
Lymph node staging with 68Ga-PSMA PET in patients with intermediate and high-risk prostate cancer suitable for radical prostatectomy managed in a prostate cancer unit
Highlight box
Key findings
• In our study, we reported the correlation between 68Ga-PSMA PET findings and pathology results in lymph node staging, in patients with intermediate and high-risk prostate cancer suitable for radical prostatectomy and extended pelvic lymph node dissection.
What is known and what is new?
• PSMA PET is not routinely recommended in primary staging in prostate cancer and in the decision-making process of whether or not to perform pelvic lymph node dissection.
• In our study, we confirm the high overall diagnostic value of PSMA PET for lymph node staging in patients with intermediate and high-risk prostate cancer.
What is the implication, and what should change now?
• PSMA PET in primary staging and in nomograms should be considered to better select the patients suitable for pelvic lymph node dissection.
Introduction
Prostate cancer (PCa) is the second most frequent cancer and the fifth leading cause of cancer death among men in 2020, with an estimated almost 1.4 million new cases and 375,000 deaths worldwide (1). Radical prostatectomy for the primary treatment of PCa has been increasing in the last years in all risk groups (2). Lymph node metastasis (LNM) were found in about 15% of patients with intermediate and high risk PCa undergoing radical prostatectomy and extended pelvic lymph node dissection (ePLND) for localized disease (3). Nowadays, patients with intermediate risk and Gleason score ≥7 (4+3) or International Society of Urological Pathology (ISUP) grade group ≥3 and patients with high risk PCa require metastatic screening including at least cross-sectional abdominopelvic imaging and a bone-scan (4). However, pre-operative computed tomography (CT) scan or magnetic resonance imaging (MRI) sensitivity range between 5–94% and 64–79% in detecting lymph node metastasis, respectively, due to variability in patient populations and methodologies in different studies (5). Extended pelvic lymph node dissection is still the most accurate procedure for nodal staging. However, lymphocele/lymphedema, venous thromboembolism and nerve and vascular risk of injuries are present. Furthermore, in the literature, no clear therapeutic effect or improvement in oncological outcomes are reported (6,7). Currently, according to the European Association of Urology (EAU) guidelines, patients who have a risk of nodal metastases over 5% based on validated nomograms (Briganti nomograms, Roach formula, Memorial Sloan Kettering Cancer Center-MSKCC nomograms) should undergo ePLND (4). In recent years, positron emission tomography/computed tomography (PET/CT) with 68Ga-Prostate-specific membrane antigen (PSMA)-2-hydroxy-5-(carboxyethyl)benzyl]-thylenediamine-N, N9-diacetic acid (HBED-CC) has been shown to have higher sensitivity for detecting nodal and distant metastases than conventional imaging and other PET tracers (8). Although most published data concern use of PSMA-based PET/CT in the biochemical recurrence (BCR) setting (9), the high rate of sensitivity and specificity drives increasing use of this technology also in primary staging of PCa. Despite these high sensitivity and specificity values, the use of PSMA PET/CT in primary staging is debated, due to the absence of prospective data on the correct management of patients considered to have a localized disease after conventional imaging (CT scan and bone scintigraphy) and found metastatic after PSMA PET/CT imaging. At present, we do not have the certainty that changes in therapeutic strategy correspond to a survival benefit. This is one reason for the use PSMA PET/CT in an investigative and multidisciplinary setting. We report on our experience in the use of PSMA PET/CT in patients with intermediate and high-risk PCa suitable for radical prostatectomy to evaluate the sensitivity, specificity, positive and negative predictive values of pre-operative 68Ga-PSMA PET/CT in lymph node staging. We present this article in accordance with the STARD reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-10/rc).
Methods
We analyzed in a retrospective setting all patients with intermediate and high risk PCa managed in the Prostate Cancer Unit of our hospital between April 2017 and March 2021 that were staged preoperatively through PSMA PET/CT and submitted to laparoscopic or robotic radical prostatectomy with ePLND. Ethics Committee of Azienda Ospedaliero-Universitaria of Parma approved the protocol study (No. 11033-11/03/2019/AOUPR). Informed consent was obtained from all individual participants included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Imaging protocol
The PET/CT exams were performed 30–60 days before surgery. Synthesis of 68Ga-PSMA-HBED-CC was performed using a fully automated module (Scintomics GRP®, Fuerstenfeldbruck, Germany) and a pharmaceutical grade 68Ge/68Ga generator (1850 MBq, GalliaPharm® Eckert & Ziegler, Berlin, Germany), as previously described (10). Mean radiochemical purity of the produced radiopharmaceutical was 99.90% and radiochemical yield 68.71%. Whole body PET/CT was acquired, from vertex to medium thigh of the femur 60 min after i.v. injection of 68Ga-PSMA-HBED-CC (150 MBq) on a hybrid scanner Discovery IQ (GE Healthcare®, Chicago, IL, USA). A low-dose non-enhanced CT was performed afterwards for attenuation correction and anatomical correlation. All PET images were corrected for attenuation, dead time, random events, and scatter. Reconstruction of PET images was performed with an iterative algorithm (ordered-subset expectation maximization).
Two experienced (>450 PSMA PET/CT a year) board-certified nuclear medicine physicians (MS, GB) evaluated all the images visually and semiquantitatively. Visual analysis assessed the presence of tracer uptake in the prostate bed (T), pelvic lymph nodes (N), extrapelvic nodes (M1a), and distant metastases (M). Pelvic lymph nodes were defined by side (left, right) and location. Focal tracer uptake was considered suspicious for cancer lesion when higher than surrounding background, according to PROMISE criteria (11). Semiquantitative analysis of tracer uptake was performed using spherical volumes of interest (VOIs) semi-automatically drawn on orthogonal planes. Maximum Standard Uptake Value (SUVmax) was measured in the prostate and in the target nodal or distant lesion considered as the hottest lesion.
Patients and treatment
We discussed all cases with tracer uptake at PSMA PET/CT in our multidisciplinary PCa team. In this analysis, we only considered procedures performed by expert laparoscopic or robotic surgeons. At the beginning extraperitoneal laparoscopic radical prostatectomy was performed by two experienced surgeons (UVM and SF), and since December 2019 a robotic trans-peritoneal radical prostatectomy by a single surgeon (UVM). An extended bilateral PLND was done in all cases in a standard fashion (12). Prostate and lymph node specimens were examined by a dedicated uropathologist (EMS) according to ISUP protocols (13). We considered the following pre-operative variables: age, prostate-specific antigen (PSA), ISUP grade group on prostate biopsy, risk of lymph node involvement (LNI) on MSKCC nomogram and the maximum standardized uptake value (SUVmax) of the pelvic lymph nodes showing tracer uptake on PSMA PET/CT. The final histopathology results (TNM, ISUP group, number of lymph nodes removed and number of positive lymph nodes) were also noted. Conformity between PSMA PET/CT and final histopathological examination was analyzed.
Statistical analysis
Descriptive statistics were performed for total sample and stratified by lymph nodes (LN) positivity (LN−; LN+). Quantitative data [age, initial prostate-specific antigen (iPSA), MSKCC risk of LNI and total LN resected] were synthesized by calculating median and interquartile range (IQR). These measures were compared between groups (LN− vs. LN+) using the Mann-Whitney test. Categorical data (Gleason Score, ISUP classification and pathologic TNM staging) were showed as frequencies and percentages. A potential association between these qualitative variables and LN positivity was explored using the Chi-squared test or the Fisher’s exact test depending on the expected frequencies. Sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of PSMA PET/CT for the detection of regional LN metastases were computed considering pathology at radical prostatectomy as standard. Interrater reliability for PSMA PET/CT was measured as percent agreement and Cohen’s kappa. For all the analyses a α=0.05 was considered, and the statistical software used was STATA 13.1 version.
Results
Forty-two patients met the inclusion criteria. Mean age was 65.5 years (range, 49–76 years), mean and median preoperative serum PSA were 14.5 and 13 ng/mL (IQR, 8.1–20 ng/mL), respectively. Patients in the high-risk group were 23 (54.7%). The remainders were in the intermediate risk group (45.3%). The mean risk of LNI using the MSKCC-nomogram was 20%. The most common ISUP grade was 3 (26.19%) after prostate biopsy. PSMA PET/CT showed focal prostatic uptake in 28 patients with a mean value of SUVmax 18.5 (range, 3.8–75.3) and weak diffuse uptake in 9 patients. In 6 cases (14.3%) PSMA PET/CT showed tracer accumulation in pelvic lymph nodes defined as metastases (an example is presented in Figure 1), with a median value of SUVmax 4.5. The correct value is IQR (2-6.9). All of them presented focal uptake in the prostate (mean SUVmax 12.3). In 5 patients PET/CT was completely negative for tracer uptake in the prostate, LNs or distant lesions. The median number of removed lymph nodes was 11 (IQR, 5–17). At the histopathological examination, lymph node metastases were detected in 7 patients (16.6%) and the median percentage of lymph nodes involvement was 16% in patients with LNM. Six patients with PSMA PET/CT uptake at pelvic lymph node had metastases at the histopathological examination. Only one patient with negative PSMA PET/CT had LNI. This patient had a high-risk PCa with a PSA value of 20 ng/mL, and an ISUP grade 3. In this case, only one metastatic lymph node out of 10 removed lymph nodes was found and pathology examination disclosed micrometastasis. In patients with LNM and positive PSMA PET/CT mean preoperative PSA was 17.9 ng/mL, ISUP group was 2, 4 and 5 in 2, 2 and 2 cases, respectively, with a LNI median risk of 25%. The only difference between LN− and LN+ was the pathological T stage, which was higher in LN+ patients, as reported in Table 1. In our series, following pathology confirmation, sensitivity, specificity, positive and negative predictive values of pre-operative 68Ga-PSMA PET/CT were 85.7%, 100%, 100% and 97.2%, respectively. All patients were re-evaluated 4–6 months after surgery and all of them tested negative for biochemical or clinical recurrence of disease.
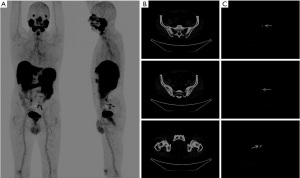
Table 1
| Characteristics | Total sample (n=42) | N | P value* | |
|---|---|---|---|---|
| N− (n=35) | N+ (n=7) | |||
| Age, years | – | 67.5 [59.5–71.5] | 70 [68–71] | 0.28 |
| iPSA | – | 12.5 [8.1–19.9] | 16 [8.3–20] | 0.46 |
| MSKCC risk of LNI | – | 12 [5–45] | 25 [5–45] | 0.55 |
| Total LN resected | – | 11 [5–14], 10.2±5.8 | 14.5 [10–18], 14±7.3 | 0.227 |
| GS biopsy | 0.5 | |||
| 6 (3+3) | 7 (16.7) | 7 (20.0) | 0 (0) | |
| 7 (3+4) | 8 (19.1) | 7 (20.0) | 1 (14.3) | |
| 7 (4+3) | 11 (26.2) | 9 (25.9) | 2 (28.6) | |
| 8 (4+4) | 10 (23.8) | 8 (22.8) | 2 (28.6) | |
| 9 (4+5) | 5 (11.9) | 3 (8.5) | 2 (28.6) | |
| 9 (5+4) | 0 (0) | 0 (0) | 0 (0) | |
| 10 (5+5) | 1 (1.4) | 1 (2.8) | 0 (0) | |
| ISUP biopsy | 0.547 | |||
| 1 | 7 (16.7) | 7 (20.0) | 0 (0) | |
| 2 | 8 (19.1) | 7 (20.0) | 1 (14.3) | |
| 3 | 11 (26.2) | 9 (25.9) | 2 (28.6) | |
| 4 | 10 (23.8) | 8 (22.8) | 2 (28.6) | |
| 5 | 6 (14.3) | 4 (11.3) | 2 (28.6) | |
| Pre-operative risk group | 0.76 | |||
| Favorable IR | 9 (21.4) | 7 (20.0) | 2 (28.6) | |
| Unfavorable IR | 10 (23.8) | 9 (25.7) | 1 (14.3) | |
| High risk | 23 (54.8) | 19 (54.3) | 4 (57.1) | |
| pGS | 0.12 | |||
| 6 (3+3) | 3 (7.1) | 3 (8.5) | 0 (0) | |
| 7 (3+4) | 12 (28.6) | 10 (28.6) | 2 (28.6) | |
| 7 (4+3) | 12 (28.6) | 11 (31.5) | 1 (14.3) | |
| 8 (4+4) | 8 (19.1) | 7 (20.0) | 1 (14.3) | |
| 9 (4+5) | 6 (14.3) | 3 (8.5) | 3 (42.8) | |
| 9 (5+4) | 1 (2.4) | 1 (2.8) | 0 (0) | |
| 10 (5+5) | 0 (0) | 0 (0) | 0 (0) | |
| pISUP | 0.14 | |||
| 1 | 3 (7.1) | 3 (8.5) | 0 (0) | |
| 2 | 12 (28.6) | 10 (28.6) | 2 (28.6) | |
| 3 | 12 (28.6) | 11 (31.5) | 1 (14.3) | |
| 4 | 8 (19.1) | 7 (20.0) | 1 (14.3) | |
| 5 | 7 (16.7) | 4 (11.4) | 3 (42.8) | |
| pT | 0.016 | |||
| T2 | 17 (40.5) | 17 (48.5) | 0 (0) | |
| T3a | 14 (33.3) | 11 (31.5) | 3 (42.8) | |
| T3b | 11 (26.2) | 7 (20.0) | 4 (57.2) | |
Values are presented as n (%), mean ± SD or median [IQR]. *, P value is significant (<0.05). iPSA, initial prostate specific antigen; MSKCC, Memorial Sloan Kettering Cancer Center; LNI, lymph node involvement; LN, lymph node, GS, Gleason score, ISUP, International Society of Urological Pathology; IR, intermediate risk, IQR, interquartile range; SD, standard deviation; N, node.
Discussion
A more precise lymph node staging is the way to avoid unnecessary lymphadenectomy. Nowadays all the patients with more than 5% risk of LNI on nomogram must undergo extended pelvic lymph node dissection. However, this cut-off value overestimates the real risk of LNI, as reported by a study of the EAU Young Academic Urologist-Robotic Section. In this paper the authors, by analyzing the results of five high volume European centers, reported a rate of 77.8% of ePLND in patients with LNI >5% with only 4.1% of nodal metastasis detected (14). Moreover, 85% of negative lymph nodes specimens after PLND in patients with intermediate and high risk PCa reported by other authors (3) suggest that most pelvic lymphadenectomies performed during radical prostatectomy may be not necessary. Recently, PET/CT imaging with PSMA-based radioligands has been showed in several retrospective and prospective studies to be superior compared to CT and MRI exams (15,16), bone scintigraphy (17,18) and PET/CT with 18F-fluciclovine (15,19-21) or radiolabeled choline (22,23). Application of PSMA PET/CT in the primary staging of PCa have recently emerged due to the essential role of imaging for risk stratification and treatment decisions (15,24,25). In the proPSMA randomized control study the use of PSMA PET/CT as first-line imaging changed treatment in 28% of patients (compared to 15% following conventional imaging) with a lower radiation burden (15). Moreover, a recent analysis based on the proPSMA Trial results has demonstrated that PSMA PET/CT is less costly than conventional imaging for initial staging (26). However, diagnostic accuracy of PSMA PET/CT varies across different series, as shown in a recent systematic review on 27 studies including 2,832 patients. The variations may be related to technical aspects of the PET/CT tomograph and image reconstruction methods, to the reader and urologist experience, to the surgical procedure and to handling of pathology specimens (27).
To reduce the potential bias linked to the retrospective nature of our study, we chose to analyze patients handled by skilled urologists/pathologists in the context of the Prostate Cancer Unit of our hospital. Moreover, PET/CT readers of our institution are highly confident in PSMA-based imaging with more than 450 scans/year (10) and reporter agreement was very high for both nodal side and pelvic location (kappa 1, 100%). The increasing interest for the application of PSMA PET/CT in primary staging is related to the high specificity and negative predictive value for local lymph node metastases (28-35), as confirmed in our study. Results of different series about specificity are similar, but there are great differences about sensitivity, ranging from 33% to 100% (36), due to the small sample size, heterogeneity of risk groups, limited number of lymph nodes removed, and presence of lymph nodes micrometastasis.
In the review by Stabile et al., the authors suggest that due to the high NPV of PSMA PET/CT in men with a lower risk of LNI might be employed to reduce the number of ePLND. Instead, ePLND should be performed in high-risk patients, even with a negative PSMA PET/CT (27).
In our experience PSMA PET/CT revealed optimal specificity but also good sensitivity (83%) and the presence of lymph node PSMA uptake has appeared as a strong indication to perform ePLND. However, a negative 68Ga-PSMA PET/CT does not exclude microscopic lymph node metastasis at all. Indeed, in our experience pathology demonstrated the presence of micrometastasis in the only case of false negative PSMA PET/CT, who is a patient with high-risk disease. The retrospective nature and the enrollment of selected patients with intermediate and high risk Pca represent limitations to this study. Moreover, the sample size was relatively small (42 patients submitted to radical prostatectomy) with LNI in 7 patients (16.6%) and only one case of false negative PSMA PET/CT.
In one of the large prospective primary staging series of intermediate and high-risk PCa with PSMA PET/CT undergoing Radical Prostatectomy (RP) and ePLND (262 patients), the authors reported a 41% sensitivity, which is lower than ours (85.7%) (37). This aspect may be explained by the difference in sensitivity and NPV in high-risk patients, as reported by Stabile et al. (27), due to the presence of a higher number of patients in the high-risk group (81%) compared to our series (54.7%). Nowadays, compared to conventional imaging, PSMA PET/CT provides superior assessment of patients’ risk, and it should be considered as a tool to guide treatment. The future aim will be to integrate PSMA PET/CT with PSA levels, histopathological and mpMRI results, to improve selection of candidates to ePLND. The study by Franklin et al. (31) suggested that patients with a negative preoperative 68Ga-PSMA PET/CT, ISUP grade <5 and Prostate imaging-reporting and data system (PI-RADS) <5 on mpMRI or ISUP Grade 5 with PI-RADS <4 have a risk of LNI <5% than predicted with nomograms and in these cases an ePLND is not necessary. Finally, the increasing accessibility to PSMA-based imaging due to the approval of 68Ga-PSMA-11 injection by Food and Drug Administration (FDA) (38) and the publication of the specific Monograph in the European Pharmacopeia (39), may allow larger validation studies in the setting of primary staging.
Conclusions
In our Prostate Cancer Unit’s experience, 68Ga-PSMA PET/CT demonstrated high overall diagnostic value for lymph node staging in intermediate and high-risk PCa. Staging accuracy may depend on lymph node-size. PSMA positive lymph nodes have to be included in the dissection field.
Acknowledgments
These data were first presented at the XCIV Congress of the Italian Urology Society, Riccione 16-19 October 2021 and at the XXXI Congress of the Italian Society of Uro-Oncology (virtual) 30 September to 02 October 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-10/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-10/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-10/coif). DC serves as an unpaid editorial board member of Chinese Clinical Oncology from August 2022 to July 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics Committee of Azienda Ospedaliero-Universitaria of Parma approved the protocol study (No. 11033-11/03/2019/AOUPR). Informed consent was obtained from all individual participants included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Kaps B, Leapman M, An Y. Trends in prostatectomy utilization: Increasing upfront prostatectomy and postprostatectomy radiotherapy for high-risk prostate cancer. Cancer Med 2020;9:8754-64. [Crossref] [PubMed]
- Abdollah F, Suardi N, Gallina A, et al. Extended pelvic lymph node dissection in prostate cancer: a 20-year audit in a single center. Ann Oncol 2013;24:1459-66. [Crossref] [PubMed]
- EAU Guidelines. Edn. presented at the EAU Annual Congress Milan 2023. ISBN 978-94-92671-19-6.
- Lebastchi AH, Gupta N, DiBianco JM, et al. Comparison of cross-sectional imaging techniques for the detection of prostate cancer lymph node metastasis: a critical review. Transl Androl Urol 2020;9:1415-27. [Crossref] [PubMed]
- Cacciamani GE, Maas M, Nassiri N, et al. Impact of Pelvic Lymph Node Dissection and Its Extent on Perioperative Morbidity in Patients Undergoing Radical Prostatectomy for Prostate Cancer: A Comprehensive Systematic Review and Meta-analysis. Eur Urol Oncol 2021;4:134-49. [Crossref] [PubMed]
- Preisser F, van den Bergh RCN, Gandaglia G, et al. Effect of Extended Pelvic Lymph Node Dissection on Oncologic Outcomes in Patients with D'Amico Intermediate and High Risk Prostate Cancer Treated with Radical Prostatectomy: A Multi-Institutional Study. J Urol 2020;203:338-43. [Crossref] [PubMed]
- Bagguley D, Ong S, Buteau JP, et al. Role of PSMA PET/CT imaging in the diagnosis, staging and restaging of prostate cancer. Future Oncol 2021;17:2225-41. [Crossref] [PubMed]
- Perera M, Papa N, Roberts M, et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol 2020;77:403-17. [Crossref] [PubMed]
- Migliari S, Sammartano A, Scarlattei M, et al. Development and Validation of a High-Pressure Liquid Chromatography Method for the Determination of Chemical Purity and Radiochemical Purity of a [68Ga]-Labeled Glu-Urea-Lys(Ahx)-HBED-CC (Positron Emission Tomography) Tracer. ACS Omega 2017;2:7120-26. [Crossref] [PubMed]
- Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J Nucl Med 2018;59:469-78. [Crossref] [PubMed]
- Pini G, Matin SF, Suardi N, et al. Robot assisted lymphadenectomy in urology: pelvic, retroperitoneal and inguinal. Minerva Urol Nefrol 2017;69:38-55. [PubMed]
- Epstein JI, Amin MB, Reuter VE, et al. Contemporary Gleason Grading of Prostatic Carcinoma: An Update With Discussion on Practical Issues to Implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 2017;41:e1-7. [Crossref] [PubMed]
- Suardi N, Larcher A, Haese A, et al. Indication for and extension of pelvic lymph node dissection during robot-assisted radical prostatectomy: an analysis of five European institutions. Eur Urol 2014;66:635-43. [Crossref] [PubMed]
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395:1208-16. [Crossref] [PubMed]
- Sawicki LM, Kirchner J, Buddensieck C, et al. Prospective comparison of whole-body MRI and (68)Ga-PSMA PET/CT for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging 2019;46:1542-50. [Crossref] [PubMed]
- Acar E, Bekiş R, Polack B. Comparison of Bone Uptake in Bone Scan and Ga-68 PSMA PET/CT Images in Patients with Prostate Cancer. Curr Med Imaging Rev 2019;15:589-94. [Crossref] [PubMed]
- Simsek DH, Sanli Y, Civan C, et al. Does bone scintigraphy still have a role in the era of 68 Ga-PSMA PET/CT in prostate cancer? Ann Nucl Med 2020;34:476-85. [Crossref] [PubMed]
- Pernthaler B, Kulnik R, Gstettner C, et al. A Prospective Head-to-Head Comparison of 18F-Fluciclovine With 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT. Clin Nucl Med 2019;44:e566-73. [Crossref] [PubMed]
- Calais J, Fendler WP, Herrmann K, et al. Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a Case Series of 10 Patients with Prostate Cancer Recurrence. J Nucl Med 2018;59:789-94. [Crossref] [PubMed]
- Savir-Baruch B, Choyke PL, Rowe SP, et al. Role of (18)F-Fluciclovine and Prostate-Specific Membrane Antigen PET/CT in Guiding Management of Oligometastatic Prostate Cancer: AJR Expert Panel Narrative Review. AJR Am J Roentgenol 2021;216:851-9. [Crossref] [PubMed]
- Jilg CA, Drendel V, Rischke HC, et al. Detection Rate of (18)F-Choline PET/CT and (68)Ga-PSMA-HBED-CC PET/CT for Prostate Cancer Lymph Node Metastases with Direct Link from PET to Histopathology: Dependence on the Size of Tumor Deposits in Lymph Nodes. J Nucl Med 2019;60:971-7. [Crossref] [PubMed]
- Puterman C, Bjöersdorff M, Amidi J, et al. A retrospective study assessing the accuracy of [18F]-fluorocholine PET/CT for primary staging of lymph node metastases in intermediate and high-risk prostate cancer patients undergoing robotic-assisted laparoscopic prostatectomy with extended lymph node dissection. Scand J Urol 2021;55:293-7. [Crossref] [PubMed]
- Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol 2016;195:1436-43. [Crossref] [PubMed]
- Donswijk ML, van Leeuwen PJ, Vegt E, et al. Clinical impact of PSMA PET/CT in primary prostate cancer compared to conventional nodal and distant staging: a retrospective single center study. BMC Cancer 2020;20:723. [Crossref] [PubMed]
- de Feria Cardet RE, Hofman MS, Segard T, et al. Is Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Imaging Cost-effective in Prostate Cancer: An Analysis Informed by the proPSMA Trial. Eur Urol 2021;79:413-8. [Crossref] [PubMed]
- Stabile A, Pellegrino A, Mazzone E, et al. Can Negative Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Avoid the Need for Pelvic Lymph Node Dissection in Newly Diagnosed Prostate Cancer Patients? A Systematic Review and Meta-analysis with Backup Histology as Reference Standard. Eur Urol Oncol 2022;5:1-17. [Crossref] [PubMed]
- Corona-Montes VE, González-Cuenca E, Fernández-Noyola G, et al. Primary lymph-node staging with (68)Ga-PSMA PET in high-risk prostate cancer: pathologic correlation with extended pelvic lymphadenectomy specimens. Urol Oncol 2021;39:494.e1-6. [Crossref] [PubMed]
- Gultekin MH, Demirci E, Turegun FA, et al. The Role of 68GA-PSMA PET/CT Scan In Patients with Prostate Adenocarcinoma who Underwent Radical Prostatectomy. Urol J 2020;18:58-65. [PubMed]
- Çelen S, Gültekin A, Özlülerden Y, et al. Comparison of 68Ga-PSMA-I/T PET-CT and Multiparametric MRI for Locoregional Staging of Prostate Cancer Patients: A Pilot Study. Urol Int 2020;104:684-91. [Crossref] [PubMed]
- Franklin A, Yaxley WJ, Raveenthiran S, et al. Histological comparison between predictive value of preoperative 3-T multiparametric MRI and (68) Ga-PSMA PET/CT scan for pathological outcomes at radical prostatectomy and pelvic lymph node dissection for prostate cancer. BJU Int 2021;127:71-9. [Crossref] [PubMed]
- Dekalo S, Kuten J, Mintz I, et al. Preoperative 68Ga-PSMA PET/CT defines a subgroup of high-risk prostate cancer patients with favorable outcomes after radical prostatectomy and lymph node dissection. Prostate Cancer Prostatic Dis 2021;24:910-6. [Crossref] [PubMed]
- Esen T, Falay O, Tarim K, et al. (68)Ga-PSMA-11 Positron Emission Tomography/Computed Tomography for Primary Lymph Node Staging Before Radical Prostatectomy: Central Review of Imaging and Comparison with Histopathology of Extended Lymphadenectomy. Eur Urol Focus 2021;7:288-93. [Crossref] [PubMed]
- Kopp D, Kopp J, Bernhardt E, et al. 68Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography-Computed Tomography-Based Primary Staging and Histological Correlation after Extended Pelvic Lymph Node Dissection in Intermediate-Risk Prostate Cancer. Urol Int 2022;106:56-62. [Crossref] [PubMed]
- Erdem S, Simsek DH, Degirmenci E, et al. How accurate is (68)Gallium-prostate specific membrane antigen positron emission tomography / computed tomography ((68)Ga-PSMA PET/CT) on primary lymph node staging before radical prostatectomy in intermediate and high risk prostate cancer? A study of patient- and lymph node- based analyses. Urol Oncol 2022;40:6.e1-9. [Crossref] [PubMed]
- Luiting HB, van Leeuwen PJ, Busstra MB, et al. Use of gallium-68 prostate specific membrane antigen positron-emission tomography for detecting lymph node metastases in primary and recurrent prostate cancer and location of recurrence after radical prostatectomy: an overview of the current literature. BJU Int 2020;125:206-14. [Crossref] [PubMed]
- Hope TA, Eiber M, Armstrong WR, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol 2021;7:1635-42. [Crossref] [PubMed]
- Hennrich U, Eder M. [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals (Basel) 2021;14:713. [Crossref] [PubMed]
- Pharmeuropa 29.4: PA/PH/Exp. 14/T (16) 45 ANP Available online: https://docplayer.net/87757254-Pharmeuropa-reference-pa-ph-exp-14-t-16-45-anp.html


