
The incorporation of cognitive-sparing techniques into prophylactic cranial irradiation in the management of small cell lung cancer
Introduction
Small cell lung cancer (SCLC) is a poorly differentiated, high-grade neuroendocrine tumor that occurs primarily in the central airways of tobacco users. It represents approximately 15% of all lung cancers, with approximately 30,000 cases diagnosed each year in the United States alone (1). Due to its aggressive nature, SCLC has a modified staging system, originally introduced by the Veterans Affairs Lung Study Group, which simplifies staging and dichotomizes SCLC into two categories: limited stage (LS-SCLC) and extensive stage (ES-SCLC), with the former referring to an extent of disease that can be treated with a “tolerable radiation portal.”
Approximately 30% of SCLC cases are diagnosed as LS-SCLC and 70% as ES-SCLC with a median overall survival (OS) of 20 and 10 months, respectively (2). Brain metastases are common in SCLC, with up to 18% of patients diagnosed with brain metastases at initial presentation (3,4). Therefore, magnetic resonance imaging (MRI) of the brain is recommended as part of work-up, restaging, and post-treatment surveillance in patients with LS and ES-SCLC (1). Since many patients develop brain metastases, prophylactic cranial irradiation (PCI) is commonly offered following primary treatment in patients with a complete or partial response with the goal of decreasing the risk of developing intracranial metastases and prolonging OS (4).
While PCI has demonstrated improvements in OS and intracranial disease control in randomized trials (4,5), it is now commonly deferred owing to its association with cognitive sequelae in favor of MRI surveillance (6,7). Multiple randomized trials in patients with brain metastases have demonstrated reduced rates of cognitive decline following whole brain radiation therapy (WBRT) with the incorporation of memantine with or without the addition of hippocampal avoidance (HA) (8-10). To date, published randomized trials incorporating HA into PCI (HA-PCI) have had mixed results; potentially related to several factors, including the lack of memantine, enrolling relatively fewer patients, and other inherent limitations (11-14). In this review, we provide a detailed overview of clinical data and considerations for the use of PCI in SCLC, as well as novel treatment strategies and ongoing clinical trials.
PCI in limited stage SCLC
Historically, PCI was often used for LS-SCLC and its role was solidified in 1999 when an individual patient data meta-analysis of seven trials comprising 987 patients with SCLC (85% LS-SCLC and 15% ES-SCLC) who had a complete response following definitive treatment treated with PCI or no PCI was published (4). The 3-year OS was 15.3% versus 20.7% for the no PCI and PCI groups, respectively (P=0.03), as shown in Table 1 (15). Significant improvements in disease-free survival (DFS) and a lower cumulative incidence of new brain metastases favoring the PCI arm were also observed. Although this study provides some of the strongest data supporting the role of PCI, the trials included in this meta-analysis do not fully reflect modern practice as they predate the common use of staging with brain MRI and PET/CT. Therefore, it is possible that the observed benefits of PCI in LS-SCLC patients resulted partly from the inclusion of patients who, with the benefit of modern staging practices, would have been diagnosed with asymptomatic brain metastases and been classified as ES-SCLC.
Table 1
| Author | Trial group | N | Patients | Methods | Results | Notes |
|---|---|---|---|---|---|---|
| Auperin et al., N Engl J Med, 1999 | PCI Collaborative Group Gustave-Roussy | 987 | LS-SCLC (85%) and ES-SCLC (15%) after CR to chemotherapy | Meta-analysis of 7 trials comparing PCI vs. no PCI in SCLC | 3-year OS: 20.7% PCI vs. 15.3% no PCI (P=0.03) | Demonstrated improved OS with use of PCI over no PCI |
| 3-year brain metastases: 33.3% PCI vs. 58.6% no PCI [RR: 0.46 (P<0.001)] | Study was performed prior to inclusion of brain MRI as staging workup and follow-up procedures | |||||
| DFS: RR: 0.75 (P<0.001) | Different radiation dosing allowed | |||||
| Earlier PCI led to fewer brain metastases (P=0.01) | No reporting on chemotherapy | |||||
| Dose increase led to fewer metastases (P=0.02) | ||||||
| No difference in OS (P=0.89) | ||||||
| Slotman et al., N Engl J Med, 2007 | EORTC 08993/22993 | 286 | ES-SCLC who responded to platinum-based chemotherapy | Phase 3, randomized trial: PCI vs. observation | Median OS: 6.7 months PCI vs. 5.4 months observation (P=0.003) | Multiple dosing fractionation schedules allowed (20–30 Gy in 5–12 fractions; 20 Gy in 5 fractions most common) |
| 1-year OS: 27.1% vs. 13.3% (P=0.003) | Study was performed prior to inclusion of brain MRI as staging workup and follow-up procedures | |||||
| 1-year symptomatic brain metastases: 14.6% PCI vs. 40.4% observation (P<0.001) | ||||||
| Median DFS: 14.7 weeks vs. 12.0 weeks (P=0.02) | ||||||
| Takahashi et al., Lancet Oncol, 2017 | National Kyushu Cancer Center, Fukuoka, Japan | 224 | ES-SCLC who responded to platinum-based doublet chemotherapy; absence of brain metastases on MRI | Phase 3, randomized trial: PCI (25 Gy in 10) fractions vs. observation | Median OS: 11.6 months PCI 13.7 months observation (P=0.094) | Trial was closed early due to predicted probability of superiority of PCI arm over observation arm |
| 1-year OS: 48.4% PCI vs. 53.6% observation (P=0.094) | No benefit for OS | |||||
| 2-year OS: 15.0% PCI vs. 18.8% observation (P=0.094) | Cognitive decline was measured by MMSE (less thorough tool for assessing neurocognitive function) | |||||
| Median PFS: 2.3 months PCI vs. 2.4 months observation (P=0.75) | ||||||
| 1-year brain metastases: 32.9% PCI vs. 59.0% observation (P<0.0001) | ||||||
| Rodríguez de Dios et al., J Clin Oncol, 2021 | PREMER | 120 | LS-SCLC (71%) and ES-SCLC without brain metastases (29%) | Phase 3, randomized trial: IMRT HA-PCI vs. PCI (25 Gy in 10 fractions) | Decline of delayed free recall at 3 months: 5.8% HA-PCI vs. 23.5% PCI (P=0.003)* | Central review was performed |
| Decline of total recall at 24 months: 14.2% HA-PCI vs. 47.6% PCI (P=0.019) | No use of memantine | |||||
| No difference in intracranial failure rates, OS or quality of life | ||||||
| Belderbos et al., J Thorac Oncol, 2021 | NCT01780675 | 168 | LS-SCLC (70%) or ES-SCLC without brain metastases (30%) | Phase 3, randomized trial: HA-PCI vs. PCI (25 Gy in 10 fractions) | Total recall at 4 months: 28% HA-PCI vs. 29% PCI (P=1.000)** | No central review performed |
| No difference in performance on other cognitive tests, incidence of intracranial failure, or OS | Weekly, instead of daily, image guidance | |||||
| No use of memantine | ||||||
| NRG CC003 (NCT02635009) | SCLC with at least PR to chemotherapy | Ongoing phase 2/3 trial randomizing: HA-PCI vs. PCI | Primary endpoint: 6-month deterioration in HVLT-R delayed recall and 12-month intracranial relapse | Memantine use allowed | ||
| MAVERICK trial (SWOG S1827) | LS-SCLC and ES-SCLC after response to first-line therapy | Ongoing phase 3 trial randomizing: MRI brain surveillance vs. PCI with MRI surveillance | Primary endpoint: OS | HA allowed | ||
| Memantine use allowed | ||||||
| NRG CC009 | ES-SCLC with 10 or fewer brain metastases | Ongoing phase 3 trial randomizing: SRS vs. HA-WBRT with memantine | Primary endpoint: time to neurocognitive decline on HVLT-R, COWA test or TMT |
*, as measured on Free and Cued Selective Reminding Test; **, as measured on Hopkins Verbal Learning Test. PCI, prophylactic cranial irradiation; SCLC, small cell lung cancer; LS-SCLC, limited stage small cell lung cancer; ES-SCLC, extensive stage small cell lung cancer; CR, complete response; OS, overall survival; RR, risk ratio; DFS, disease free survival; MMSE, mini mental status exam; IMRT, intensity modulated radiation therapy; HA, hippocampal avoidance; PR, partial response; HVLT-R, Hopkins Verbal Learning Test-Revised; WBRT, whole brain radiation therapy; COWA, controlled oral word association; SRS, stereotactic radiosurgery; TMT, trail making test.
In 2009, Le Péchoux et al. published a randomized trial of 720 patients with LS-SCLC who were in complete remission following definitive chemoradiotherapy to PCI: (I) 25 Gy in 10 fractions; (II) 36 Gy in 18 daily fractions; or (III) 36 Gy given in twice-daily fractions of 1.5 Gy (16). The 2-year OS was 42% and 37% in the 25 Gy and 36 Gy groups, respectively (P=0.05) (15). No significant difference in the development of brain metastases was noted between the treatment arms. Thus, 25 Gy delivered in 10 daily fractions remains the currently favored PCI dose.
In appropriately selected LS-SCLC patients, PCI typically follows primary treatment after a partial or complete response to treatment (1). LS-SCLC patients can be divided into two groups which differ with respect to both treatment recommendations, and consensus on the necessity of PCI. Patients with cT1-2aN0M0 (AJCC 8th Edition) disease undergo mediastinal staging. If negative, these patients are typically treated with primary surgery followed by chemotherapy with or without the addition of radiation therapy (RT). Adjuvant RT to the mediastinum is recommended if the surgical pathology reveals the presence of nodal disease and/or positive microscopic or gross margins (17). An alternative to primary surgery for cT1-2aN0M0 disease is definitive stereotactic body radiation therapy (SBRT) followed by chemotherapy. All other LS-SCLC patients with good performance status typically receive concurrent chemoradiation.
The National Cancer Comprehensive Network (NCCN) presently recommends PCI in patients with LS-SCLC who have a complete or partial response following definitive treatment (1). However, patients with very early-stage LS-SCLC who undergo definitive surgery or SBRT are known to have a lower risk of developing brain metastases and may derive minimal benefit from PCI (18). Therefore, the NCCN recommends that LS-SCLC patients who will not receive PCI undergo close MRI surveillance (1).
PCI in extensive stage SCLC
The role of PCI in ES-SCLC remains an area of significant debate within the oncology community. In 2007, Slotman et al. published an important phase 3 trial (5), where 286 patients with ES-SCLC who had a response to chemotherapy were randomized to: (I) PCI; or (II) observation. Multiple PCI doses were approved, which ranged from 20–30 Gy in 5–12 fractions with the most common dose being 20 Gy in 5 fractions. The 1-year OS favored the PCI arm: 6.7 versus 5.4 months (P=0.003). Additionally, there were statistically significant improvements in the cumulative incidence of symptomatic brain metastases and DFS, both favoring the PCI arm. However, this study did have its limitations. First, brain imaging was not required for staging and follow-up procedures; thus, patients with existing brain metastases may have been included in the study. Second, these patients were treated prior to the introduction of contemporary systemic regimens, such as PD-L1 inhibitors, which are now a fundamental component of the ES-SCLC armamentarium (19-21).
In 2017, Takahashi et al. published the results of a phase 3 trial of 224 patients with ES-SCLC who were randomized to: (I) PCI to 25 Gy in 10 daily fractions; or (II) observation. Brain MRI was required prior to enrollment and at regular intervals (7). No statistically significant differences were observed in rates of OS and progression-free survival (PFS) in the PCI and observation arms. This trial was closed early due to futility of the PCI arm being superior to the observation arm in demonstrating a survival advantage. Additionally, while no differences in cognitive decline were observed between the two study arms, this trial utilized the mini mental status exam (MMSE), which is typically used as a screening test and does not provide a robust assessment of neurocognitive functioning (6). Taken together, the findings of these two studies demonstrated that observation over PCI may be an acceptable strategy in patients with ES-SCLC (22).
The standard primary treatment in patients with ES-SCLC involves the use of platinum-based chemotherapy with concurrent anti-PD-L1 therapy, followed by maintenance anti-PD-L1 therapy (1,19-21). While thoracic radiotherapy has demonstrated a modest OS benefit in patients who have a response following systemic therapy, this is yet to be validated in the era of immunotherapy and remains a topic of ongoing study (23,24). Thorough consideration of individual patient characteristics are essential when making recommendations for or against the use of PCI in ES-SCLC. Important points of consideration are: response and treatment tolerability of systemic therapy, performance status and medical comorbidities, and baseline neurocognitive functioning, as PCI is not recommended in the setting of poor performance status or impaired baseline neurocognitive function (1).
Cognitive decline following PCI
PCI is associated with a constellation of acute side effects, such as fatigue, nausea, alopecia, headache, and skin erythema, and the potential for chronic effects such as cognitive decline. In 2011, Wolfson et al. published the results of Radiation Therapy Oncology Group (RTOG) 0212 which randomized patients with LS-SCLC undergoing PCI to: (I) 25 Gy in 10 daily fractions; (II) 36 Gy in 18 daily fractions; or (III) 36 Gy in 24 BID fractions of 1.5 Gy (25). The goal of this study was to determine the impact of PCI dosing on neurocognitive functioning. A comprehensive neuropsychological testing battery was utilized at regular intervals following PCI. A higher incidence of neurocognitive decline following PCI, most notably on the Hopkins Verbal Learning Test (HVLT) was observed in patients receiving higher dose PCI (i.e., 36 Gy). The pathophysiology of cognitive decline following conventional WBRT and PCI is believed to be related to multiple mechanisms, such as cerebrovascular damage, neuroanatomical changes, impairment of neurogenesis, and neuroinflammation (6). Due to its association with quality of life (26), strategies to mitigate cognitive decline following brain-directed radiation therapy are essential.
Memantine and hippocampal avoidance
Memantine is an oral antagonist of the N-methyl-D-aspartate (NMDA) receptor, widely used in the management of Alzheimer dementia. The role of memantine in mitigating cognitive decline following WBRT was first validated in the randomized setting on RTOG 0614 (8). The study enrolled 508 patients with brain metastases to WBRT (37.5 Gy in 15 fractions) with or without the addition of memantine for 24 weeks. A comprehensive battery of neuropsychological assessments was performed at regular intervals. The study demonstrated a statistically significant benefit in time to cognitive failure across cognitive domains in the memantine arm without significant differences in OS, PFS, or grade 3–4 toxicity. This benefit was most pronounced in patients living at least 3 months following WBRT.
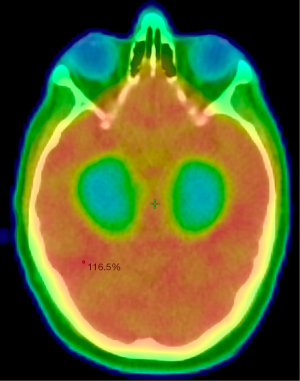
The hippocampus plays an essential role in learning and memory. Therefore, RT delivery strategies that minimize dose to the hippocampus have been of major interest to minimize cognitive decline following WBRT and PCI. The first major trial incorporating hippocampal avoidance WBRT (HA-WBRT) in patients with brain metastases was RTOG 0933 (8,9), see Figure 1. This was a phase 2 trial, in which 100 patients with brain metastases, not within 5 mm of the bilateral hippocampi, underwent HA-WBRT to 30 Gy in 10 fractions. Cognitive outcomes were compared to a historical WBRT cohort with identical inclusion criteria using comprehensive neuropsychological assessments at regular intervals following treatment. HA-WBRT was associated with significant improvements in delayed recall on the HVLT compared to the historical cohort. As was observed on RTOG 0614, these improvements were most notable in patients with a minimum life expectancy of 4 months.
The role of memantine and HA-WBRT in patients with brain metastases was validated in the randomized setting in 2020, when Brown et al. published the results of NRG CC001 (10). This study randomized 518 patients to: (I) WBRT with memantine or (II) HA-WBRT with memantine. The primary endpoint, which evaluated time to cognitive failure and incorporated all neurocognitive testing, was statistically significant, favoring the HA-WBRT arm (HR: 0.76; P=0.03). While approximately 80% of the enrolled patients in each arm had died by 6-months following treatment, survivors who received HA-WBRT had fewer memory complaints (P=0.01) and neurocognitive symptoms (P=0.01).
Due to the positive findings of these studies in the setting of brain metastases, HA-WBRT and the incorporation of memantine into PCI remains a topic of continued study. The PREMER trial was a phase 3 trial that randomized 150 patients with LS-SCLC or ES-SCLC (without brain metastases) to: (I) standard PCI (25 Gy in 10 daily fractions); or (II) HA-PCI (25 Gy in 10 daily fractions) (11). Patients underwent comprehensive neuropsychological assessments using the Free and Cued Selective Reminding Test. Each participating center was required to perform a practice run on a HA-PCI treatment of two cases to be approved for inclusion in the trial. Additionally, central review was conducted within days of the treatment in order to provide feedback to the investigators. The use of memantine was not mandated or assessed in the study. With a median follow-up of 40.4 months among living patients, there was less decline of delayed free recall from baseline to 3-months in the HA-PCI arm (5.8%) versus the conventional PCI arm (23.5%) (P=0.003). There were no significant differences in intracranial failure rates, OS, or quality of life between the two arms.
Interestingly, around the same time as the PREMER study, Belderbos et al. published a phase 3 trial of 168 patients with LS-SCLC or ES-SCLC (without brain metastases), where patients were randomized to PCI or HA-PCI (both to 25 Gy in 10 fractions) (12). Patients underwent regular comprehensive neuropsychological testing; the primary endpoint was the HVLT total recall at 4-month post-treatment, with a drop of at least 5 points considered a failure. With a median follow-up time of 26.2 months, the investigators did not observe any significant differences in the primary endpoint, as well as performance on other cognitive tests, OS, or the cumulative incidence of brain metastases.
There are multiple possible explanations for the conflicting results observed by Belderbos et al. compared to the PREMER study. First, the cognitive benefit observed with HA-WBRT and the addition of memantine on NRG CC001 was conducted in a much larger group of patients (10); therefore, the PREMER study may have been underpowered. Second, centralized pretreatment review was not mandated on the Belderbos et al. study, and it has been demonstrated that up to 25% of plans utilizing HA can have unacceptable deviations from published HA guidelines (27). Third, plans utilizing volume modulated arc therapy require daily image guidance in order to ensure accuracy of patient setup and treatment delivery; the study by Belderbos et al. only mandated weekly image guidance. Lastly, it is important to note that neither the Belderbos et al. or the PREMER trials required the use of memantine.
While these two studies confirm the safety of HA in PCI, the conflicting results on neurocognitive function begs the question on the benefit of HA-PCI (28). The recently closed trial NRG CC003 (NCT02635009) will determine if HA-PCI should be standard of care treatment and assess the benefit of memantine with inclusion as a stratification variable (29). The ongoing phase III EORTC PRIMALung trial is randomizing patients with ES-SCLC or LS-SCLC to MRI surveillance versus MRI surveillance plus PCI. The primary endpoint is non-inferiority of OS of MRI surveillance alone compared to MRI surveillance with PCI, with stratification for memantine (30). Finally, the ongoing MAVERICK trial (SWOG S1827) is investigating if (I) surveillance MRI is non-inferior to (II) PCI (HA optional) with respect to OS for LS-SCLC and ES-SCLC (without brain metastases after first line therapy) with a secondary endpoint of cognitive function (31).
Salvage strategies after PCI failure and stereotactic radiosurgery
Given the evolution of immunotherapy and patients living longer with SCLC, studies have explored the use of salvage therapy after PCI or WBRT failure. Several small, retrospective studies suggest the use of stereotactic radiosurgery (SRS) as salvage treatment after PCI may be reasonable (32,33). These studies are often mixed studies of patients who underwent SRS for upfront definitive and salvage treatment, and given their retrospective nature must be further explored to validate the use of SRS in this setting. In the randomized clinical trial by Takahashi et al., 46% of patients in the PCI group ultimately received fractionated radiation therapy for new brain metastases versus 83% of patients in the non-PCI group (7).
More commonly, there has been growing interest in the potential role of SRS in SCLC as a definitive treatment modality for existing brain metastases in order to delay use of PCI to improve long-term cognition and quality of life. In 2020, the FIRE-SCLC study was published, which was a multicenter retrospective study that compared outcomes in 710 patients following SRS to a historical WBRT cohort (34). On propensity score-matched analyses comparing SRS with WBRT, WBRT was associated with improved time to central nervous system (CNS) progression (HR: 0.38; P<0.001). Superior OS was noted following SRS versus WBRT: 6.5 months for SRS versus 5.2 months for WBRT (P=0.003). There was no difference in CNS PFS: 4.0 versus 3.8 months for SRS and WBRT, respectively (P=0.79). The ongoing NRG CC009 phase 3 study is further investigating the role of SRS in this setting. The study is randomizing patients with ES-SCLC with 10 brain metastases or fewer to SRS or HA-WBRT with memantine (35).
Conclusions
Prophylactic cranial irradiation plays an important role in the management of SCLC. The use of PCI in LS-SCLC and ES-SCLC has been demonstrated to improve OS and decrease the development of brain metastases, which is being re-assessed in the modern era of improved imaging modalities and systemic therapies. Neurotoxicity secondary to PCI use can potentially be partially ameliorated with avoidance of critical brain structures such as the hippocampus and the use of pharmaceuticals, such as memantine. Ongoing clinical trials in the era of modern systemic therapies will help to better inform the role of incorporating HA-PCI or, potentially, an imaging surveillance strategy over PCI.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-12/coif). RMS reports a research grant from the NIH unrelated to the present work. PDB is a contributor and receives an honorarium from UpToDate, which is outside submitted work. DMT receives institutional support from Novocure Ltd. and is a consultant for Boston Scientific Corporation, both for unrelated research. JDP receives personal fees from Varian Medical Systems, he serves as a consultant for Huron Consulting group, he is on the advisory board for Novocure, and has research grants from Kroger and the National Institutes of Health, outside submitted work. WGB is on the scientific advisory board and serves as a consultant to GE Healthcare, all funds are paid to institution, unrelated to the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ganti AKP, Loo BW, Bassetti M, et al. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:1441-64. [Crossref] [PubMed]
- Kalemkerian GP, Gadgeel SM. Modern staging of small cell lung cancer. J Natl Compr Canc Netw 2013;11:99-104. [Crossref] [PubMed]
- Seute T, Leffers P, ten Velde GP, et al. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer 2004;100:801-6. [Crossref] [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [Crossref] [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [Crossref] [PubMed]
- Lehrer EJ, Jones BM, Dickstein DR, et al. The Cognitive Effects of Radiotherapy for Brain Metastases. Front Oncol 2022;12:893264. [Crossref] [PubMed]
- Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:663-71. [Crossref] [PubMed]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Brown PD, Gondi V, Pugh S, et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol 2020;38:1019-29. [Crossref] [PubMed]
- Rodríguez de Dios N, Couñago F, Murcia-Mejía M, et al. Randomized Phase III Trial of Prophylactic Cranial Irradiation With or Without Hippocampal Avoidance for Small-Cell Lung Cancer (PREMER): A GICOR-GOECP-SEOR Study. J Clin Oncol 2021;39:3118-27. [Crossref] [PubMed]
- Belderbos JSA, De Ruysscher DKM, De Jaeger K, et al. Phase 3 Randomized Trial of Prophylactic Cranial Irradiation With or Without Hippocampus Avoidance in SCLC (NCT01780675). J Thorac Oncol 2021;16:840-9. [Crossref] [PubMed]
- Breen WG, Brown PD, Laack NN. Hippocampal Avoidance Prophylactic Cranial Irradiation for SCLC. J Thorac Oncol 2021;16:e41-2. [Crossref] [PubMed]
- Brown PD, Parsons MW, Rusthoven CG, et al. Hippocampal Avoidance Prophylactic Cranial Irradiation: A New Standard of Care? J Clin Oncol 2021;39:3093-6. [Crossref] [PubMed]
- Tomassen ML, Pomp J, van der Stap J, et al. The overall survival impact of prophylactic cranial irradiation in limited-stage small-cell lung cancer: A systematic review and meta-analysis. Clin Transl Radiat Oncol 2022;33:145-52. [Crossref] [PubMed]
- Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467-74. [Crossref] [PubMed]
- Simone CB 2nd, Bogart JA, Cabrera AR, et al. Radiation Therapy for Small Cell Lung Cancer: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol 2020;10:158-73. [Crossref] [PubMed]
- Yang Y, Zhang D, Zhou X, et al. Prophylactic cranial irradiation in resected small cell lung cancer: A systematic review with meta-analysis. J Cancer 2018;9:433-9. [Crossref] [PubMed]
- Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. [Crossref] [PubMed]
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021;39:619-30. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Chu X, Zhu Z. Prophylactic cranial irradiation in small cell lung cancer: an update. Curr Opin Oncol 2023;35:61-7. [Crossref] [PubMed]
- Yu NY, Sio TT, Ernani V, et al. Role of Prophylactic Cranial Irradiation in Extensive-Stage Small Cell Lung Cancer. J Natl Compr Canc Netw 2021;19:1465-9. [Crossref] [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [Crossref] [PubMed]
- Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:77-84. [Crossref] [PubMed]
- Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys 2008;71:64-70. [Crossref] [PubMed]
- Gondi V, Cui Y, Mehta MP, et al. Real-time pretreatment review limits unacceptable deviations on a cooperative group radiation therapy technique trial: quality assurance results of RTOG 0933. Int J Radiat Oncol Biol Phys 2015;91:564-70. [Crossref] [PubMed]
- Maragkoudakis E, Kouloulias V, Grenzelia M, et al. Impact of Hippocampal Avoidance - Prophylactic Cranial Irradiation in Small Cell Lung Cancer Patients. Cancer Diagn Progn 2022;2:279-84. [Crossref] [PubMed]
- Gondi V, Pugh SL, Mehta MP, et al. NRG Oncology CC003: A randomized phase II/III trial of prophylactic cranial irradiation with or without hippocampal avoidance for small cell lung cancer. J Clin Oncol 2019;37:TPS8578.
- PRophylactic Cerebral Irradiation or Active MAgnetic Resonance Imaging Surveillance in Small-cell Lung Cancer Patients (PRIMALung Study) - Full Text View - ClinicalTrials.gov [Internet]. [cited 2023 Jun 23]. Available online: https://clinicaltrials.gov/ct2/show/NCT04790253
- Southwest Oncology Group. MRI Brain Surveillance Alone Versus MRI Surveillance and Prophylactic Cranial Irradiation (PCI): A Randomized Phase III Trial in Small-Cell Lung Cancer (MAVERICK) [Internet]. clinicaltrials.gov; 2022 Jul [cited 2023 Feb 1]. Report No.: NCT04155034. Available online: https://clinicaltrials.gov/ct2/show/NCT04155034
- Faramand A, Niranjan A, Kano H, et al. Primary or salvage stereotactic radiosurgery for brain metastatic small cell lung cancer. J Neurooncol 2019;144:217-25. [Crossref] [PubMed]
- Yomo S, Hayashi M. Is stereotactic radiosurgery a rational treatment option for brain metastases from small cell lung cancer? A retrospective analysis of 70 consecutive patients. BMC Cancer 2015;15:95. [Crossref] [PubMed]
- Rusthoven CG, Yamamoto M, Bernhardt D, et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol 2020;6:1028-37. [Crossref] [PubMed]
- NRG Oncology. Phase III Trial of Stereotactic Radiosurgery (SRS) Versus Hippocampal-Avoidant Whole Brain Radiotherapy (HA-WBRT) for 10 or Fewer Brain Metastases From Small Cell Lung Cancer [Internet]. clinicaltrials.gov; 2022 Apr [cited 2023 Feb 5]. Report No.: NCT04804644. Available online: https://clinicaltrials.gov/ct2/show/NCT04804644


