
Sequencing systemic therapy in hormone-receptor positive metastatic breast cancer: a modern paradigm
Introduction
Breast cancer (BC) is the most common malignancy among women and second most common cancer diagnosed worldwide. According to GLOBOCAN 2020, about 2 million new BCs were diagnosed and >685,000 BC related deaths occurred (1), an 18% increase in the risk of BC-related death in the 4 years from 2008–2012 (2). Over the last decade, the improvements in survival achieved with new therapies for metastatic breast cancer (MBC) have been groundbreaking.
With the continued success of cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors, an emerging generation of selective estrogen receptor degraders (SERDs), and new antibody drug-conjugates (ADCs), the landscape and sequencing of hormone receptor positive (HR+) MBC has become increasingly complicated. Here we aim to review current approaches to the sequential management of HR+ MBC.
Diagnostic approach
Together, estrogen receptor (ER) and/or progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) constitute the core prognostic and predictive markers in the treatment of both early stage and advanced BC, although other markers continue to emerge. Given a small but significant rate of discordant expression in prognostic markers of approximately 15%, biopsy at metastatic presentation is recommended (3). Breast cancer expressing either ER and/or PR, hereafter HR+, BC constitutes about 65% of all BC. About 20% of HR+ BC will also be positive for HER2, either by immunohistochemistry (IHC, scored 3+) or in situ hybridization (ISH) (4). Where HER2 expression has previously been considered binary, the advent of trastuzumab deruxtecan (T-DXd) has introduced HER2-Low as a new entity, which accounts for as much as 60% of BC and includes many cases of HR+ disease. HER2-Low BC is defined as BC scored IHC 1+ or both IHC 2+ and ISH negative.
Standard first-line therapy is defined by HR and HER2, but early coordination of care can ensure patients are appropriately selected for second line therapy (5). As such, at metastatic diagnosis, early testing for PIK3CA mutation should be performed. PIK3CA mutations are activating mutations present in 40% of BCs that play a key role in cellular proliferation and angiogenesis (6). As an activating mutation, it is often present throughout the disease course rather than as a mutation that emerges as a mechanism of resistance. Additionally, germline genetic testing should be obtained if it has not previously been considered. The presence of breast cancer susceptibility gene 1 or 2 (BRCA1 or BRCA2) pathogenic variants (BRCApv, previously “mutations”) creates therapeutic options in the form of poly[ADP-ribose] polymerase (PARP) inhibitors as well as expands options for appropriate counseling, testing, and screening for family members (7,8). Together with physical exam, systemic imaging, comprehensive laboratory assessment, and assessment of performance status, this constitutes our initial diagnostic approach in HR+ MBC.
Initial management
As with early-stage disease, multidisciplinary care is critical to best practice in advanced breast cancer and should be incorporated early. While the role of palliative and supportive care, social work, financial counselors, support groups, patient advocate groups, physical therapy, nutrition, and patient navigation, among others, are outside the scope of this article, they bear mentioning (9,10). Additionally, for patients with bone metastases, addition of an osteoclast inhibitor or bone modifying agent such as bisphosphonates or denosumab is recommended given that they have been shown to significantly reduce the time frequency and time to onset of skeletal related events, which come with significant morbidity to patients (11,12). Finally, clinical trials are an appropriate treatment option for patients at any time point in the cancer care continuum and should always be considered (13,14).
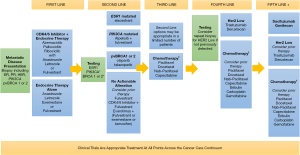
Standard first-line therapy for HR+HER2− MBC includes aromatase inhibitor (AI) therapy in combination with CDK4/6 inhibitor rather than AI monotherapy (Figure 1). This approach has many advantages including delaying time to cytotoxic chemotherapy, the freedom of oral therapy, reduced disease burden and improved progression free and overall survival (OS). For pre-menopausal women, combination with either surgical oophorectomy or ovarian function suppression using luteinizing hormone agonists is recommended.
Three CDK4/6 inhibitors are available, including abemaciclib, palbociclib, and ribociclib (Table 1). Abemaciclib was evaluated in the MONARCH 3 trial in combination with letrozole or anastrozole in first-line HR+HER2− MBC and found to improve median progression-free survival (PFS), the primary endpoint, as compared to letrozole or anastrozole alone [28.1 vs. 14.7 months, hazards ratio =0.54, 95% confidence interval (CI): 0.41–0.69] (15,27). Unique to abemaciclib, there was a notable risk of diarrhea (9.6%, grade 3) and like other CDK4/6 inhibitors, neutropenia (21%, grade 3). At the European Society of Clinical Oncology Congress 2022, interim analysis update showed a favorable trend towards (OS), but it had not yet met data maturity for reporting (median, 65.1 months with combination vs. 48.8 months with AI alone, hazards ratio =0.70, 95% CI: 0.50–0.98) (28). Although immature, this hazard ratio for OS and PFS with abemaciclib parallels the reports for ribociclib (17,29).
Table 1
| Therapy (target, if any) | Preferred line | Combined with | Approved dose | Notable adverse events* | Key endpoint |
|---|---|---|---|---|---|
| Abemaciclib | 1 | AI or fulvestrant | 150 mg PO twice daily (15) | Diarrhea, neutropenia, infections, nausea, abdominal pain, anemia, vomiting, alopecia, decreased appetite, leukopenia, increased ALT | mPFS hazards ratio =0.54 (abemaciclib + AI vs. AI alone) (15) |
| Palbociclib | 1 | AI or fulvestrant | 125 mg PO once daily for 21 days, followed by 7 days off, repeat every 28 days (16) | Neutropenia, leukopenia, fatigue, nausea, arthralgia, alopecia, diarrhea, cough, anemia, back pain, headache, hot flush | mPFS hazards ratio =0.58 (palbociclib + letrozole vs. letrozole alone) (16) |
| Ribociclib | 1 | AI or fulvestrant | 600 mg PO once daily for 21 days, followed by a 7-day rest period to complete a 28-day treatment cycle (17) | Neutropenia, nausea, fatigue, diarrhea, alopecia, vomiting, arthralgia, leukopenia, constipation, headache, hot flash, back pain, cough, rash, anemia, decreased appetite, abnormal LFTs | mOS hazards ratio =0.76 (ribociclib + letrozole vs. letrozole alone) (17) |
| Alpelisib (PIK3CA mutation) | 2 | Fulvestant | 300 mg PO once daily (18) | Hyperglycemia, diarrhea, nausea, decreased appetite, rash, vomiting, weight loss, stomatitis, fatigue, asthenia | mOS hazards ratio =0.86 (alpelisib + fulvestrant vs. fulvestrant alone) (18) |
| Elacestrant (ESR1 mutation) | 2 | Alone | 345 mg PO once daily (19) | Nausea | mPFS hazards ratio =0.55 (elacestrant vs. fulvestrant or aromatase inhibitor) (19) |
| Olaparib (pvBRCA1 or 2) | 2 | Alone | 300 mg PO twice daily (20) | Nausea, anemia, neutropenia, vomiting, fatigue, diarrhea | mPFS hazards ratio =0.58 (olaparib vs. TPC) (20) |
| Talazoparib (pvBRCA1 or 2) | 2 | Alone | 1 mg PO once daily (8) | Anemia, neutropenia, thrombocytopenia, leukopenia, fatigue, nausea, headache, alopecia, vomiting, diarrhea, constipation, decreased appetite, back pain | mPFS hazards ratio =0.54 (talozoparib vs. TPC) (8) |
| Fulvestrant | 1 or 2 | Alone or with other agents as indicated | 500 mg IM on days 1, 15, and 29; maintenance: 500 mg IM once monthly (21) | No notable adverse events* | mPFS hazards ratio =0.79 (fulvestrant vs. anastrozole) (21) |
| Everolimus | 2 | AI or fulvestrant | 10 mg PO once daily (22) | Oral mucositis, fatigue, rash, anemia, diarrhea, pneumonitis | mPFS hazards ratio =0.61 (everolimus + fulvestrant vs. fulvestrant alone) (23) |
| Trastuzumab deruxtecan (HER2 low) | 4 | Alone | 5.4 mg/kg IV once every 3 weeks (24) | Neutropenia, anemia, thrombocytopenia, leucopenia, nausea, vomiting, diarrhea, constipation, increased AST, fatigue, decreased appetite, alopecia, interstitial lung disease/pneumonitis, decreased left ventricular ejection fraction | mOS hazards ratio =0.64 (trastuzumab deruxtecan vs. TPC) (24) |
| Sacituzumab govitecan | 4 or 5 | Alone | 10 mg/kg IV on days 1 and 8 of a 21-day treatment cycle (25) | Neutropenia, anemia, diarrhea, nausea, vomiting, fatigue, alopecia, decreased appetite, nervous system disorders, febrile neutropenia (26) | mOS hazards ratio =0.78 (sacituzumab govitecan vs. TPC) (25) |
*, notable AE include any grade AE occurring in ≥20% of patients and/or G3 AE occurring in ≥5%. List of approved therapies for HR+ MBC and any associated target indicated in their approval (parentheses). Includes preferred line of therapy for treatment consideration and dosing partner, if any, it is recommended therapy be combined with as well as approved starting dose (columns 2–4, respectively). Notable AEs are listed which include all-grade AEs occurring in ≥20% of patients or grade 3 toxicities occurring in ≥5% of patients. Key endpoint includes best published endpoint for study drug—including mPFS, or, where available, mOS. HR, hormone receptor; MBC, metastatic breast cancer; AI, aromatase inhibitor; PO, Per Os (by mouth); ALT, alanine aminotransferase; AEs, adverse events; mPFS, median progression free survival; mOS, median overall survival; LFT, liver function tests; PIK3CA, phosphatidylinositol-4,5-bisphosphate-3-kinase catalytic subunit alpha; ESR1, estrogen receptor 1; pvBRCA1 or 2, pathogenic variant BReast Cancer gene 1 or 2; TPC, treatment of physician’s choice; IM, intramuscular; IV, intravenous; HER2, human epidermal growth factor receptor 2; AST, aspartate transaminase.
MONALEESA-2 showed an improvement in the median PFS (mPFS) from 16.0 months to 25.3 months for the addition of ribociclib to letrozole compared to letrozole alone (hazards ratio =0.56, 95% CI: 0.45–0.70) (29). When mature OS was reported, median OS was 63.9 months with ribociclib plus letrozole and 51.4 months letrozole alone (hazards ratio =0.76; 95% CI: 0.63–0.93; P=0.008) (17). Ribociclib also had significant rates of grade 3 neutropenia (52%), notable rates of abnormal liver function tests (grade 3, 8%) and QT-prolongation (all grade, 3.6%), however diarrhea was not commonly seen.
In PALOMA-2, which randomized patients to palbociclib and letrozole or letrozole alone, similar mPFS to MONALEESA-2 and MONARCH-3 was observed, with palbociclib improving PFS from letrozole alone (median, 14.5 vs. 24.8 months; hazards ratio =0.58, 95% CI: 0.46–0.72) (16). While a trend towards prolonged OS was seen favoring the combination, this did not reach statistical significance (53.9 vs. 51.2 months, hazards ratio =0.956, 95% CI: 0.77–1.17) (30). PALOMA-2 was the first of the three CDK4/6 inhibitor trials, and as such the negative result may have been impacted by unique challenges including that it was underpowered for how well patients ultimately performed on CDK4/6 inhibitors, and there was a high rate of crossover. Subsequent real-world data has suggested that Palbociclib does confer an OS advantage (31).
Whether there is a singular best in class CDK4/6 inhibitor is not clear as they have never been compared head-to-head. Currently, the National Comprehensive Cancer Network guidelines differentiate between ribociclib (category 1) and palbociclib or abemaciclib (category 2A) owing to the OS data from phase III trials (13). Other factors that continue to weigh in shared decision making include financial toxicity and side effect profile for each individual patient.
The above data is relevant to patients who experience progressive disease >12 months after discontinuation of adjuvant endocrine therapy. However, for patients who present with metastatic disease ≤12 months after discontinuing AI in the adjuvant setting or who progress on adjuvant AI therapy, CDK4/6 inhibitor therapy in combination with fulvestrant is often utilized in the first-line MBC setting. All three CDK4/6 inhibitors, abemaciclib, palbociclib, and ribociclib have data supporting their efficacy in this setting (21,32-34).
Interval assessments
It is our standard approach to do systemic imaging every 3 to 6 months. Options for systemic imaging include computed tomography (CT) of the Chest, Abdomen, and Pelvis with Technetium (Tc99) Bone Scan or integrated positron emission tomography (PET)/CT. We engage in shared decision making regarding best interval of imaging and optimal imaging strategy, based on factors including disease history and response, financial barriers, and disease burden. Imaging is always paired with regular laboratory assessments and clinical examinations including history, physical, and review of systems; any clinical concern for disease progression should prompt earlier evaluation. Decision to change to discontinue therapy is driven by many factors. If at any time the patient experiences diminished quality of life due to adverse effects of cancer treatment not alleviated by supportive care and dose modification, discontinuation or change is appropriate. Evidence of disease progression should prompt change in therapy, although consideration for whether a subtle difference in measurements (a few millimeters for example) meets RECIST criteria for disease progression or is clinically relevant to warrant a change in therapy for a patient tolerating current therapy with few side effects is another opportunity for shared decision making. For patients with MBC showing oligometastatic progression, sometimes local therapy with radiation or interventional radiology technique, may be utilized to maintain current systemic therapy, again particularly in the setting of prolonged response and good tolerance.
For patients with MBC and rapid, perhaps unexpected progression, considerations should include: assessment to adherence to oral therapy, markers of resistance (ESR1), and/or tumor heterogeneity (repeat biopsy to confirm ER/PR/HER2 status). Patients with rapidly progressive MBC may not be ideal candidates for second-line endocrine therapy, and if utilized, should be followed judiciously.
Second-line management
Beyond progression on first-line CDK4/6 inhibitor therapy for MBC, the treatment landscape becomes complex and requires and nuanced understanding of both one’s patient’s personal preferences and disease biology. Germline BRCA status, presence of either PIK3CA or ESR1 mutations, and patient preferences around side effects and oral therapy all can factor into shared decision making (Figure 1 and Table 1).
Continuation of CDK4/6 inhibitor beyond progression has been questioned as one strategy to extend hormone-directed therapy in the metastatic setting. Two trials have reported initial findings and a third trial is ongoing. The first, the MAINTAIN trial, randomized patients to exemestance or fulvestrant with or without ribociclib after progression on any CDK4/6 inhibitor in combination with an AI (35). Approximately 86% of patients had received prior palbociclib, 60% had visceral metastasis, and the median duration of CDK4/6 inhibitor therapy was 17 months in the placebo arm and 15.5 months in the ribociclib arm. PFS showed a benefit for the addition of ribociclib (median, 5.29 vs. 2.76 months, hazards ratio =0.57, 95% CI: 0.39–0.95). Conversely, the PACE trial included patients who progressed on palbociclib and AI, and randomized them to fulvestrant with or without palbociclib. Over 50% of patients had visceral disease, and >75% had been on first-line palbociclib for >1 year. There was no difference in mPFS for continuation of palbociclib (4.6 months with, 4.8 months without; hazards ratio =1.11, 95% CI: 0.74–1.66). The ongoing postMONARCH trial (NCT05169567) will look at abemaciclib plus fulvestrant vs. placebo/fulvestrant for patients who have previously progressed on any CDK4/6 inhibitor and AI (36). Currently, we are not routinely utilizing CDK4/6 inhibitors beyond progression outside of a clinical trial.
For patients who have tumors with activating PIK3CA mutations, the US Food and Drug Administration have approved alpelisib in combination with fulvestrant. Alpelisib was first tested in the SOLAR-1 trial in combination with fulvestrant vs. fulvestrant alone and found to improve mPFS among patients with PIK3CA mutated cancers (median, 11.0 vs. 5.7 months; hazards ratio =0.65; 95% CI: 0.50–0.85; P<0.001) (37). Subsequent follow-up showed that although OS did not meet the pre-specified statistical endpoints, alpelisib did confer a 7.9-month numeric improvement in OS (39.3 months for alpelisib-fulvestrant vs. 31.4 months fulvestrant alone, hazards ratio =0.86 (95% CI: 0.64–1.15; P=0.15) (18). One of the common criticisms of the SOLAR-1 data is that it was collected in a pre-CDK4/6 inhibitor era, so the BYLieve study was designed to provide outcomes data after progression on CDK4/6 inhibitor therapy. A non-randomized, phase II design, BYLieve showed a mPFS of 7.3 months and median overall survival (mOS) of 17.3 months (38). This compares favorably to historical standards of fulvestrant as a single agent after progression on CDK4/6 inhibitor. Another frequent criticism of alpelisib is toxicity. SOLAR-1 reported significant rates of grade 3 hyperglycemia (36.6%), rash (9.9%), and diarrhea (6.7%); 25% of patients discontinued alpelisib due to adverse events (AE). However, with interventions like the addition of non-sedating antihistamines (cetirizine, loratadine or fexofenadine) for the first 8 weeks of therapy to reduce incidence of rash, and metformin prescribed prior to initiation of alpelisib for prevention of hyperglycemia adverse event mitigation is possible (5,39). Given lead-time anticipating second-line therapy with aleplisib, partnering with primary care and physical therapy to optimize insulin resistance and physical exercise before alpelisisb is also likely to yield improved tolerance.
Germline pathogenic variants in BRCA1 or BRCA2 (pvBRCA1/2) are actionable in HR+ MBC with PARP inhibitors, which allows for accumulation of DNA damage ultimately leading to cell death. Talazoparib, as compared to chemotherapy (capecitabine, gemcitabine or vinorelbine) improved mPFS from 5.6 months for chemotherapy to 8.6 months for the PARP inhibitor (hazards ratio =0.54, 95% CI: 0.41–0.71). A proportion of 55.9% of patients enrolled were HR+ and favored talazoparib on subgroup analysis (8). Similarly, olaparib, when compared to the same chemotherapeutics, improved PFS from 4.2 to 7.0 months (hazards ratio =0.58, 95% CI: 0.43–0.80) (7). About half the patients enrolled were HR+ and subgroup analysis favored olaparib. Neither trial showed a statistically significant difference in OS (8,20).
After progression on a CDK4/6 inhibitor, particularly if a patient is deemed not a good candidate for treatment that targets PIK3CA or PARP, it is important to test for the acquired mutation of estrogen receptor 1 (ESR1). In the phase III EMERALD trial, patients were randomized after disease progression on CDK4/6 inhibitor and AI to fulvestrant vs. elacestrant, an oral SERD (19). All patients enrolled were centrally tested for the presence of cell-free circulating DNA for ESR1, which was a stratification variable. A proportion of 47.8% of patients had an ESR1 mutation. Median PFS for the study population and ESR1 mutation population were reported. For the study population, elacestrant vs. fulvestrant improved mPFS to 2.8 months from 1.9 months (hazards ratio =0.70, 95% CI: 0.55–0.88, P=0.0018), and for those with ESR1 mutated tumors elacestrant 3.8 months vs. fulvestrant 1.9 months (hazards ratio =0.55, 95% CI: 0.39–0.77, P=0.0005). Given significant rates of progression across both arms in the initial weeks of the study, suggesting high rates of endocrine resistance in this second-line setting, 6-month PFS was reported as well, which specifically among those with ESR1 mutations was 40.8% for elacestrant and 19.1% for fulvestrant. Nausea, vomiting, decreased appetite, fatigue, and arthralgias are common AE. Earlier work has suggested it is potentially fruitful to switch therapy from AI to fulvestrant with the emergence of ESR1 mutations and may warrant further investigation now that ESR1 directed treatments are approved (40).
Many other SERDs are emerging in clinical trials. Camizestrant was compared at two dose levels (75 and 150 mg) to fulvestrant in SERENA-2 (NCT04214288), and both doses improved mPFS (7.2 months for 75 mg, 7.7 months for 150 mg) compared to fulvestrant (3.7 months, hazards ratio =0.58, 90% CI: 0.41–0.81 at 75 mg and hazards ratio =0.67, 90% CI: 0.48–0.92 at 150 mg). Those enrolled with an ESR1 mutation, 36.7% of patients showed a greater benefit to camizestrant than the overall population (41). Imlunestrant, another oral SERD in development, in the phase 1b dose expansion trial, EMBER (NCT04188548), showed an objective response rate (ORR) of 36% in combination with abemaciclib and 44% in combination with both abemaciclib and AI (42). The phase 3 EMBER-3 trial (NCT04975308) is currently enrolling to explore the combination. Lasofoxifene is a tissue-selective estrogen antagonist that does not degrade the receptor thought to have affinity for cancers with ESR1 mutations (43). In the phase 2 ELAINE 1 study (NCT03781063), patients with ESR1 mutated HR+ MBC were randomized between lasofoxifene and fulvestrant. Lasofoxifene improved mPFS from 16.2 to 24.2 weeks (hazards ratio =0.699, 95% CI: 0.434–1.125). As different hormone-driven therapies emerge for the second-line treatment of HR+ MBC, it is likely that cross comparison will be difficult owing to variable populations and subtle differences in both drug affinity and mechanism of action. However, having other choices will continue to create opportunities to tailor therapy to patients’ unique preferences based on efficacy, toxicity profiles, and cost.
For patients whose tumors do not have an actionable alteration, addition of everolimus to fulvestrant is yet another option. Building on the earlier results of BOLERO-II study, the randomized phase II PrE0102 trial compared fulvestrant alone to everolimus plus fulvestrant, with the addition of everolimus doubling PFS (5.1 vs. 10.3 months, hazards ratio =0.61, 95% CI: 0.4–0.92) (22,23). With the addition of a prophylactic oral dexamethasone mouth rinse, the risk of grade 2 mucositis at 8 weeks fell from 33% in BOLERO-2 to 2%, suggesting the toxicity of everolimus can be medically managed (44).
Emerging in this space is the pan-AKT kinase inhibitor, capivasertib. The AKT pathway has been implicated in resistance to endocrine therapy and, as such, is an attractive target (45). Capivasertib was previously studied in the phase 2 FAKTION trial among HR+ MBC. Patients randomized to capivasertib plus fulvestrant as compared to placebo plus fulvestrant demonstrated a 5.5-month improvement in mPFS and a similar increase in mOS (46). Hypertension, rash, and diarrhea stand out as significant toxicities. The phase 3 CAPItello-291 continued the same randomization schema and design, again including patients regardless of phosphatidylinositol-3 kinase /AKT/phosphatase and tensin homolog (PI3K/AKT/PTEN or AKT pathway) pathway status, and ultimately 41% of enrolled patients had AKT pathway-altered tumors on central testing. Median PFS was 7.2 months with capivasertib and fulvestrant compared to 3.6 months for placebo-fulvestrant [hazards ratio =0.60; 95% CI: 0.51–0.71; P<0.001 (47)]. The benefit was maintained the AKT-pathway altered population, however in an exploratory analysis of patients with AKT-non-altered tumors and known sequencing results, the margin of benefit seemed to significantly diminish [hazards ratio =0.79, 95% CI: 0.61–1.02 (47)]. However, clinical utility may be limited by significant grade 3 AE including diarrhea (9.3%), hyperglycemia (2.3%), and stomatitis (2.0%) (47).
Third-line/endocrine-refractory management
Beyond endocrine-driven therapy, it is usually necessary to turn to chemotherapy for continued treatment. While some patients may demonstrate endocrine sensitivity and be appropriate for endocrine-driven treatment beyond second-line, most will not (Figure 1). As such, choosing therapy based on toxicity profile as well as efficacy remains crucial. Single agent approaches rather than multi-agent chemotherapy regimens are preferred. Taxanes and capecitabine stand out as effective, reasonably tolerated agents in this setting and allow for patient preference to factor into decision-making (48-51). Considerations include that paclitaxel can be given with a degree of hepatic impairment, but also requires pre-medications given risk of infusion reactions; docetaxel has a higher degree of myelosuppression and fluid retention, which requires steroid premedication; and while with nab-paclitaxel patients may avoid infusion reactions and pre-medications, both paclitaxel and nab-paclitaxel seem to carry a higher risk of neuropathy and myalgias than docetaxel.
Alternatively, oral capecitabine causes little neuropathy or alopecia and has demonstrated some activity penetrating the blood-brain barrier but carries a risk of significant diarrhea and hand-foot syndrome (51,52). Prior studies have shown capecitabine to be effective therapy in hormone-resistant MBC (51,52).
While some scenarios such as visceral crisis or rapidly progressive disease may warrant consideration of multi-agent approaches in the metastatic, refractory setting, newer evidence suggests these combinations may not be more effective than even non-cytotoxic therapies (53). Combination options include doxorubicin and cyclophosphamide, epirubicin and cyclophosphamide, CMF (cyclophosphamide, methotrexate, fluorouracil), docetaxel and capecitabine, gemcitabine with either paclitaxel or carboplatin, and carboplatin with either paclitaxel or albumin-bound paclitaxel (13).
ADC
DESTINY-Breast-04 introduced HER2-low BC into parlance and practice. The trial, enrolling patients with Her2-low MBC, defined previously, that had previously progressed on 1–2 prior lines of cytotoxic chemotherapy as well endocrine therapy randomized patients between treatment of physician’s choice (TPC, capecitabine, eribulin, gemcitabine, paclitaxel, or nab-paclitaxel) or trastuzumab deruxtecan (T-DXd) (24). T-DXd is an ADC that through a cleavable linker combines the humanized anti-HER2 monoclonal antibody to a topoisomerase-I inhibitor payload. It is through the cleavable linker and high ratio of cytotoxic payload deliver (8:1) that the ADC is thought to garner its potency, creating a bystander effect in neighboring tumors cells despite heterogenous target expression (54).
DESTINY-Breast-04 enrolled predominately patients with HR+ disease (88.7%) (24). Compared to chemotherapy, T-DXd significant improved PFS among patients with HR+ HER2-low MBC (10.1 vs. 5.4 months, hazards ratio =0.51; P<0.001) and OS (23.9 vs. 17 months, hazards ratio =0.64; P=0.003). Interstitial lung disease, a potentially fatal adverse event with T-DXd, occurred in 12.1% of the study population (all grades) with 0.8% experiencing grade 5 events. Judicious monitoring for early pulmonary symptoms, coupled with prompt dose interruption and early initiation of glucocorticoids to minimize the risk of serious AE is advised. Given these remarkable results, ongoing trials are exploring earlier line utilization of T-DXd for HER2-low MBC (DESTINY-Breast06, NCT04494425), but at present represents a standard approach in fourth-line metastatic therapy for patients who have HER2-Low MBC (Figure 1, Table 1).
A second ADC, sacituzumab govitecan, originally approved for triple negative breast cancer, has also shown efficacy in HR+ MBC (25,55). Sacituzumab govitecan is an ADC that targets the human trophoblast cell-surface antigen 2 (Trop-2) expressed in most MBC with a topoisomerase-I inhibitor payload bound with a hydrolysable linker. TROPiCS-02 compared sacituzumab govitecan vs. TPC (eribulin, vinorelbine, gemcitabine, or capecitabine) in patients with HR+ MBC who have received ≤2 to ≤4 prior lines of chemotherapy (25). Of note, prior lines of endocrine therapy in the metastatic setting did not count towards lines of chemotherapy for eligibility. Compared to standard chemotherapy, sacituzumab govitecan improved PFS as compared to TPC (median, 5.5 vs. 4.0 months, hazards ratio =0.661, 95% CI: 0.529–0.826) and OS (median, 14.2 vs. 11.2 months; hazards ratio =0.789, 95% CI: 0.646–0.964). Significant AEs (grade 3–4) include diarrhea (10%), fatigue (8%), and cytopenias (leukopenia, 38%; neutropenia, 53%; and lymphopenia 21%) (25). As such, sacituzumab govitecan is an option for patients in the 4th or 5th line setting and beyond (Figure 1, Table 1).
While it may be easy to draw comparison between the reported mPFS, mOS, and adverse event profiles of T-DXd and sacituzumab govitecan, it is important to note key differences in the trials. Whereas DESTINY-Breast04 accrued patients “with a median of 3 prior lines of therapy” that included endocrine therapy, 58% and 41% of the study population had only 1 or 2 prior lines of systemic chemotherapy for MBC, respectively (56). Conversely, in TROPiCS-02, patients had a median of 7 lines of prior therapy when endocrine therapy was considered, and 3 prior lines of systemic chemotherapy for MBC, making the population a much more heavily pre-treated group of patients (26). Additionally, if demographic differences had an impact, it will be difficult to determine. DESTINY-Breast 04 included 40% Asian patients and 70% of patients had liver metastasis and 30% lung metastasis—although overlap between groups is not reported; compared to TROPiCS-02 which included only 3% Asian patients and 95% visceral metastasis (24,25). Additional efforts to understand these drugs in sequence and in earlier lines of therapy are underway.
Another ADC on the horizon is datopotamab deruxtecan (Dato-DXd), a monoclonal antibody targeting Trop-2 attached via a stable cleavable linker to a topoisomerase-I inhibitor payload. Results from the phase I TROPION-PanTumor01 study exploring the HR+ MBC cohort were presented in 2022. Among 41 patients with HR+ MBC, all heavily pre-treated with a median of 5 prior lines of therapy, Dato-DXd resulted in an ORR of 27% and a clinical benefit rate (CBR) of 44%; median PFS was 8.3 months (95% CI: 5.5–11.1) (57). Safety analysis showed significant toxicities (≥ grade 3) including anemia (7%), stomatitis (10%), and lymphopenia (15%). Notable all-grade toxicities included ocular toxicities, GI toxicities, and fatigue. Dato-DXd is being evaluated now in TROPION-Breast01 (NCT05104866), a phase 3 randomized trial evaluating Dato-DXd compared to TPC (eribulin, capecitabine, vinorelbine, or gemcitabine) in HR+ MBC in second-line chemotherapy or beyond.
Conclusions
Increasingly, the modern paradigm of treatment sequencing in HR+ MBC allows for tremendous shared-decision making and personalization between patient and physician. Whether considering which CDK4/6 inhibitor based on efficacy, dosing, AEs and cost is best for an individual patient or utilizing advanced genetic and genomic testing together with many of the same factors to drive decision making the second-line, therapy is increasingly tailored.
As a patient shifts from endocrine-based treatment to cytotoxic chemotherapy or ADCs, again, latitude for dosing schedule, side effect profile, and patient preference exists. Future directions including targeted oral therapies to overcome endocrine resistance and the next-generation of ADC continue to expand the possibilities.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-22/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-22/coif). DSD received consulting fees from Data Safety Monitoring Boards for AstraZeneca and GSK, and speaker fees from Kronos Biotech. He owns stock options in Doximity and MIDI, and is the Co-Chief Medical Officer of Global Cancer Institute and a member of Hope Foundation. SLG reports consulting or advisory role for Pfizer, Daiichi Sankyo, Eli Lilly, AstraZeneca, Genentech, SeaGen, Novartis and Menarini; stock ownership for HCA Healthcare; and writing support from AstraZeneca/Daiichi Sankyo; travel support from Paxman; non-profit leadership position (paid) as a Medical Advisor of Dr. Susan Love Foundation for Breast Cancer Research. SAH has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- DeSantis CE, Bray F, Ferlay J, et al. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer Epidemiol Biomarkers Prev 2015;24:1495-506. [Crossref] [PubMed]
- Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 2012;30:2601-8. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 2018;142:1364-82. [Crossref] [PubMed]
- Rugo HS, Lacouture ME, Goncalves MD, et al. A multidisciplinary approach to optimizing care of patients treated with alpelisib. Breast 2022;61:156-67. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012;490:61-70. [Crossref] [PubMed]
- Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017;377:523-33. Erratum in: N Engl J Med 2017;377:1700. [Crossref] [PubMed]
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753-63. [Crossref] [PubMed]
- Anampa-Guzmán A, Freeman-Daily J, Fisch M, et al. The Rise of the Expert Patient in Cancer: From Backseat Passenger to Co-navigator. JCO Oncol Pract 2022;18:578-83. [Crossref] [PubMed]
- Osman H, Shrestha S, Temin S, et al. Palliative Care in the Global Setting: ASCO Resource-Stratified Practice Guideline. J Glob Oncol 2018;4:1-24. [Crossref] [PubMed]
- Coleman R, Hadji P, Body JJ, et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol 2020;31:1650-63. [Crossref] [PubMed]
- Van Poznak C, Somerfield MR, Barlow WE, et al. Role of Bone-Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology-Cancer Care Ontario Focused Guideline Update. J Clin Oncol 2017;35:3978-86. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer Guideline, Version 2.2023, updated 02/07/23, accessed online February 10, 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Weber JS, Levit LA, Adamson PC, et al. American Society of Clinical Oncology policy statement update: the critical role of phase I trials in cancer research and treatment. J Clin Oncol 2015;33:278-84. Erratum in: J Clin Oncol 2019;37:353. [Crossref] [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N Engl J Med 2022;386:942-50. [Crossref] [PubMed]
- André F, Ciruelos EM, Juric D, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol 2021;32:208-17. [Crossref] [PubMed]
- Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol 2022;40:3246-56. [Crossref] [PubMed]
- Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;30:558-66. [Crossref] [PubMed]
- Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016;388:2997-3005. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Kornblum N, Zhao F, Manola J, et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J Clin Oncol 2018;36:1556-63. [Crossref] [PubMed]
- Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022;387:9-20. [Crossref] [PubMed]
- Rugo HS, Bardia A, Marmé F, et al. LBA76 Overall survival (OS) results from the phase III TROPiCS-02 study of sacituzumab govitecan (SG) vs treatment of physician's choice (TPC) in patients (pts) with HR+/HER2- metastatic breast cancer (mBC). Ann Oncol 2022;33:S1386.
- Trodelvy. Prescribing Information. Gilead Oncology U.S. Accessed February 11, 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761115s035lbl.pdf
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;5:5. [Crossref] [PubMed]
- Goetz MP, Toi M, Huober J, et al. MONARCH 3: Interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann Oncol 2022;33:S808-69.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018;29:1541-7. [Crossref] [PubMed]
- Finn RS, Rugo HS, Dieras VS, et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J Clin Oncol 2022;40:LBA1003.
- Rugo HS, Brufsky A, Liu X, et al. Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer 2022;8:114. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol 2020;6:116-24. [Crossref] [PubMed]
- Cristofanilli M, Rugo HS, Im SA, et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2- ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study. Clin Cancer Res 2022;28:3433-42. [Crossref] [PubMed]
- Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol 2021;32:1015-24. [Crossref] [PubMed]
- Kalinsky K, Accordino MK, Chiuzan C, et al. Randomized Phase II Trial of Endocrine Therapy With or Without Ribociclib After Progression on Cyclin-Dependent Kinase 4/6 Inhibition in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: MAINTAIN Trial. J Clin Oncol 2023; Epub ahead of print. [Crossref]
- Kalinsky K, Layman RM, Kaufman PA, et al. postMONARCH: A phase 3 study of abemaciclib plus fulvestrant vs placebo plus fulvestrant in patients with HR+, HER2-, metastatic breast cancer following progression on a CDK4 & 6 inhibitor and endocrine therapy. J Clin Oncol 2022;40:TPS1117.
- André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2019;380:1929-40. [Crossref] [PubMed]
- Turner S, Chia S, Kanakamedala H, et al. Effectiveness of Alpelisib + Fulvestrant Compared with Real-World Standard Treatment Among Patients with HR+, HER2-, PIK3CA-Mutated Breast Cancer. Oncologist 2021;26:e1133-42. [Crossref] [PubMed]
- Borrego MR, Tolosa P, Blanch S, et al. Abstract PD8-02: Metformin (MET) for the prevention of Alpelisib (ALP)-related Hyperglycemia (HG) in PIK3CA-mutated, Hormone Receptor-Positive (HR[+]) HER2-Negative (HER2[-]) Advanced Breast Cancer (ABC): The METALLICA study. Cancer Res 2023;83:PD8-02.
- Bidard FC, Hardy-Bessard AC, Dalenc F, et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2022;23:1367-77. [Crossref] [PubMed]
- Oliveira M, Pominchuck D, Nowecki Z, et al. GS3-02 Camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: Results of the randomized, multi-dose Phase 2 SERENA-2 trial. Cancer Res 2023;83:GS3-02.
- Jhaveri K, Wang HC, Ma C, et al. PD13-12 Imlunestrant, an oral selective estrogen receptor degrader, in combination with abemaciclib with or without an aromatase inhibitor, in estrogen receptor-positive advanced breast cancer: Results from the phase 1a/b EMBER study. Cancer Res 2023;83:PD13-12.
- Pickar JH, MacNeil T, Ohleth K. SERMs: progress and future perspectives. Maturitas 2010;67:129-38. [Crossref] [PubMed]
- Rugo HS, Seneviratne L, Beck JT, et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol 2017;18:654-62. [Crossref] [PubMed]
- Toss A, Piacentini F, Cortesi L, et al. Genomic alterations at the basis of treatment resistance in metastatic breast cancer: clinical applications. Oncotarget 2018;9:31606-19. [Crossref] [PubMed]
- Howell SJ, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol 2022;23:851-64. [Crossref] [PubMed]
- Turner NC, Oliveira M, Howell SJ, et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2023;388:2058-70. [Crossref] [PubMed]
- Mauri D, Kamposioras K, Tsali L, et al. Overall survival benefit for weekly vs. three-weekly taxanes regimens in advanced breast cancer: A meta-analysis. Cancer Treat Rev 2010;36:69-74. [Crossref] [PubMed]
- Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 2009;27:3611-9. Erratum in: J Clin Oncol 2011;29:2739. [Crossref] [PubMed]
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794-803. [Crossref] [PubMed]
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol 2004;22:3608-17. [Crossref] [PubMed]
- Oshaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol 2001;12:1247-54. [Crossref] [PubMed]
- Lu YS, Bin Mohd Mahidin EI, Azim H, et al. Primary results from the randomized phase II RIGHT Choice trial of premenopausal patients with aggressive hormone receptor–positive HER2-negative advanced breast cancer treated with ribociclib + endocrine therapy vs physician’s choice combination chemotherapy. 2022 San Antonio Breast Cancer Symposium. Abstract GS1-10. Presented December 6, 2022.
- Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016;107:1039-46. [Crossref] [PubMed]
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 2021;384:1529-41. [Crossref] [PubMed]
- Enhertu. Prescribing Information. Astra-Zeneca U.S. Accessed February 11, 2023 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761139s022lbl.pdf
- Meric-Bernstam F, Krop I, Dejan J, et al. Phase 1 TROPION-PanTumor01 Study Evaluating Datopotamab Deruxtecan (Dato-DXd) in Unresectable or Metastatic Hormone Receptor–Positive/HER2-Negative Breast Cancer. Cancer Res 2023;83:PD13-08.


