
Geographical, ethnic, and genetic differences in pancreatic cancer predisposition
Background
Pancreatic ductal adenocarcinoma (PDAC) consists of 85–90% of pancreatic neoplasms and is the most common histologic subtype. Globally, there has been an increasing burden of PDAC (1) over the years—this is expected to continue with a shift in lifestyle habits, ageing populations, and improved diagnostic tools. Despite medical advancements, PDAC is often diagnosed late with locally advanced, unresectable, or metastatic disease with poor clinical outcomes. It remains the seventh leading cause of worldwide cancer-related deaths (2) in 2020. An understanding of risk factors for PDAC is crucial for early detection and treatment.
Predisposition to PDAC can be attributed to modifiable and non-modifiable risk factors, including genetic factors. Ethnicity and geography affect the prevalence and significance of each risk factor and should be taken into consideration for effective screening and prevention programmes.
This review aims to describe the differences in ethnic and geographical distribution of PDAC and provide a comprehensive overview of the modifiable and non-modifiable risk factors and genetic predisposition syndromes for PDAC. It will highlight recent findings of interest, point out gaps in understanding, and suggest potential ways forward to improve screening and outcomes for patients with PDAC.
Incidence and mortality
Geography
Worldwide, high-income regions such as Europe, North America, Australia/New Zealand and Japan have higher incidence of PDAC ranging from 7.9 to 9.9 per 100,000 people (Figures 1,2). Conversely, low-income regions of Africa, Central America and South Asia have the lowest incidence at 1.5 to 4.6 per 100,000 people (2). This difference can be due to increased prevalence of risk factors associated with higher incomes such as alcohol use, obesity and diabetes, as well as ageing populations (1). This could also be confounded by the scarcity of high-quality data in low-income regions due to reduced access to advanced diagnostic tools and imaging, thus potentially causing a discrepancy in actual epidemiology.

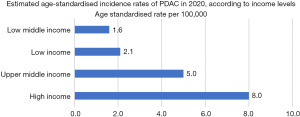
Ethnicity
Within a geographic region, there is a difference in the incidence of PDAC amongst different ethnic groups. In the United States, many studies have identified a higher incidence of PDAC in non-Hispanic African populations compared to non-Hispanic European populations (3,4). Despite lower income levels amongst non-Hispanic Africans compared to non-Hispanic Europeans, differences in diet and lifestyle lead to higher rates of PDAC risk factors such as smoking, diabetes and obesity (5) in the non-Hispanic African group. However, a study done by Huang et al. (6) showed that non-Hispanic Africans had a 20% greater risk of PDAC compared to non-Hispanic Europeans even after adjusting for dietary and lifestyle differences, thus alluding to other factors at play. One such factor could be that of biological differences that cause varying susceptibility to developing PDAC—research suggests that the non-Hispanic Africans are slower at metabolizing carcinogens from tobacco (7), and that PDAC in non-Hispanic Africans have increased K-ras mutations (8).
Socioeconomic factors also result in poorer overall survival for non-Hispanic Africans—a recent study has also showed that non-Hispanic Africans with PDAC had lower education and income level compared to non-Hispanic Europeans, and this correlated with more advanced stage at diagnosis, a lower likelihood of receiving treatment, and a longer time to commencement of treatment (9).
There have been fewer studies examining the rate of PDAC in other ethnic minorities, such as in Hispanic and Asian populations (6). In one US-based study, Asian populations had lower rates of smoking and obesity compared with other ethnic groups, which may contribute to their lower pancreatic cancer rates. Asian populations also have a higher survival rate compared to non-Asian populations (10), and there can be genetic factors behind this. Secreted protein acidic and rich in cysteine (SPARC), a protein that has been found to independently predict for poor disease-free survival and overall survival for patients with PDAC, was found by a recent study to have a lower stromal expression in Japanese patients and could be a potential factor contributing to better outcomes in this Asian population (11,12).
Trends
Incidence of PDAC has been on a gradual uptrend, with cases rising from 460,000 worldwide in 2018 to 496,000 in 2020 (2). Both the incidence and mortality of PDAC are expected to rise, and this likely has to do with its associated risk factors—ageing populations, lifestyle changes such as smoking, reduced physical activity and consumption of calorie-rich food (13). Improved diagnostic tools and technology, especially in developing regions, are also detecting more cases that would have otherwise been missed. PDAC has been projected to surpass breast cancer as the third leading cause of cancer death by 2025 (14). Comparing the incidence of PDAC from 2018 to 2020, the global distribution of proportion of newly diagnosed PDAC remains similar.
Risk factors (Table 1)
Table 1
| Risk factor | Relative risk of PDAC | Geographical regions and ethnic groups with higher prevalence |
|---|---|---|
| Smoking | 2 (15,16) | Central Europe, Western Europe and Southern Latin America amongst females, and Southeast/East Asian and Oceania amongst males |
| Obesity and physical inactivity | 1.72 (17) | Americas, Europe |
| Heavy alcohol consumption | 1.15–1.6 (18-20) | Russia, Europe; East Asia: ALDH2*2 allele* |
| Ageing population | – | Europe, North America, East Asia |
| Male gender | 1.39 (2) | – |
| Diabetes mellitus | 1.82 (21) | North America, Russia, Asia (China, India, South-east Asia) |
| Chronic pancreatitis | Up to 16.16 (22) | Non-Hispanic Africans |
Smoking
Smoking is a notable risk factor for PDAC. Multiple studies have shown that there is an association between smoking and increased risk of death from PDAC, up to two times higher in smokers compared to non-smokers (15,16). The estimated attributable fraction of PDAC deaths due to smoking is 11–32% (25). Of note, the risk of PDAC decreases upon cessation of smoking—with a 30% risk reduction for pancreatic cancer with more than 10 years of cessation.
A systematic analysis in 2019 found that regions with the highest prevalence of smoking were that of Europe, Asia and Oceania, while the lowest prevalence of smoking were in Latin America and Sub-Saharan Africa (26). This distribution correlates well with the geographic distribution of PDAC, suggesting that smoking is indeed a strong independent risk factor.
Obesity and physical inactivity
There is a robust causal association between increasing body mass index (BMI) and PDAC risk [relative risk (RR) 1.72] (17). Overweight or obese individuals develop PDAC at a younger age, and have a lower overall survival rate (27). On the other hand, physical activity is inversely associated with risk of PDAC among individuals with a BMI of more than 25 kg/m2 (RR 0.59) (28). The global prevalence of obesity has doubled since 1980 (29)—as society gets increasingly re-engineered to minimize movement, sedentary lifestyles become easier to adopt and this poses an increasing health risk (30).
In a study that analyzed the epidemiology of obesity from 1980 to 2015 (29), the Americas and Europe emerged with the highest rates of obesity and Southeast Asia and West Pacific with the lowest, correlating closely with the pattern of incidence of PDAC. However, there were discrepancies such as countries like Austria and Japan, which had below-average obesity rates but high PDAC rates (2,31). These discrepancies could be due to other contributing risk factors such as higher alcohol consumption (32,33) and an ageing population (34) in these countries.
Alcohol
There is conflicting evidence regarding the association of alcohol intake and risk of PDAC. Several studies have shown that heavy alcohol consumption was associated with a 1.15 to 1.6 times increased risk of PDAC (18-20), but there is a lack of evidence to determine the association between low-to-moderate alcohol intake and PDAC. Liquor has been associated with PDAC more so than other types of alcohol. Increased alcohol consumption is also an established risk factor of pancreatitis (35), which in turn is a risk factor for PDAC.
Globally, the average per capita alcohol consumption has increased over the past two decades, with the lowest amount occurring in the Middle East and Northern Africa, and the highest in Russia and Europe. Interestingly, there is a decreasing trend of overall alcohol consumption in Europe and Russia and an increasing one in the Western Pacific and Southeast Asia regions, which is not in tandem with the trend in incidence of PDAC, suggesting again other confounding factors. In East Asian countries, 30–50% of the population carry the ALDH2*2 allele, which is associated with inefficient enzyme metabolism of acetaldehyde, a metabolite of ethanol. Individuals carrying the ALDH2*2 allele were found to have a higher risk of developing alcohol-related cancers such as pancreatic, oesophageal and liver cancer (23,24), suggesting that alcohol consumption may play a more significant role in PDAC development in the East Asian population.
Age
Pancreatic cancer incidence increases with age, with 90% of newly diagnosed patients aged 55 years and above (36), and the highest incidence of PDAC reported in people over 70 years old (37). It is estimated that the proportion of the world’s population over 60 years will double from 11% in 2015 to 22% in 2050 (38). Many aging populations around the world also have high PDAC rates—for example, Japan had the highest proportion of elderly aged 65 and above (28%), and the third highest incidence of PDAC (9.9 per 100,000 people) in 2020. Other countries like Germany, Malta and Hungary, with fast aging populations (20% and above) also had a high incidence of PDAC (8.8 per 100,000 people and above). Geographically, aging populations are concentrated in the regions of Europe, North America and Eastern Asia, which coincides with the geographical distribution of high PDAC incidence (2,39).
Gender
PDAC is more commonly found in males than females—this is consistent across various regions (Figure 3). The worldwide incidence of PDAC in 2020 is 5.7 per 100,000 for males and 4.1 per 100,000 for females (2). While the disparity could be attributed to differences in lifestyle factors, especially that of higher rates of smoking in men compared to women, there are intrinsic biological differences between the genders. Several studies suggest that the female sex hormone estrogen decreases pancreatic cancer growth (40-42), and a study using The Cancer Genome Atlas (TCGA) data has also revealed that there are distinct molecular differences between male and female patients across a broad range of cancer types (43). Another study found that Kaiso, a bi-modal transcription factor regulating gene expression, predicts for more aggressive pancreatic cancer when found in male versus female patients’ tumor samples (44).

Diabetes mellitus (DM)
DM is a well-established risk factor for PDAC. There is a bidirectional relationship between DM and PDAC. One meta-analysis revealed an odds ratio (OR) of 1.82 for PDAC in individuals with type 2 DM (21). Newly diagnosed diabetics are also at 50% higher risk of developing PDAC compared to those with long-standing diabetes (45), possibly due to DM being one of the early manifestations of PDAC. Furthermore, the mortality rate of diabetics is twice as high compared to non-diabetics. DM can be the first presentation, and complication of PDAC (46).
North America, South-east Asia, Russia and some Asian countries like China and India have the highest prevalence of DM correlating with lifestyle and dietary factors, whereas Europe and Oceania have a lower prevalence (47). This distribution differs from that of global PDAC incidence, in which Europe and Oceania had higher incidence of PDAC than South-east Asia, China and India.
Pancreatitis
Chronic pancreatitis is a strong risk factor for PDAC, due to progressive inflammation and fibrosis. Rijkers et al. reported that whilst patients with a first episode of acute pancreatitis had a 0.4% risk of developing PDAC, this risk increased 9-fold for patients who progress to chronic pancreatitis (48). This risk increases in the first 5 years after diagnosis of chronic pancreatitis, thereafter decreases, suggesting that the initial few years of diagnosis are crucial for close follow up (49).
Lew et al. (50) found that 0.78% of patients admitted for chronic pancreatitis in a United States-based population also had PDAC. Blacks, men, age 40–59, and being overweight were significantly associated with chronic pancreatitis. Interesting, non-Hispanic Africans had a higher risk for chronic pancreatitis which did not translate into having a higher association of chronic pancreatitis with PDAC. Patients who were found to have both chronic pancreatitis and PDAC were predominantly non-Hispanic Europeans who were overweight and of older age. This correlated with higher incomes, better chances of getting insured and higher rates of being admitted to large urban teaching hospitals in the non-Hispanic European population.
With regards to the etiology of chronic pancreatitis, Wilcox et al. (51) reported that non-Hispanic Africans were twice as likely as non-Hispanic Europeans to be diagnosed with chronic pancreatitis attributed to alcohol or smoking, while genetic, idiopathic and autoimmune etiologies were more significant in non-Hispanic Europeans. Non-Hispanic Africans also had a longer duration of disease (8.6 versus 6.97 years) and significantly higher frequencies of severe and consistent pain, disability, and advanced pancreatic morphological changes, demonstrating different degrees of access to healthcare according to ethnicity.
Genetics
Approximately 10% to 15% of PDAC has a familial and/or underlying genetic predisposition, of which familial pancreatic cancer (FPC) constitutes 7% and those with known genetic predisposition syndromes constitute 3% (52) (Table 2). The most frequent genetic alterations are of BRCA2, PALB2, ATM, and CDKN2A, with less common alterations including BRCA1, APC, MLH1, MSH2, MSH6, PMS2 and PRSS1. Of the patients with PDAC without significant family history, 5–8% are carriers of a germline mutation that predisposes to PDAC (64,65), explaining the trend and importance of multigene panel testing in patients diagnosed with FPC regardless of age or family history (22). In contrast, FPC is defined by PDAC developing in the context of a strong family history without a known causative germline pathogenic variant (PV) (66).
Table 2
| Gene | Relative risk of PDAC | Chromosome | Syndrome associated with increased risk of PDAC | Typical inheritance pattern | Phenotype |
|---|---|---|---|---|---|
| PRSS1 | 87 (53) | 7q35 | Hereditary pancreatitis | Autosomal dominant | Pancreatitis |
| SPINK1 | 5q32 | Hereditary pancreatitis | Autosomal dominant | Pancreatitis | |
| CFTR | 7q31.2 | Cystic fibrosis | Autosomal recessive | Pancreatitis, sinopulmonary disease, cirrhosis, infertility | |
| TP53 | 7.3 (54) | 17p13.1 | Li-Fraumeni syndrome | Autosomal dominant | Breast cancer, soft tissue sarcomas, osteosarcomas, adrenocortical carcinoma, central nervous system tumors |
| ATM | 2.41 (55) | 11q22 | Hereditary breast and ovarian cancer syndrome | Autosomal dominant | Multiple and early-onset breast and ovarian cancersPancreas, prostate, melanoma and gastric cancer |
| CDKN2A | 38 (56) | 9p21 | Familial atypical multiple mole melanoma | Autosomal dominant | Multiple atypical naevi progressing to melanomaBreast, lung and endometrial cancer |
| STK11 | 132 (57) | 19p13.3 | Peutz-Jeghers syndrome | Autosomal dominant | Gastrointestinal hamartomatous polyps, mucocutaneous pigmentationBreast, colon, pancreatic, stomach, ovarian cancer |
| BRCA1, BRCA2 | 3.1 (58), 3.51–4.1 (59,60), up to 21.7 (61) | 17q12-21; 13q12-13 | Hereditary breast and ovarian cancer syndrome | Autosomal dominant | Multiple and early-onset breast and ovarian cancersPancreas, prostate, melanoma and gastric cancer |
| PALB2 | 6 (62) | 16p12.2 | Hereditary breast and ovarian cancer syndrome | Autosomal dominant | Multiple and early-onset breast and ovarian cancersPancreas, prostate, melanoma and gastric cancer |
| MLH1, MSH2, MSH6, PMS2 | 8.6 (63) | 3p21.3, 2p22-p21, 2p16, 7p22 | Hereditary non-polyposis colorectal cancer aka Lynch syndrome | Autosomal dominant | Colorectal cancer, extra-colorectal cancers—pancreatic, endometrial, ovarian, stomach, bile duct, small bowel, pancreatic, ureter and renal pelvis cancerMuir-Torre syndrome: skin cancer (sebaceous tumors)Turcot syndrome: central nervous system tumors |
PDAC, pancreatic ductal adenocarcinoma.
With time, germline PV may result in carcinogenesis due to the mechanisms of cell injury, dysregulation of cell growth, dysfunctional DNA repair, and disruption of cell mobility and adhesion.
Cell injury
Hereditary pancreatitis accounts for a very small fraction of PDAC but is associated with a markedly increased risk of PDAC (53), as chronic inflammation leads to accelerated mutation accumulation and clonal expansion. Increasingly more germline PV have been implicated in hereditary pancreatitis that progress into PDAC, the most well-studied being PRSS1, SPINK1, and CFTR alterations. Affected individuals develop chronic pancreatitis before the age of 20 and lifetime risk of PDAC is 25% to 44%, with a RR of 87 for developing PDAC (53,67).
PRSS1
PRSS1 on chromosome 7q35 is encoded by the cationic trypsinogen gene. Gain-of-function PRSS1 variants are associated with autosomal dominant (AD) hereditary pancreatitis, with variable penetrance rates of 40–93% depending on variant (68-70). PRSS1-related hereditary pancreatitis has a prevalence of up to 12.4% in populations with chronic pancreatitis (71).
SPINK1
SPINK1 on chromosome 5q32 codes for serine peptidase inhibitor Kazal type 1. It is upregulated with inflammation to protect the pancreas from autodigestion by trypsin and other pancreatic enzymes. SPINK1 germline mutation related-pancreatitis is associated with 12 times higher rate of PDAC than patients with idiopathic pancreatitis (72).
CFTR
CFTR on chromosome 7q31.2 codes for the cystic fibrosis transmembrane conductance regulator protein. Mutations in CFTR cause classic cystic fibrosis, an autosomal recessive disorder in which chloride and bicarbonate conductance is impaired, resulting in viscous fluid secretion in organs leading to sinopulmonary disease, cirrhosis, and infertility. In the pancreas, this causes retained trypsin and hence pancreatic inflammation. Heterozygous CFTR carriers also have an increased risk of recurrent acute and chronic pancreatitis. A study by Hamoir et al. (73) reported that those with CFTR-related chronic pancreatitis had a standardized incidence ratio (SIR) of 26.5 for PDAC.
The CFTR PVs occur most commonly in Europe, North America and Australia amongst European populations, but is rare amongst Asian populations (74). Its incidence likely remains underreported in regions such as Latin and South America, India and Africa due to lack of registries.
Cell growth
TP53
Tumor protein p53 (TP53) on chromosome 17p13.1 codes for a tumor suppressor that regulates cell proliferation, DNA repair and apoptosis in response to cellular stress. Mutations in TP53 cause Li-Fraumeni syndrome, an AD disorder characterized by high risk for cancer—often multiple and at early age (75). The most common tumors in children are osteosarcoma, adrenocortical carcinoma, central nervous system tumors, and soft tissue sarcoma, and in adults breast cancer in women and soft tissue sarcoma (76). Ruijs et al. estimated that the TP53 mutation concurs a RR of 7.3 for PDAC (54).
ATM
ATM on chromosome 11q22 codes for a protein kinase that regulates cell proliferation and detects DNA damage (77). Biallelic loss-of-function mutations of ATM result in Ataxia-telangiectasia (AT), a rare autosomal recessive disorder characterized by progressive ataxia, telangiectasias, immune deficiency, and increased risk of malignancies—particularly leukemias and lymphomas (78). Instead of having classic manifestations of AT, heterozygote carriers of the ATM mutation are at increased risk for coronary heart disease and solid organ malignancies, particularly that of breast and pancreatic cancer (79). In a study of 4,607 ATM PV carriers, carriers were at moderate-to-high risk for PDAC (OR 4.21) (55). A United Kingdom study of 1,160 individuals estimated that heterozygous carriers of ATM mutation have a RR of 2.41 for developing PDAC (80). ATM is a well-established breast cancer susceptibility gene, with heterozygote carriers having more than twice the risk of the average population of developing breast cancer and a cumulative lifetime breast cancer incidence of 20–40% (81). Mutations in ATM should be considered in patients with PDAC that have a family history of breast cancer.
CDKN2A
CDKN2A on chromosome 9p21 codes for proteins p16INK4A and p14ARF. Germline CDKN2A mutations are usually missense or nonsense variants (82), permitting inappropriate progression through the cell cycle. Prevalence of CDKN2A mutations in the general population is low at about 0.05% (83). Familial atypical multiple mole melanoma (FAMMM) is an AD condition associated with CDKN2A mutations, but with incomplete penetrance and variable expressivity. It is characterized by multiple atypical naevi progressing to melanoma (84), and increased risk for internal malignancies such as head and neck and esophageal squamous cell carcinomas, non-small cell lung cancers and pancreatic cancer (85). CDKN2A-associated FAMMM is associated with an elevated risk of developing PDAC, RR 13–22 (56), with variants affecting p16INK4A more frequently observed with pancreatic cancer compared to p14ARF (86).
Germline CDKN2A mutations are more prevalent in families in Europe, North America, and Australia (82), and in Dutch populations a CDKN2A mutation variant known as p16-Lieden is known to carry a particularly higher risk of PDAC, with a cumulative risk of 17% at 75 years of age (87).
STK11
STK11 (also known as LKB1) on chromosome 19p13.3 codes for a kinase that regulates AMP-activated protein kinase family members, which control multiple cellular processes including cell polarity, metabolism, and apoptosis (88). Mutations in STK11 cause Peutz-Jeghers syndrome (PJS), an AD disorder characterized by gastrointestinal hamartomatous polyps and mucocutaneous pigmentation. Individuals with PJS have elevated cancer risks, most commonly that of breast and colon cancer, followed by pancreatic, stomach and ovarian cancer. The cumulative risk of developing any cancer and specifically PDAC at 70 years of age is 85% and 11% respectively, with a RR of 132 of developing PDAC (89).
DNA repair
BRCA1 and BRCA2
BRCA1 and BRCA2 on chromosome 17q12-21 and 13q12-13 are DNA damage repair genes which code for proteins that function in homologous recombination repair (90,91). BRCA1 also functions in DNA damage signalling, chromatin remodelling, and transcriptional regulation. Mutations in BRCA1/2 can cause hereditary breast and ovarian cancer syndrome (HBOC), one of the more common referrals for cancer genetic testing (92). HBOC is an AD syndrome characterized by multiple and early-onset breast and ovarian cancers, and increased risk for other cancers such as pancreas, prostate and melanoma. The incidence of germline BRCA1/2 PVs in PDAC is 5–9% (93). BRCA2 is the most frequently mutated gene (6–19%) in patients with PDAC associated with germline mutations even in the absence of breast cancer (59). BRCA2 mutation confers a RR of 3.51–4.1 (59,60) for PDAC, with Mersch et al. even reporting an increased risk of PDAC up to 21.7 folds (61). On the other hand, BRCA1 mutation carriers have a lower predilection for pancreatic malignancy (59,61), with RR of 3.1 (58).
BRCA1/2 founder mutations have been identified in groups of Ashkenazi Jews, French Canadians, Hispanics, and African Americans, and in the geographical regions of Netherlands, Sweden, Hungary, Iceland, Italy, France, South Africa, Pakistan and Asia (94). An analysis of the POLO trial cohort revealed that 5.9% of people with previously unknown BRCA status had a newly identified BRCA mutation, with rates highest in the United States, France, and Israel at 9.5%, 7.6%, and 7.4%, respectively (95). The highest rate of newly identified BRCA mutation prevalence was observed in African American patients (10.7%), although this could have been confounded by a small population size and potential disparities in uptake of genetic testing. Biallelic mutations cause Fanconi anemia (96), a syndrome characterized by bone marrow failure, predisposition to malignancy particularly that of acute myeloid leukemia, and physical abnormalities including short stature, microcephaly, developmental delay, café-au-lait skin lesions, and malformations belonging to the VACTERL-H association.
PALB2
PALB2 on chromosome 16p12.2 encodes a protein that contributes to the cellular machinery for DNA repair by homologous recombination (97). Heterozygous mutations in carriers are significantly associated with breast cancers at an OR of 3.1 to 9.2 (98), which is comparable with that of BRCA1/2. Among the breast cancer susceptibility genes like BRCA1/2, PALB2 is also considered a high penetrance gene for breast cancer. Several studies have found that PALB2-mutated breast cancers are associated with aggressive features, such as higher rates of triple-negative phenotype, advanced disease stage, and higher Ki67 level (99). While the prevalence of PALB2 variants is not high, there is emerging evidence supporting PALB2 as a susceptibility gene for PDAC (100). PALB2 mutation confers a 6-fold increased PDAC risk (62), with a significantly earlier mean age of onset (101).
MLH1, MSH2, MSH6, PMS2, EPCAM
Mismatch repair (MMR) genes MLH1, MSH2, MSH6, PMS2, EPCAM maintain genomic integrity by correcting base substitution and small insertion-deletion mismatches during DNA replication. Lynch syndrome, also known as hereditary non-polyposis colorectal cancer (HNPCC), is an AD condition caused by mutations in the MMR genes, or deletion in the EPCAM gene resulting in silencing of the downstream MSH2 (102). Cancer develops according to the two-hit hypothesis, when the first allele is affected by the germline mutation and the second allele is inactivated by a somatic mutation, resulting in defective DNA repair and microsatellite instability. Affected individuals have an increased risk of colorectal cancer and other malignancies such as pancreatic, endometrial, ovarian, gastric, bile duct, small bowel, ureter and genitourinary cancers. The variants Muir-Torre syndrome and Turcot syndrome predispose to sebaceous tumors and central nervous system tumors (glioblastomas and astrocytomas) respectively (63,103). Kastrinos et al. described an increased PDAC risk by 8.6-fold and a cumulative PDAC risk of 3.7% at 70 years of age for individuals with HNPCC (63). There is recent evidence linking this increased risk of PDAC in HNPCC particularly with MLH1 PV carriers (104)—Møller et al. observed the cumulative incidence of PDAC to be 6.2% by 75 years of age in MLH1 PV carriers, compared to 0.5%, 1.4% and 0% for MSH2, MSH6 and PMS2 respectively.
Founder mutations of MMR genes have been found in Finland, Iceland, Ashkenazi Jews, French Canadian and Amish populations (105).
Cell mobility and adhesion
APC
APC on chromosome 5q21–22 codes for a tumor suppressor that helps to control cell proliferation, stabilize microtubules, and mediate cell migration and adhesion (106). PVs cause Familial adenomatous polyposis (FAP), an AD syndrome classically characterized by the development of hundreds to thousands of colorectal adenomas, typically by late adolescence, which inevitably progress to colon cancer without intervention. FAP is also associated with extracolonic tumors including hepatoblastoma, duodenal, thyroid, bile duct and brain adenocarcinoma. While FAP has historically been thought of as a predisposing condition for PDAC (107), the incidence of classical exocrine PDAC in this population has likely been overreported in literature and we now know that the association of APC mutations with PDAC is not strong (108).
FPC
FPC is defined as families with two or more first-degree relatives with PDAC in the absence of a known PDAC-associated hereditary syndrome (22). It consists of 7% of PDACs (66), suggesting that there is much more to be discovered about the genetic, epigenetic, and environmental factors that contribute to the development of PDAC. European registries have observed an anticipation phenomenon in FPC (109), and every year there have been more discoveries of potential predisposing germline PVs for PDAC.
Large-scale population-based genome-wide association studies have identified common variants in several genomic regions associated with PDAC risk, particularly in the European (110-112), Chinese (113) and Japanese (114,115) populations. These variants individually have a small effect on PDAC risk, but each additional copy of a risk allele is associated with a 10–30% increase in the risk of PDAC and the cumulative effects can be significant. Studies are underway to better understand the mechanisms underlying carcinogenesis and to increase the diversity of genomic studies of PDAC.
The International Cancer of the Pancreas Screening (CAPS) Consortium (116) has put forth consensus guidelines recommending that in addition to individuals with known germline mutations in susceptibility genes, individuals who are FPC kindred should also undergo pancreatic surveillance to detect early pancreatic cancer and its high-grade precursors. This criterion is met by having at least one first-degree relative with pancreatic cancer who in turn also has a first-degree relative with pancreatic cancer. A 2015 systematic review by Lu et al. (117) found that PDAC screening in individuals with FPC resulted in a higher curative resection rate (60% versus 25%) and longer median survival time (14.5 versus 4 months) compared with the control group. Canto et al. observed that most PDACs detected during surveillance of high-risk individuals with FPC were resectable (9 out of 10), and 85% of these patients survived for 3 years (118). This is in contrast to the general population that typically present late, with only 15% to 20% of patients being candidates for pancreatectomy (119).
Conclusions
In this review, we have examined and summarized the geographical, ethnic and genetic factors that predispose to PDAC carcinogenesis. Incidence of PDAC has been on an uptrend worldwide, with high-income regions such as Europe, North America, Australia/New Zealand and Japan and ethnicities such as the African population experiencing higher incidence rates of PDAC. This is a result of an interplay between varying prevalence and trends of certain established risk factors—including diabetes, obesity, aging, smoking, alcohol consumption and chronic pancreatitis. Genetic factors also play an important role in PDAC predisposition, including germline PDAC, familial basis, and epigenetics involvement. With increasing uptake of large-scale population-based genome-wide association studies, future efforts of research could be directed to identifying more predisposing genetic mutations and understanding their ethnic and geographical variations, as the knowledge base on this is at present still scarce. Early detection and treatment of PDAC results in significantly better outcomes yet the majority currently are only detected at a late and advanced stage. Future studies should consider detailed evaluation of interethnic, environmental, behavioural and genetic data to further elucidate discrepancies between different populations. From there, a better understanding of nuances pertaining to PDAC predisposition will allow more effective and efficient measures for individualised and prompt detection and treatment of PDAC, to improve outcomes for patients with PDAC.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-8/coif). ZZ received honorarium and overseas conference sponsorship from Astra Zeneca. JYYN received the research funding from Astra Zeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wong MCS, Jiang JY, Liang M, et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep 2017;7:3165. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Cervantes A, Waymouth EK, Petrov MS. African-Americans and Indigenous Peoples Have Increased Burden of Diseases of the Exocrine Pancreas: A Systematic Review and Meta-Analysis. Dig Dis Sci 2019;64:249-61. [Crossref] [PubMed]
- Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970-2009. J Natl Cancer Inst 2013;105:1694-700. [Crossref] [PubMed]
- Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis 2010;28:645-56. [Crossref] [PubMed]
- Huang BZ, Stram DO, Le Marchand L, et al. Interethnic differences in pancreatic cancer incidence and risk factors: The Multiethnic Cohort. Cancer Med 2019;8:3592-603. [Crossref] [PubMed]
- Muscat JE, Djordjevic MV, Colosimo S, et al. Racial differences in exposure and glucuronidation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cancer 2005;103:1420-6. [Crossref] [PubMed]
- Pernick NL, Sarkar FH, Philip PA, et al. Clinicopathologic analysis of pancreatic adenocarcinoma in African Americans and Caucasians. Pancreas 2003;26:28-32. [Crossref] [PubMed]
- Zhu F, Wang H, Ashamalla H. Racial and Socioeconomic Disparities in the Treatments and Outcomes of Pancreatic Cancer Among Different Treatment Facility Types. Pancreas 2020;49:1355-63. [Crossref] [PubMed]
- Gad MM, Găman MA, Saad AM, et al. Temporal trends of incidence and mortality in Asian-Americans with pancreatic adenocarcinoma: an epidemiological study. Ann Gastroenterol 2020;33:210-8. [Crossref] [PubMed]
- Murakawa M, Aoyama T, Miyagi Y, et al. The impact of SPARC expression on the survival of pancreatic ductal adenocarcinoma patients after curative resection. J Cancer 2019;10:627-33. [Crossref] [PubMed]
- Gundewar C, Sasor A, Hilmersson KS, et al. The role of SPARC expression in pancreatic cancer progression and patient survival. Scand J Gastroenterol 2015;50:1170-4. [Crossref] [PubMed]
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- Nguyen SM, Deppen S, Nguyen GH, et al. Projecting Cancer Incidence for 2025 in the 2 Largest Populated Cities in Vietnam. Cancer Control 2019;26:1073274819865274. [Crossref] [PubMed]
- Kuzmickiene I, Everatt R, Virviciute D, et al. Smoking and other risk factors for pancreatic cancer: a cohort study in men in Lithuania. Cancer Epidemiol 2013;37:133-9. [Crossref] [PubMed]
- Lin Y, Tamakoshi A, Kawamura T, et al. A prospective cohort study of cigarette smoking and pancreatic cancer in Japan. Cancer Causes Control 2002;13:249-54. [Crossref] [PubMed]
- Carreras-Torres R, Johansson M, Gaborieau V, et al. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J Natl Cancer Inst 2017;109:djx012. [Crossref] [PubMed]
- Wang YT, Gou YW, Jin WW, et al. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer 2016;16:212. [Crossref] [PubMed]
- Tramacere I, Scotti L, Jenab M, et al. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer 2010;126:1474-86. [Crossref] [PubMed]
- Rahman F, Cotterchio M, Cleary SP, et al. Association between alcohol consumption and pancreatic cancer risk: a case-control study. PLoS One 2015;10:e0124489. [Crossref] [PubMed]
- Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076-83. [Crossref] [PubMed]
- Stoffel EM, McKernin SE, Brand R, et al. Evaluating Susceptibility to Pancreatic Cancer: ASCO Provisional Clinical Opinion. J Clin Oncol 2019;37:153-64. [Crossref] [PubMed]
- Chang JS, Hsiao JR, Chen CH. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci 2017;24:19. [Crossref] [PubMed]
- Kanda J, Matsuo K, Suzuki T, et al. Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Sci 2009;100:296-302. [Crossref] [PubMed]
- Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol 2015;44:186-98. [Crossref] [PubMed]
- Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021;397:2337-60. [Crossref] [PubMed]
- Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553-62. [Crossref] [PubMed]
- Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921-9. [Crossref] [PubMed]
- Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 2019;92:6-10. [Crossref] [PubMed]
- Owen N, Sparling PB, Healy GN, et al. Sedentary behavior: emerging evidence for a new health risk. Mayo Clin Proc 2010;85:1138-41. [Crossref] [PubMed]
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288-98. [Crossref] [PubMed]
- GBD 2020 Alcohol Collaborators. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet 2022;400:185-235. [Crossref] [PubMed]
- Alcohol consumption [Internet]. 2018. Available online: https://www.oecd-ilibrary.org/content/data/e6895909-en
- Nakatani H. Population aging in Japan: policy transformation, sustainable development goals, universal health coverage, and social determinates of health. Glob Health Med 2019;1:3-10. [Crossref] [PubMed]
- Hanck C, Whitcomb DC. Alcoholic pancreatitis. Gastroenterol Clin North Am 2004;33:751-65. [Crossref] [PubMed]
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- Bosetti C, Bertuccio P, Negri E, et al. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog 2012;51:3-13. [Crossref] [PubMed]
- Kanasi E, Ayilavarapu S, Jones J. The aging population: demographics and the biology of aging. Periodontol 2000 2016;72:13-8. [Crossref] [PubMed]
- Fitzmaurice C, Abate D, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Lee E, Horn-Ross PL, Rull RP, et al. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol 2013;178:1403-13. [Crossref] [PubMed]
- Sadr-Azodi O, Konings P, Brusselaers N. Menopausal hormone therapy and pancreatic cancer risk in women: a population-based matched cohort study. United European Gastroenterol J 2017;5:1123-8. [Crossref] [PubMed]
- Andersson G, Borgquist S, Jirström K. Hormonal factors and pancreatic cancer risk in women: The Malmö Diet and Cancer Study. Int J Cancer 2018;143:52-62. [Crossref] [PubMed]
- Yuan Y, Liu L, Chen H, et al. Comprehensive Characterization of Molecular Differences in Cancer between Male and Female Patients. Cancer Cell 2016;29:711-22. [Crossref] [PubMed]
- Jones J, Mukherjee A, Karanam B, et al. African Americans with pancreatic ductal adenocarcinoma exhibit gender differences in Kaiso expression. Cancer Lett 2016;380:513-22. [Crossref] [PubMed]
- Li D, Tang H, Hassan MM, et al. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control 2011;22:189-97. [Crossref] [PubMed]
- Salvatore T, Marfella R, Rizzo MR, et al. Pancreatic cancer and diabetes: A two-way relationship in the perspective of diabetologist. Int J Surg 2015;21:S72-7. [Crossref] [PubMed]
- Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep 2020;10:14790. [Crossref] [PubMed]
- Rijkers AP, Bakker OJ, Ahmed Ali U, et al. Risk of Pancreatic Cancer After a Primary Episode of Acute Pancreatitis. Pancreas 2017;46:1018-22. [Crossref] [PubMed]
- Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol 2017;112:1366-72. [Crossref] [PubMed]
- Lew D, Kamal F, Phan K, et al. Epidemiologic risk factors for patients admitted with chronic pancreatitis and pancreatic ductal adenocarcinoma in the United States. World J Clin Oncol 2022;13:907-17. [Crossref] [PubMed]
- Wilcox CM, Sandhu BS, Singh V, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol 2016;111:1488-96. [Crossref] [PubMed]
- Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog 2012;51:14-24. [Crossref] [PubMed]
- Rebours V, Lévy P, Ruszniewski P. An overview of hereditary pancreatitis. Dig Liver Dis 2012;44:8-15. [Crossref] [PubMed]
- Ruijs MW, Verhoef S, Rookus MA, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet 2010;47:421-8. [Crossref] [PubMed]
- Hall MJ, Bernhisel R, Hughes E, et al. Germline Pathogenic Variants in the Ataxia Telangiectasia Mutated (ATM) Gene are Associated with High and Moderate Risks for Multiple Cancers. Cancer Prev Res (Phila) 2021;14:433-40. [Crossref] [PubMed]
- Soura E, Eliades PJ, Shannon K, et al. Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome. J Am Acad Dermatol 2016;74:395-407; quiz 408-10. [Crossref] [PubMed]
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006;12:3209-15. [Crossref] [PubMed]
- Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107:2005-9. [Crossref] [PubMed]
- Moran A, O'Hara C, Khan S, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer 2012;11:235-42. [Crossref] [PubMed]
- Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2007;16:342-6. [Crossref] [PubMed]
- Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015;121:269-75. [Crossref] [PubMed]
- Casadei S, Norquist BM, Walsh T, et al. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res 2011;71:2222-9. [Crossref] [PubMed]
- Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA 2009;302:1790-5. [Crossref] [PubMed]
- Shindo K, Yu J, Suenaga M, et al. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J Clin Oncol 2017;35:3382-90. [Crossref] [PubMed]
- Young EL, Thompson BA, Neklason DW, et al. Pancreatic cancer as a sentinel for hereditary cancer predisposition. BMC Cancer 2018;18:697. [Crossref] [PubMed]
- Ohmoto A, Yachida S, Morizane C. Genomic Features and Clinical Management of Patients with Hereditary Pancreatic Cancer Syndromes and Familial Pancreatic Cancer. Int J Mol Sci 2019;20:561. [Crossref] [PubMed]
- Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993;328:1433-7. [Crossref] [PubMed]
- Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut 2009;58:97-103. [Crossref] [PubMed]
- de las Heras-Castaño G, Castro-Senosiaín B, Fontalba A, et al. Hereditary pancreatitis: clinical features and inheritance characteristics of the R122C mutation in the cationic trypsinogen gene (PRSS1) in six Spanish families. JOP 2009;10:249-55. [PubMed]
- Grocock CJ, Rebours V, Delhaye MN, et al. The variable phenotype of the p.A16V mutation of cationic trypsinogen (PRSS1) in pancreatitis families. Gut 2010;59:357-63. [Crossref] [PubMed]
- Phillips AE, LaRusch J, Greer P, et al. Known genetic susceptibility factors for chronic pancreatitis in patients of European ancestry are rare in patients of African ancestry. Pancreatology 2018;18:528-35. [Crossref] [PubMed]
- Suzuki M, Shimizu T. Is SPINK1 gene mutation associated with development of pancreatic cancer? New insight from a large retrospective study. EBioMedicine 2019;50:5-6. [Crossref] [PubMed]
- Hamoir C, Pepermans X, Piessevaux H, et al. Clinical and morphological characteristics of sporadic genetically determined pancreatitis as compared to idiopathic pancreatitis: higher risk of pancreatic cancer in CFTR variants. Digestion 2013;87:229-39. [Crossref] [PubMed]
- Scotet V, L'Hostis C, Férec C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes (Basel) 2020;11:589. [Crossref] [PubMed]
- Miranda Alcalde B, Villa Alcázar M, Martínez Romera I, et al. The importance of Li-Fraumeni syndrome, a hereditary cancer predisposition disorder. Arch Argent Pediatr 2021;119:e11-7. [PubMed]
- Bougeard G, Renaux-Petel M, Flaman JM, et al. Revisiting Li-Fraumeni Syndrome From TP53 Mutation Carriers. J Clin Oncol 2015;33:2345-52. [Crossref] [PubMed]
- Awasthi P, Foiani M, Kumar A. ATM and ATR signaling at a glance. J Cell Sci 2015;128:4255-62. [Crossref] [PubMed]
- Dutzmann CM, Spix C, Popp I, et al. Cancer in Children With Fanconi Anemia and Ataxia-Telangiectasia-A Nationwide Register-Based Cohort Study in Germany. J Clin Oncol 2022;40:32-9. [Crossref] [PubMed]
- Hsu FC, Roberts NJ, Childs E, et al. Risk of Pancreatic Cancer Among Individuals With Pathogenic Variants in the ATM Gene. JAMA Oncol 2021;7:1664-8. [Crossref] [PubMed]
- Thompson D, Duedal S, Kirner J, et al. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst 2005;97:813-22. [Crossref] [PubMed]
- Jerzak KJ, Mancuso T, Eisen A. Ataxia-telangiectasia gene (ATM) mutation heterozygosity in breast cancer: a narrative review. Curr Oncol 2018;25:e176-80. [Crossref] [PubMed]
- Goldstein AM, Chan M, Harland M, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet 2007;44:99-106. [Crossref] [PubMed]
- Astiazaran-Symonds E, Kim J, Haley JS, et al. A genome-first approach to estimate prevalence of germline pathogenic variants and risk of pancreatic cancer in select cancer susceptibility genes. Cancers (Basel) 2022;14:3257. [Crossref] [PubMed]
- Lynch HT, Fusaro RM, Lynch JF, et al. Pancreatic cancer and the FAMMM syndrome. Fam Cancer 2008;7:103-12. [Crossref] [PubMed]
- Zhao R, Choi BY, Lee MH, et al. Implications of genetic and epigenetic alterations of CDKN2A (p16(INK4a)) in cancer. EBioMedicine 2016;8:30-9. [Crossref] [PubMed]
- Chan SH, Chiang J, Ngeow J. CDKN2A germline alterations and the relevance of genotype-phenotype associations in cancer predisposition. Hered Cancer Clin Pract 2021;19:21. [Crossref] [PubMed]
- Vasen HF, Gruis NA, Frants RR, et al. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer 2000;87:809-11. [Crossref] [PubMed]
- Kullmann L, Krahn MP. Controlling the master-upstream regulation of the tumor suppressor LKB1. Oncogene 2018;37:3045-57. [Crossref] [PubMed]
- van Lier MG, Wagner A, Mathus-Vliegen EM, et al. High cancer risk in Peutz-Jeghers syndrome: a systematic review and surveillance recommendations. Am J Gastroenterol 2010;105:1258-64; author reply 1265. [Crossref] [PubMed]
- Savage KI, Harkin DP. BRCA1, a 'complex' protein involved in the maintenance of genomic stability. FEBS J 2015;282:630-46. [Crossref] [PubMed]
- Fradet-Turcotte A, Sitz J, Grapton D, et al. BRCA2 functions: from DNA repair to replication fork stabilization. Endocr Relat Cancer 2016;23:T1-T17. [Crossref] [PubMed]
- Chiang J, Ngeow J. The management of BRCA1 and BRCA2 carriers in Singapore. Chin Clin Oncol 2020;9:62. [Crossref] [PubMed]
- Wong W, Raufi AG, Safyan RA, et al. BRCA mutations in pancreas cancer: spectrum, current management, challenges and future prospects. Cancer Manag Res 2020;12:2731-42. [Crossref] [PubMed]
- Ferla R, Calò V, Cascio S, et al. Founder mutations in BRCA1 and BRCA2 genes. Ann Oncol 2007;18:vi93-8. [Crossref] [PubMed]
- Golan T, Kindler HL, Park JO, et al. Geographic and Ethnic Heterogeneity of Germline BRCA1 or BRCA2 Mutation Prevalence Among Patients With Metastatic Pancreatic Cancer Screened for Entry Into the POLO Trial. J Clin Oncol 2020;38:1442-54. [Crossref] [PubMed]
- Reid S, Schindler D, Hanenberg H, et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 2007;39:162-4. [Crossref] [PubMed]
- Hanenberg H, Andreassen PR. PALB2 (partner and localizer of BRCA2). Atlas Genet Cytogenet Oncol Haematol 2018;22:484-90. [PubMed]
- Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med 2021;384:440-51. [Crossref] [PubMed]
- Wu S, Zhou J, Zhang K, et al. Molecular Mechanisms of PALB2 Function and Its Role in Breast Cancer Management. Front Oncol 2020;10:301. [Crossref] [PubMed]
- Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009;324:217. [Crossref] [PubMed]
- Borecka M, Zemankova P, Vocka M, et al. Mutation analysis of the PALB2 gene in unselected pancreatic cancer patients in the Czech Republic. Cancer Genet 2016;209:199-204. [Crossref] [PubMed]
- Kempers MJ, Kuiper RP, Ockeloen CW, et al. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol 2011;12:49-55. [Crossref] [PubMed]
- Win AK, Young JP, Lindor NM, et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol 2012;30:958-64. [Crossref] [PubMed]
- Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 2018;67:1306-16. [Crossref] [PubMed]
- Lynch HT, Coronel SM, Okimoto R, et al. A founder mutation of the MSH2 gene and hereditary nonpolyposis colorectal cancer in the United States. JAMA 2004;291:718-24. [Crossref] [PubMed]
- Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst 2017;109:djw332. [Crossref] [PubMed]
- Giardiello FM, Offerhaus GJ, Lee DH, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993;34:1394-6. [Crossref] [PubMed]
- Moussata D, Senouci L, Berger F, et al. Familial adenomatous polyposis and pancreatic cancer. Pancreas 2015;44:512-3. [Crossref] [PubMed]
- McFaul CD, Greenhalf W, Earl J, et al. Anticipation in familial pancreatic cancer. Gut 2006;55:252-8. [Crossref] [PubMed]
- Childs EJ, Mocci E, Campa D, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet 2015;47:911-6. [Crossref] [PubMed]
- Klein AP, Wolpin BM, Risch HA, et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun 2018;9:556. [Crossref] [PubMed]
- Liu D, Zhou D, Sun Y, et al. A Transcriptome-wide association study identifies candidate susceptibility genes for pancreatic cancer risk. Cancer Res 2020;80:4346-54. [Crossref] [PubMed]
- Wu C, Miao X, Huang L, et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet 2011;44:62-6. [Crossref] [PubMed]
- Low SK, Kuchiba A, Zembutsu H, et al. Genome-wide association study of pancreatic cancer in Japanese population. PLoS One 2010;5:e11824. [Crossref] [PubMed]
- Lin Y, Nakatochi M, Hosono Y, et al. Genome-wide association meta-analysis identifies GP2 gene risk variants for pancreatic cancer. Nat Commun 2020;11:3175. [Crossref] [PubMed]
- Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7-17. [Crossref] [PubMed]
- Lu C, Xu CF, Wan XY, et al. Screening for pancreatic cancer in familial high-risk individuals: a systematic review. World J Gastroenterol 2015;21:8678-86. [Crossref] [PubMed]
- Canto MI, Almario JA, Schulick RD, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 2018;155:740-751.e2. [Crossref] [PubMed]
- Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017;265:185-91.


