopy for detection of occult gastric carcinoma in CDH1 variant carriers. J Gastrointest Oncol 2021;12:216-25. [Crossref] [PubMed]
- Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol 2013;26:11-22. [PubMed]
- Asif B, Sarvestani AL, Gamble LA, et al. Cancer surveillance as an alternative to prophylactic total gastrectomy in hereditary diffuse gastric cancer: a prospective cohort study. Lancet Oncol 2023;24:383-91. [Crossref] [PubMed]
- van der Kaaij RT, van Kessel JP, van Dieren JM, et al. Outcomes after prophylactic gastrectomy for hereditary diffuse gastric cancer. Br J Surg 2018;105:e176-82. [Crossref] [PubMed]
- Strong VE, Gholami S, Shah MA, et al. Total Gastrectomy for Hereditary Diffuse Gastric Cancer at a Single Center: Postsurgical Outcomes in 41 Patients. Ann Surg 2017;266:1006-12. [Crossref] [PubMed]
- Davis JL, Ripley RT. Postgastrectomy Syndromes and Nutritional Considerations Following Gastric Surgery. Surg Clin North Am 2017;97:277-93. [Crossref] [PubMed]
- Stillman MD, Kusche N, Toledano S, et al. Short and long-term outcomes of prophylactic total gastrectomy in 54 consecutive individuals with germline pathogenic mutations in the CDH1 gene. J Surg Oncol 2022;126:1413-22. [Crossref] [PubMed]
- Laszkowska M, Silver ER, Schrope B, et al. Optimal Timing of Total Gastrectomy to Prevent Diffuse Gastric Cancer in Individuals With Pathogenic Variants in CDH1. Clin Gastroenterol Hepatol 2020;18:822-829.e4. [Crossref] [PubMed]
- Haverkamp L, Weijs TJ, van der Sluis PC, et al. Laparoscopic total gastrectomy versus open total gastrectomy for cancer: a systematic review and meta-analysis. Surg Endosc 2013;27:1509-20. [Crossref] [PubMed]
- Forrester JD, Foster D, Ford JM, et al. Surgery for Hereditary Diffuse Gastric Cancer: Long-Term Outcomes. Cancers (Basel) 2022;14:728. [Crossref] [PubMed]
- Vos EL, Salo-Mullen EE, Tang LH, et al. Indications for Total Gastrectomy in CDH1 Mutation Carriers and Outcomes of Risk-Reducing Minimally Invasive and Open Gastrectomies. JAMA Surg 2020;155:1050-7. [Crossref] [PubMed]
- Kaurah P, Talhouk A, MacMillan A, et al. Hereditary diffuse gastric cancer: cancer risk and the personal cost of preventive surgery. Fam Cancer 2019;18:429-38. [Crossref] [PubMed]
- Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer 2001;37:1331-4. [Crossref] [PubMed]
- Irene Gullo CO, van der Post RS, van Dieren JM, et al. Updated perspective and directions on hereditary diffuse gastric cancer. Research and Clinical Applications of Targeting Gastric Neoplasms. Elsevier Inc.; 2021.
- Han MA, Oh MG, Choi IJ, et al. Association of family history with cancer recurrence and survival in patients with gastric cancer. J Clin Oncol 2012;30:701-8. [Crossref] [PubMed]
- Kakiuchi M, Nishizawa T, Ueda H, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet 2014;46:583-7. [Crossref] [PubMed]
- Zhang H, Schaefer A, Wang Y, et al. Gain-of-Function RHOA Mutations Promote Focal Adhesion Kinase Activation and Dependency in Diffuse Gastric Cancer. Cancer Discov 2020;10:288-305. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015;18:476-84. [Crossref] [PubMed]
- Bonnot PE, Piessen G, Kepenekian V, et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol 2019;37:2028-40. [Crossref] [PubMed]
- Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370-7. [Crossref] [PubMed]
- Rau B, Brandl A, Piso P, et al. Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer 2020;23:11-22. [Crossref] [PubMed]
- Alyami M, Bonnot PE, Mercier F, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur J Surg Oncol 2021;47:123-7. [Crossref] [PubMed]
- Michael M, Garzoli E, Reiner CS. Mammography, sonography and MRI for detection and characterization of invasive lobular carcinoma of the breast. Breast Dis 2008-2009;30:21-30. [Crossref] [PubMed]
- van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 2015;52:361-74. [Crossref] [PubMed]
- Pharoah PD, Guilford P, Caldas C, et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001;121:1348-53. [Crossref] [PubMed]
- Benusiglio PR, Malka D, De Pauw A, et al. Germline Mutations in CDH1 and the Hereditary Diffuse Gastric and Lobular Breast Cancer Syndrome. Ann Oncol 2012;23:ix175-ix177. [Crossref]
- Silva R, Susy C, Carneiro F, et al. Genetic profile and morphological features of breast cancers in the setting of Hereditary Breast Cancer (HBC) syndrome. Virchows Arch 2014;465:S111.
- Hamilton LE, Jones K, Church N, et al. Synchronous appendiceal and intramucosal gastric signet ring cell carcinomas in an individual with CDH1-associated hereditary diffuse gastric carcinoma: a case report of a novel association and review of the literature. BMC Gastroenterol 2013;13:114. [Crossref] [PubMed]
- Roberts ME, Ranola JMO, Marshall ML, et al. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol 2019;5:1325-31. [Crossref] [PubMed]



