
Hippocampal sparing radiation therapy for brain metastases: treatment techniques and clinical implementation
Introduction
Background
Brain metastasis treatment is challenging not only because it is located at one of the human’s most important organs, but also because its treatment outcomes will severely affect a patient’s living capabilities. Gamma Knife stereotactic radiosurgery (GK-SRS) (1), linear accelerator (LINAC)-based SRS, intensity-modulated radiation therapy (IMRT), volumetric-modulated arc therapy (VMAT), or whole brain radiation therapy (WBRT) with or without surgical resection has been the historical treatment option for patients with a limited number of brain metastases, and WBRT alone for those with multiple brain metastases (2,3). WBRT has long been regarded as a practical therapeutic choice for various settings of management in radiation oncology such as the presence of a large number of brain metastases or leptomeningeal diseases (4). Although tumor control is often the most important factor for treatment decisions (5), a significant decline in neurocognitive function can occur after multiple SRS treatments or WBRT where the hippocampal region is overly irradiated (6-8). Because GK-SRS and LINAC-based SRS can only treat focal lesions, most brain metastasis patients requiring large-regional volume irradiation will be treated with VMAT, IMRT, or WBRT techniques. This review article focuses on the hippocampal-sparing techniques used in a variety of SRS and WBRT settings, with the purpose of providing a “how-to” resource from a technological and physical standpoint to guide clinical practice. Thus, the primary target audience of this study is physicists and dosimetrists, while also considering the educational demands of physicians and radiation oncology residents.
Rationale and knowledge gap
The benefits of hippocampal sparing were only recently validated with clinical data. To achieve acceptable hippocampal sparing, special attention is required from radiation oncologists, physicists, and dosimetrists, including contouring accurate hippocampal volumes, selecting optimal planning parameters and dose limits, and calculating appropriate dose metrics for reporting and evaluation. To the authors’ best knowledge, an up-to-date overview of available hippocampal-sparing techniques and practical recommendations is not yet available in the current literature.
Objective
This review aims to provide a current, comprehensive summary of planning techniques and dose constraints, and a practical workflow specifically for physicists and dosimetrists driving the clinical implementation of these techniques.
The hippocampus
Function and delineation
The hippocampus is an S-shaped structure located in the medial region of the temporal lobes. Humans have two hippocampi, one on each side of the brain. The hippocampus is a known region for neurogenesis (9) and plays an integral role in memory, navigation, and cognition (10). Better preserved cognitive function including less deterioration in executive function, learning, and memory were observed among patients treated with hippocampal avoidance WBRT (HA-WBRT) plus memantine (11).
The hippocampus is best delineated on a T1-weighted magnetic resonance imaging (MRI) axial sequence. An MRI of 1.25 mm slice thickness or less is preferred to contour the hippocampus accurately. To begin contouring the hippocampus, locate the caudal most extent of the temporal horn and contour the gray matter inside the curve of the temporal horn. Continue contouring postero-cranially until the top-most extent of the hippocampus which stops at the lateral edges of the quadrigeminal cisterns. At this level, the crux of the fornix can be visualized anteriorly and the splenium of the corpus callosum emerges posteriorly. Hippocampal avoidance zones are generated using a 5 mm volumetric expansion on the hippocampus contours and the planning target volume (PTV) is generated by subtracting the hippocampal avoidance region from the existing brain contour (12,13). The increasing availability of high-quality automated contouring tools also has the potential to increase the efficiency and reproducibility of hippocampal contouring.
Additionally, it is important to distinguish between left and right hippocampus and to document dose to each hippocampus respectively. Studies of temporal lobectomy patients suggest that the left hippocampus is crucial for verbal memory whereas the right hippocampus correlates with visuo-spatial memory (14). Correspondingly, radiation injury to the left, usually dominant, hippocampus may have increased effects on verbal memory formation (15).
Radiation effects and dose constraints
The protective effects of hippocampal avoidance in brain irradiation continue to be investigated in multiple prospective clinical trials including Canadian Cancer Trials Group CE.7 and NRG Oncology trials BN009 and CC009 (16). Regarding verbal memory, minimum dose (Dmin), D10%, D50%, and D80% to bilateral hippocampi of 12.60, 8.81, 7.45, and 5.83 Gy, respectively, were significantly associated with neurocognitive preservation indicated by the immediate recall of Word List Test of Wechsler Memory Scale-III (17). Le Fèvre et al. demonstrated a continuous decrease in hippocampal volumes when D40% ranged from 7.5 to 50 Gy and reported a reduction rate of 5.55% when D40% >50 Gy (18). Seibert et al. observed a one-year hippocampal atrophy rate of 6% when mean dose (Dmean) >40 Gy (19), and Gondi et al. reported that bilateral hippocampi D40% ≥7.3 Gy was associated with long-term impairment in list-learning delayed verbal recall (17). All the above constraints are given for standard dose fractionation or equivalent dose in 2-Gy fractions (EQD2) with an α/β ratio of 2 Gy.
The Radiation Therapy Oncology Group (RTOG) and European Organization of Research and Treatment of Cancer (EORTC) jointly conducted a phase II clinical trial (RTOG 0933) where the feasibility of reducing dose to hippocampus in WBRT was evaluated (20). In this trial, the recommended (primary) hippocampal dose constraints were Dmin ≤9 Gy and maximum dose (Dmax) ≤16 Gy, with acceptable variations of up to Dmin ≤10 Gy and Dmax ≤17 Gy (with a prescription dose of 30 Gy in 10 fractions). All critical structure constraints for this trial are given in Table 1. Other clinical trials including CC001 (11), SWOG 1827 (21), and a phase III clinical trial conducted by Sahgal et al. also applied the same hippocampal dose constraints for bilateral hippocampi sparing in WBRT (22).
Table 1
| Structure | Metric | Per protocol | Variation acceptable |
|---|---|---|---|
| PTV | V30 | ≥95% | >90% |
| D2% | ≤37.5 Gy | ≤40 Gy | |
| D98% | ≥25 Gy | <25 Gy | |
| Hippocampus | Dmin | ≤9 Gy | ≤10 Gy |
| Dmax | ≤16 Gy | ≤17 Gy |
Constraints given for 3 Gy/fraction. PTV, planning target volume; V30, volume covered by 30 Gy isodose line; D2%, maximum dose to 2% of the structure; D98%, maximum dose to 98% of the structure; Dmin, minimum dose; Dmax, maximum dose.
The hippocampal dose constraints from RTOG 0933 are also applied in hippocampal avoidance SRS (HA-SRS) planning. However, since SRS plans deliver higher dose in fewer fractions than conventional fractionated plans, for comparison these constraints need to be calculated as EQD2 (α/β =2 Gy) (13,22-24). Therefore, the Dmin and Dmax from RTOG0933 in EQD2 are 4.21 and 6.65 Gy, respectively. Zhang et al. used similar hippocampal dose constraints of EQD2(40%) ≤4.5 Gy (for either hippocampus) and EQD2(max) ≤6.6 Gy for SRS plans with different treatment modalities (e.g., LINAC-based, GK, TomoTherapy) (25).
In addition to these bilateral constraints, studies found that dosimetric parameters (e.g., Dmean, Dmin) specific to the left sided hippocampus exerted an influence on immediate recall of verbal memory (15,26). Tsai et al. reported that EQD2(max) ≥12.4 Gy to the left hippocampus was significantly associated with functional preservation in preservative errors of Wisconsin Card Sorting test (15). Le Fèvre et al. stated that, if EQD2(mean) to the left hippocampus is increased by 1 Gy, there may be four-fold increase in the risk of neurocognitive decline in immediate recall of verbal memory (18). These studies suggest that in cases where bilateral hippocampal sparing may not be feasible, sparing of the left hippocampus should be prioritized.
In general, the RTOG 0933 hippocampal dose constraints of EQD2(min) ≤9 Gy and EQD2(max) ≤16 Gy are most used in HA-WBRT planning. Correspondingly, EQD2(min) ≤4.2 Gy and EQD2(max) ≤6.7 Gy are widely used in SRS planning. Similar constraints based on the results of the RTOG 0933 study as well as Birer et al. (24) (discussed in Section LINAC-based SRS) are recommended in French clinical practice guidelines (27).
Simulation
The first step in the radiation therapy workflow is to perform a computed tomography (CT) simulation to acquire images for treatment planning. In addition, other medical imaging modalities such as MRI are ordered for enhanced soft-tissue contrast and better visualization of the patient’s anatomy (e.g., T1-weighted MRI for T1 enhancing lesions or other functional disorders, and T2-weighted fluid attenuated inversion recovery (FLAIR) to delineate edema) (28). These supplemental image sets are registered to the primary planning CT via rigid non-deformable registration and used to assist in the delineation of the target structures and organs-at-risk (OARs) (29).
The typical patient setup for WBRT or LINAC-based SRS consists of a frameless external mask (thermoplastic mask molded to the patient’s head) and headrest. A low-density headrest should be considered to minimize dosimetric effects such as beam attenuation and increased skin dose (30). Any external fiducials to be used for patient setup or intra-fraction motion monitoring should also be placed prior to the simulation scan (31).
A head-tilting baseplate enabling head rotation about the lateral axis can also be utilized for HA-WBRT to increase hippocampal sparing (32-34). Miura et al. showed that hippocampal dose could be reduced for helical TomoTherapy HA-WBRT by utilizing a head-tilting baseplate with an angle of ~26°–48° (34). Similar reductions in hippocampus dose, as well as improved target dose conformity, can be seen with VMAT HA-WBRT planning using a 40° tilted baseplate (33).
For most GK-SRS to date and historically for LINAC-based SRS, a frame-based immobilization and registration device is attached with screws to the patient’s cranium by a neurosurgeon (35). A fiducial localizer box is then attached to the head frame for the CT simulation scan to define the stereotactic coordinate space during treatment planning. A localizer box can also be used for mask-based SRS (with GK or LINAC) for mapping the stereotactic coordinate system. These invasive head frames have been mostly superseded by the frameless approach discussed earlier for LINAC-based SRS and some GK systems, as studies have not shown any negative impacts on patient outcomes with this less invasive, frameless fixation method (36-38).
Treatment techniques
HA-WBRT
Helical TomoTherapy
The earliest HA-WBRT feasibility study from 2007 showed that helical TomoTherapy (Accuray Incorporated, Sunnyvale, CA, USA) could be used to effectively spare the hippocampus during WBRT and simultaneously boost the dose to up to 5 brain metastases (39). This study explored several different combinations of parameters for field width and pitch to assess their dosimetric impact and found that utilizing the 1.05 cm field width resulted in lower OAR doses and better target homogeneity. Although this narrower field width also significantly increases treatment times, it has been used for most subsequent studies on HA-WBRT with TomoTherapy (13,34,40-42). This was also the recommended field width for RTOG 0933 protocol planning, along with a modulation factor of 3.0, a pitch of 0.215, and a recommended set of optimization objectives. A comparison of planning parameters and corresponding hippocampal doses for several HA-WBRT TomoTherapy studies is shown in Table 2.
Table 2
| Study | Prescription dose (Gy) | Modulation factor | Pitch | Hippocampus dose (Gy) | |
|---|---|---|---|---|---|
| Mean (mean/mean ± SD) | Max (mean/mean ± SD) | ||||
| Gutiérrez 2007 (39) | 32.25 (63 to mets) | 3.5 | 0.289‡ | 5.78±1.88 (NTDmean) | 14.7 |
| Gondi 2010 (13) | 30 | 3.0 | 0.215 | 4.9±0.2 (NTDmean) | 12.8±0.7 |
| Jiang 2019 (40) | 30 (45–50 to mets) | Not given | 0.5 | 10.0±0.7 | 15.5±0.9 |
| Wang 2021 (41) | 30 | 0.2, 0.22 | 0.233 | 6.66 | 14.11 (D98%) |
| Miura 2021† (34) | 30 | 3.0 | 0.200 | 8.85±0.35, 7.97±0.24 with tilt | 15.06±0.66, 13.95±0.33 with tilt |
| Yokoyama 2022 (42) | 30 | 3.0 | 0.215 | 8.02±0.44 (D50%) | 12.63±0.60 |
| RTOG 0933 Criteria | 30 | 3.0 | 0.215 | – | ≤16 |
†, also used head-tilting baseplate (26.4–48.6 degrees); ‡, also calculated for 2.5 cm field width, not included here. SD, standard deviation; NTDmean, mean normalized total dose for 2-Gy fractions, α/β ratio of 2 Gy; D98%, maximum dose to 98% of the structure; D50%, maximum dose to 50% of the structure; RTOG, Radiation Therapy Oncology Group.
HA-WBRT planning with helical TomoTherapy has demonstrated superior hippocampal sparing to LINAC-based IMRT/VMAT in several studies (13,41,43). Yokoyama et al. found that compared to Halcyon HA-WBRT plans, TomoTherapy enabled greater sparing of the hippocampus, eyes, and lenses, with slightly inferior target coverage and significantly longer treatment times (using 1 cm field width) (42). However, in another study by Jiang et al. there were no significant differences in mean hippocampal dose between TomoTherapy, IMRT, and VMAT plans (40).
Overall, the literature suggests that increased hippocampal sparing may be possible using helical TomoTherapy rather than LINAC-based IMRT or VMAT. However, delivery time of for helical TomoTherapy HA-WBRT can be significantly longer than that of VMAT (41). This can be improved by increasing the field width from 1 to 2.5 cm, but at the expense of inferior dosimetry (39).
Static IMRT
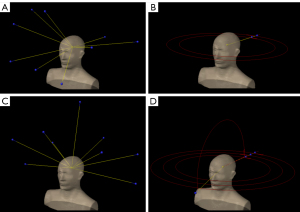
The 2010 comparison study by Gondi et al. was one of the earliest studies demonstrating the feasibility of LINAC-based static IMRT for HA-WBRT (13). The plans used nine static non-coplanar beams and were optimized with the Pinnacle3 treatment planning system (TPS) (version 8.0m; Philips, Fitchburg, WI, USA) with a set of six planning objectives for the PTV, hippocampus, eyes, and lenses. They were able to sufficiently spare the hippocampus (average normalized Dmean of 7.3 Gy for 5 plans) while achieving acceptable homogeneity and target coverage. This beam arrangement and set of planning objectives was then published by the RTOG as a recommended LINAC-based IMRT planning strategy for meeting the constraints of the 0933 trial, as well as an alternative nine-field beam arrangement option (RTOG 0933 protocol update March 31, 2011). Both recommended beam arrangements are illustrated in Figure 1A,1B.
In another HA-WBRT planning study by Nevelsky et al., plans were created with the Monaco 3.1 TPS (Elekta AB, Stockholm, Sweden) utilizing seven coplanar plus two non-coplanar beams (44). This study demonstrated that the strict constraints from RTOG 0933 could be achieved using Elekta equipment and provided planning advice for Elekta users. It also demonstrated that a more efficient beam arrangement could be utilized than that recommended in RTOG 0933, enabling shorter treatment delivery times (estimated to be ~15 minutes in this study).
HA-WBRT delivery efficiency was increased even further in Andreas et al., where 14 patients were treated on the 0933 trial using just seven coplanar beams (45). Although the authors noted some undesirable dosimetric trends with this beam arrangement, such as hotspots anterior to the hippocampus and high mean PTV doses, eleven plans met all the protocol dose criteria and three were within the “variation acceptable” criteria for the hippocampus.
VMAT
The use of VMAT enables significantly higher treatment delivery efficiency compared to static IMRT, along with potential dosimetric advantages. Many different VMAT planning approaches have been documented for HA-WBRT using one to four arcs, coplanar and non-coplanar beam angles, and full or split-field geometries, with a representative selection described in the following section.
The most conventional VMAT beam arrangement for HA-WBRT is the use of two coplanar arcs with 30°–45° collimator rotations to minimize the tongue-and-groove effect (Figure 1C). Several studies have demonstrated that this simple beam geometry is sufficient for meeting the RTOG 0933 protocol criteria while also achieving short treatment delivery times of ~3 minutes (46,47).
Shen et al. reported successful hippocampal sparing using a more complex beam arrangement with two full-rotation, partial-field arcs to target the inferior and superior halves of the PTV separately (48). Another partial-field VMAT approach, separately targeting the superior, left, and right PTV regions, has also been shown to achieve superior dosimetry to dual coplanar arcs (49). These plans reduced the maximum and mean hippocampus doses by 19% and 15%, respectively, compared to the dual coplanar arc plans.
In a study by Kazda et al., one partial vertex arc was utilized in addition to three full coplanar arcs (Figure 1D) (50). This study was unique in that they also compared these plans to “experimental” plans where only the left hippocampus was spared. Although these plans of course delivered much higher doses to the right hippocampus, they enabled reductions in mean and maximum left hippocampal dose by >10% compared to the bilateral sparing plans.
Several studies have also demonstrated the use of VMAT for successfully sparing the hippocampus while also boosting the dose to multiple metastases (51-53). The techniques used for these simultaneous integrated boost HA-WBRT plans have ranged from a single full coplanar arc (51), to three full coplanar arcs (52), to four coplanar arcs with complete blocking of radiation entering or exiting through the hippocampus (53).
These studies suggest that VMAT planning with two full coplanar arcs can effectively spare the hippocampus, but further improvements in dosimetry may be possible with the incorporation of non-coplanar fields or split-field approaches. However, utilizing non-coplanar beam angles is not always feasible (e.g., with standard helical TomoTherapy) or practical for all clinical workflows due to increased delivery complexity. Several studies have explored the use of a head-tilting baseplate, as discussed in Section Simulation, as an alternative strategy for planning HA-WBRT with non-coplanar beams. There is some evidence that utilizing a multileaf collimator (MLC) with a smaller leaf width may improve hippocampal sparing as well (46,48).
Automated planning
Advancements in treatment planning technology have enabled the use of automation to decrease HA-WBRT planning times and improve plan quality and consistency. One commercially available option is the Auto-Planning module in the Pinnacle3 TPS. In a planning study by Wang et al., Auto-Planning was used to generate both VMAT and IMRT HA-WBRT plans for 10 patients (54). All plans met the RTOG 0933 criteria and 85% of cases were generated without any planner intervention.
The RayStation TPS (RaySearch Laboratories, Stockholm, Sweden) also offers automation features for both segmentation and planning that can be utilized for HA-WBRT. The multi-criteria optimization (MCO) module was shown to significantly improve PTV coverage and reduce OAR doses (55) and the auto-contouring and auto-planning functions enable rapid (~10 minute) HA-WBRT plan generation with minor differences in dosimetry between manually and automatically generated plans (56).
For Eclipse TPS users (Varian Medical Systems, Palo Alto, CA, USA), there have been two versions of the Hippocampal Sparing Whole Brain (HSWB) RapidPlan model shared for public use. Feng et al. tested the first version of this model (HSWBv1) on 100 patients, and all plans met NRG CC001 criteria (or with “acceptable variation”) with a median processing time of ten minutes (57). An updated version of this RapidPlan model (HSWBv2) was released in 2022 by Liu et al., which enables significantly reduced hippocampus dose, improved PTV homogeneity, and reduced inter-plan variability compared to HSWBv1 (58). This article by Liu et al. presents a comparison of the PTV dose and minimum hippocampus dose for thirteen studies on HA-WBRT published between 2013 and 2022, including those referenced in this section, with HSWBv2 achieving the best PTV homogeneity (lowest D2% and highest D98%) and lowest maximum hippocampal dose.
Varian’s HyperArc platform, developed for automated planning and delivery of intracranial radiation therapy, has also been evaluated for HA-WBRT. HyperArc plans showed significant reductions in high dose brain volume and OAR doses (59), especially when combined with RapidPlan (60).
HA-SRS
GK-SRS
The efficacy of GK-SRS in treating intracranial lesions has been demonstrated (61). However, when multiple lesions are treated, there is a potential increase in cumulative dose to the hippocampi. In an early study by Chang et al. (62), researchers investigated how to minimize hippocampal dose in patients treated using single-fraction, frame-based GK-SRS. In this retrospective study, eight patients with 6–12 brain metastases, originally treated without explicit hippocampal avoidance, were replanned using GammaPlan treatment planning software’s dynamic shaping function. This proactive beam shaping method aimed to reduce direct beam irradiation to the hippocampi. The results showed that this approach decreased hippocampal dose by an average of 35% at the expense of increasing treatment time by 20%. The most significant reduction in hippocampal dose was observed when the lesions were located near the hippocampus, especially those within 10 mm. No clear correlation was found between hippocampal dose and the number or total volume of lesions.
The state-of-the-art Gamma Knife ICON (GKI), a frameless image-guided unit, allows multiple fraction SRS with thermoplastic mask immobilization and cone beam CT image guidance. Nguyen et al. proposed a novel technique called Spatially Partitioned Adaptive RadiosurgEry (SPARE) for GKI, involving multiday single-fraction SRS (GKI-Spr) (63). This technique utilizes in-house clustering software to group targets and treat them over multiple days, allowing each target to receive single-fraction SRS while limiting daily treatment time to less than 60 minutes. The authors identified 10 patients with more than 10 brain metastases treated with GKI-Spr and replanned them using GKI-Sfr (all targets treated with a single fraction in a single day) and VMAT-based HA-WBRT. Even without explicitly avoiding the hippocampi, both GKI-Spr and GKI-Sfr significantly reduced EQD2 Dmax, Dmin, Dmean, and D40% values to the hippocampi compared with HA-WBRT by more than 80%. Additionally, GKI-Spr achieved further reductions in all hippocampal dose metrics compared to GKI-Sfr. Therefore, GKI-Spr may be an optimal strategy for hippocampal avoidance in GK-SRS.
A multi-institutional study, JLGK0901, suggests that SRS alone is the preferred treatment option over WBRT for patients with 1–4 brain lesions, and emerging data supports its use for up to 10 lesions (64-66). However, the role of SRS alone in patients with more than 10 brain lesions is not well understood. Susko et al. conducted a review of patients treated with single-fraction frame-based GK-SRS to ≥10 brain lesions (67). Hippocampus contours were retrospectively generated for 62 patients who underwent up-front SRS. The results showed a mean hippocampal dose of 161 cGy, indicating that hippocampal sparing can be readily achieved in most cases with GK-SRS even without explicitly considering hippocampal avoidance.
In another study by Riina et al., the relationship between hippocampal dosimetry and the number of brain metastases was investigated (1). The authors reviewed 75 GK-SRS plans without explicit hippocampal avoidance and compared the hippocampal dosimetry between patients with 4–9 lesions and those with ≥10 lesions. The group with ≥10 lesions exhibited significantly higher median bilateral Dmean, D100%, D40%, and Dmax compared to the group with 4–9 lesions. Furthermore, the group with ≥10 lesions were less likely to meet the hippocampal constraints. Seven plans that failed to meet the constraints were successfully replanned with an increase in treatment time of eight minutes and without compromising target coverage or conformity. Therefore, patients with extensive brain metastases, particularly those with ≥10 lesions or lesions located within 5 mm of the hippocampi, may benefit from hippocampal avoidance in GK-SRS planning.
Limited data exists on the investigation of cumulative hippocampal dosimetry in patients undergoing multiple sessions of GK-SRS for extensive brain metastases. A retrospective analysis conducted by Yuan et al. (68) reviewed 10 patients who received a minimum of three GK-SRS sessions for a median of 25 brain lesions and revealed that the hippocampi received a median Dmax of 13.81 Gy and a median Dmean of 3.41 Gy. However, the study did not provide information on the tumor location relative to the hippocampi or the tumor volume. Another study by Kavi et al. (3) assessed the hippocampal doses in 89 patients receiving multiple-session GK-SRS. Their findings demonstrated a significant correlation between the median D40%, D50%, and Dmin with the number and volume of the tumors. They observed that patients with tumors located within 5 mm of the hippocampi received higher doses, with the largest difference in the maximum hippocampal dose.
LINAC-based SRS
An alternative to GK-SRS is to treat brain metastasis patients with LINAC-based SRS, which has been shown to achieve similar local control rates with significantly shorter treatment times (69). In an early study investigating the hippocampal dose in LINAC-based SRS using VMAT, the authors identified 22 patients with 1–4 brain metastases (70). They retrospectively contoured the hippocampi and found that the hippocampal Dmax and D40% constraints were exceeded in 10 out of 22 cases. After replanning with hippocampal avoidance, 50% of these plans met the hippocampal dose constraints with no significant change in conformity or homogeneity. They found no correlation between hippocampal dose and the number or total volume of lesions, but they observed that more than 50% of the plans exceeded the hippocampal dose constraints after replanning had metastases located in the proximity of the hippocampi.
Another retrospective analysis study by Daniela Falco et al. had similar findings (71). They assessed 22 patients with 1–4 metastases treated with VMAT SRS without hippocampal sparing, and nine plans with hippocampal doses exceeding the constraints were replanned. The hippocampal Dmean was reduced by an average of 35% and the max dose was met in 45% of plans after replanning. The highest doses were observed when targets were within 12 mm of the hippocampi. Both studies suggest that hippocampal sparing in VMAT-based SRS is feasible without compromising the target coverage or conformity. Hippocampal sparing should be considered particularly when the lesions are near the hippocampi.
There are other studies investigating VMAT-based SRS for patients with more extensive brain metastases. Birer et al. evaluated the hippocampal dose for patients with 4–10 metastases treated with single fraction VMAT SRS and found that a significant number of patients received hippocampal doses exceeding constraints (24). It was feasible to replan with hippocampal avoidance that substantially reduced the hippocampal dose without compromising target coverage or normal tissue constraints. Decreasing distance from the closest metastasis to the hippocampi and the total target volume were found to be risk factors associated with exceeding hippocampal constraints. However, there was no correlation between exceeding hippocampal constraints and number of metastases (4–5 vs. 6–10) or prescribed dose.
In a similar dosimetric study, Gude et al. analyzed 40 patients receiving single isocenter VMAT SRS for 4–10 lesions (72). They found eight patients (20%) with hippocampal dose exceeding the maximum biologically effective dose (BED) constraints and a trend of increasing hippocampal dose with decreasing distance from hippocampus to the nearest target. There was no difference in meeting the hippocampal maximum BED constraints between plans with standard MLC and high-definition MLC. In a planning study, Pokhrel et al. compared dynamic conformal arc VMAT (DCA-VMAT) with conventional VMAT for patients with 2–8 brain lesions (23). They found that DCA-VMAT plans provided similar tumor dose, target coverage, and conformity, with lower dose to normal brain and other OARs including hippocampi.
Zhang et al. compared four SRS techniques, including GK, single-isocenter VMAT, TomoTherapy, and CyberKnife, for patients with 3–10 brain metastases (25). They selected 10 patients with 14 separate treatments. For each SRS platform, the original plans were generated to meet the tumor coverage goal without considering hippocampal sparing. The hippocampal dose constraints were met in the majority (13 of 14) of non-hippocampal sparing SRS plans using the GK or CyberKnife, while the hippocampal dose constraints were exceeded in more than half (8 of 14) of TomoTherapy plans and nearly all (13 of 14) VMAT plans. These were re-planned with hippocampal avoidance and the Dmax and D40% were reduced, but dose constraints were still not met for one patient on any platform because the tumor was adjacent to the hippocampus. This study shed light on the importance of considering hippocampal sparing in SRS for patients with multiple brain metastases, particularly for those receiving treatment with VMAT or TomoTherapy. A comparison of studies evaluating both GK and LINAC-based HA-SRS is shown in Table 3.
Table 3
| Study | Number of metastases (range) | Number of patients | Prescription dose | Technique | Hippocampus mean dose (Gy), median (range) | Hippocampus max dose (Gy), median (range) |
|---|---|---|---|---|---|---|
| Chang 2016 (43) | 6–12 | 8 | 16–20 Gy in 1 fx | GK-SRS without HA | N/A (0.4–2.9) | N/A (0.8–9.0) |
| GK-SRS proactive beam shaping with HA | N/A (0.1–1.1) | N/A (0.5–6.2) | ||||
| Nguyen 2019 (63) | 13–31 | 10 | 30 Gy in 10 fx | HA-WBRT (median EQD2) | 11.2 (10.0–11.8) | 15.4 (14.8–15.8) |
| 15–20 Gy in 1 fx | GKI-Sfr (EQD2) | 1.1 (0.3–2.2) | 3.0 (0.7–5.8) | |||
| GKI-Spr (EQD2) | 0.8 (0.2–1.6) | 2.1 (0.6–4.0) | ||||
| Susko 2020 (67) | 11–17† | 62 | 18–19 Gy in 1 fx† | GK-SRS without HA | 1.61 (0.94–2.58)† | N/A |
| Riina 2020 (1) | 4–30 | 75 | 14–20 Gy in 1 fx | GK-SRS without HA (4–9 metastases) (n=60) | 1.0 (0.6–1.8)† | 2.0 (1.1–4.5)† |
| GK-SRS without HA (≥10 metastases) (n=15) | 2.0 (1.6–2.6)† | 4.9 (4.0–5.8)† | ||||
| Original plan without HA (n=7) | 2.5 (2.1–2.9)† | 8.6 (7.7–9.5)† | ||||
| Replan with HA (n=7) | 2.3 (1.9–2.7)† | 6.4 (6.3–6.5)† | ||||
| Yuan 2018 (68) | 10–63 | 10 | 15–24 in 1 fx | Multi-session GK-SRS | 3.4 (1.0–14.4) | 13.8 (1.5–39.3) |
| Kavi 2021 (3) | 25–116 | 82 | 10–21 Gy in 1 fx | Multi-session GK-SRS (n=82) | 3.97 (1.03–10.4) | 16.66 (2.03–40.32) |
| NTHA (n=35) | 3.07 (1.03–7.64) | 5.64 (2.03–19.59) | ||||
| THA (n=47) | 5.55 (1.43–10.4) | 23.13 (5.78–40.32) | ||||
| Di Carlo 2018 (70) | 1–4 | 22 | 20 Gy in 4 fx or 24 Gy in 3 fx | Original VMAT plan without HA (n=10) | 9.1 (4.6–13.7)‡ | 24.0 (10.5–58.9) |
| Replan with HA (n=10) | 5.6 (4.4–7.3)‡ | 20.0 (7.9–59.0) | ||||
| Daniela Falco 2018 (71) | 1–4 | 22 | 20 Gy in 4 fx or 24 Gy in 3 fx | Original VMAT plan without HA (n=9) | 9.4 (4.6–13.7)‡ | 25.1 (10.5–58.9) |
| Replan with HA (n=9) | 5.5 (4.4–7.3)‡ | 21.2 (7.9–59.0) | ||||
| Birer 2017 (24) | 4–10 | 38 | 18–20 Gy in 1 fx† | Single-isocenter VMAT (n=38) | N/A | 3.519 (2.156–6.226)† |
| Original plan without HA (n=7) | N/A | 12.12 (9.50–12.69)† | ||||
| Replan with HA (n=7) | N/A | 6.40 (5.61–7.64)† | ||||
| Gude 2021 (72) | 4–10 | 40 | 20 Gy in 1 fx or 25 in 5 fx | Original VMAT plan without HA & with HD-MLC (n=7) | 11.5 | 50.65 |
| Replan with HA & with HD-MLC (n=7) | 8.89 | 42.65 | ||||
| Original VMAT plan without HA & with SD-MLC (n=7) | 14.76 | 56.95 | ||||
| Replan with HA & with SD-MLC (n=7) | 10.5 | 42.26 | ||||
| Pokhrel 2021 (23) | 2–8 | 7 | 20 Gy in 1 fx | HyperArc VMAT | N/A | 6.6 (4.6–7.9) |
| DCA-VMAT | N/A | 4.7 (2.9–5.9) | ||||
| Zhang 2017 (25) | 3–10 | 10 | 16–20 Gy in 1 fx | GK-SRS without HA (n=3) | 2.25‡ | 7.6 |
| GK-SRS with HA (n=3) | 2.21‡ | 6.60 | ||||
| VMAT without HA (n=13) | 6.94‡ | 10.41 | ||||
| VMAT with HA (n=13) | 3.56‡ | 5.75 | ||||
| CyberKnife without HA (n=3) | 4.03‡ | 11.5 | ||||
| CyberKnife with HA (n=3) | 4.00‡ | 4.03 | ||||
| Tomo without HA (n=8) | 5.54‡ | 8.96 | ||||
| Tomo with HA (n=8) | 3.28‡ | 6.4 |
†, IQR instead of range; ‡, D40% (dose to 40% of the hippocampi) instead of Dmean (mean dose); HA-SRS, hippocampal avoidance stereotactic radiosurgery; fx, fraction(s); GK-SRS, Gamma Knife stereotactic radiosurgery; N/A, not applicable; HA-WBRT, whole brain radiation therapy with hippocampal avoidance; EQD2, equivalent dose in 2-Gy fractions; GKI-Sfr, Gamma Knife Icon single-fraction single-day plan; GKI-Spr, Gamma Knife Icon Spatially Partitioned Adaptive RadiosurgEry (SPARE) plan; NTHA, no tumors in hippocampal avoidance region; THA, tumors in hippocampal avoidance region; VMAT, volumetric-modulated arc therapy; HD/SD-MLC, high-definition/standard-definition multileaf collimator; DCA-VMAT, dynamic conformal arc VMAT; IQR, inter-quartile range.
Based on the current literature, hippocampal avoidance should be considered for SRS of multiple brain metastases, particularly when there are extensive metastases and when the metastases are close to the hippocampi. Plan comparison studies show that hippocampal sparing can be achieved in most cases without compromising the target coverage, conformity, or other OAR constraints.
Study limitations
Since the literature surrounding hippocampal sparing radiation therapy is extensive, there are inevitably some studies that were not included in this review. However, the authors feel that the articles referenced are largely representative of the current state of the technology and research related to this topic.
This review also does not address the clinical outcomes related to the discussed treatment options, as that is beyond the scope of this work. Nonetheless, it should be noted that with the publication of the American Society for Radiation Oncology’s Choosing Wisely recommendations (73) and the International Stereotactic Radiosurgery Society’s guidelines confirming the Lancet study by Chang et al. in 2009 (8), the role of WBRT in the setting of managing multiple brain metastases has changed dramatically over the past decade. Similarly, the original effort and rationale of leveraging advanced technology such as discussed above for HA-WBRT has also witnessed its decline in addressing the neurotoxicity concerns of WBRT and therefore should be exercised with great caution, especially considering encouraging results from hypo-fractionated treatment in conjunction with targeted therapy and immunotherapy agents. Several studies have estimated the potential risks of underdosing metastases to achieve hippocampal sparing (43,74,75). For any local therapy regimen (HA-WBRT, WBRT, SRS, etc.) the risks of local and distant brain recurrence and neurotoxicity must always be carefully balanced against each other, especially in managing multiple brain metastases.
Conclusions
Limiting the radiation dose to the hippocampus during radiation therapy for brain metastases aims to preserve neurocognitive function, with the RTOG 0933 hippocampal dose constraints widely regarded as practical planning parameters. For HA-WBRT, helical TomoTherapy has been shown to enable greater hippocampal sparing than static IMRT or VMAT in some studies, but at the expense of significantly longer treatment times. VMAT offers the most efficient treatment delivery, with automated planning techniques continuing to improve the quality and consistency of HA-WBRT plans. Hippocampal sparing can also be achieved by default for most GK-SRS and LINAC-based SRS plans due to limited irradiation of target volume. For the same reason, we recommend that all hippocampal sparing options be considered when treating multiple brain metastases, especially for patients with high-risk lesions near the hippocampi.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-73/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-73/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riina MD, Stambaugh CK, Huber KE. Hippocampal Dosimetry and the Necessity of Hippocampal-Sparing in Gamma Knife Stereotactic Radiosurgery for Extensive Brain Metastases. Adv Radiat Oncol 2019;5:180-8. [Crossref] [PubMed]
- Grosu AL, Frings L, Bentsalo I, et al. Whole-brain irradiation with hippocampal sparing and dose escalation on metastases: neurocognitive testing and biological imaging (HIPPORAD) – a phase II prospective randomized multicenter trial (NOA-14, ARO 2015-3, DKTK-ROG). BMC Cancer 2020;20:532. [Crossref] [PubMed]
- Kavi A, Gurewitz J, Benjamin CG, et al. Hippocampal sparing in patients receiving radiosurgery for ≥25 brain metastases. Radiother Oncol 2021;161:65-71. [Crossref] [PubMed]
- Baliga S, Adams JA, Bajaj BVM, et al. Patterns of failure in pediatric medulloblastoma and implications for hippocampal sparing. Cancer 2023;129:764-70. [Crossref] [PubMed]
- Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 2007;68:1388-95. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Welzel G, Fleckenstein K, Schaefer J, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 2008;72:1311-8. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Toda T, Parylak SL, Linker SB, et al. The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry 2019;24:67-87. [Crossref] [PubMed]
- Lisman J, Buzsáki G, Eichenbaum H, et al. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci 2017;20:1434-47. [Crossref] [PubMed]
- Brown PD, Gondi V, Pugh S, et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol 2020;38:1019-29. [Crossref] [PubMed]
- Gondi V, Tome WA, Rowley H, et al. Hippocampal contouring: a contouring atlas for RTOG 0933.
- Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1244-52. [Crossref] [PubMed]
- Lee G, Besse L, Lamba N, et al. Feasibility of hippocampal avoidance whole brain radiation in patients with hippocampal involvement: Data from a prospective study. Med Dosim 2021;46:21-8. [Crossref] [PubMed]
- Tsai PF, Yang CC, Chuang CC, et al. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: a prospective study. Radiat Oncol 2015;10:253. [Crossref] [PubMed]
- Gondi V, Meyer J, Shih HA. Advances in radiotherapy for brain metastases. Neurooncol Adv 2021;3:v26-34. [Crossref] [PubMed]
- Gondi V, Hermann BP, Mehta MP, et al. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys 2012;83:e487-93. [Crossref] [PubMed]
- Le Fèvre C, Cheng X, Loit MP, et al. Role of hippocampal location and radiation dose in glioblastoma patients with hippocampal atrophy. Radiat Oncol 2021;16:112. [Crossref] [PubMed]
- Seibert TM, Karunamuni R, Bartsch H, et al. Radiation Dose-Dependent Hippocampal Atrophy Detected With Longitudinal Volumetric Magnetic Resonance Imaging. Int J Radiat Oncol Biol Phys 2017;97:263-9. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- MRI Brain Surveillance Alone Versus MRI Surveillance and Prophylactic Cranial Irradiation (PCI): A Randomized Phase III Trial in Small-Cell Lung Cancer (MAVERICK). 2020. Available online: https://www.swog.org/clinical-trials/s1827
- Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2015;91:710-7. [Crossref] [PubMed]
- Pokhrel D, Palmiero AN, Bernard ME, et al. Dynamic conformal arcs-based single-isocenter VMAT planning technique for radiosurgery of multiple brain metastases. Med Dosim 2021;46:195-200. [Crossref] [PubMed]
- Birer SR, Olson AC, Adamson J, et al. Hippocampal dose from stereotactic radiosurgery for 4 to 10 brain metastases: Risk factors, feasibility of dose reduction via re-optimization, and patient outcomes. Med Dosim 2017;42:310-6. [Crossref] [PubMed]
- Zhang I, Antone J, Li J, et al. Hippocampal-sparing and target volume coverage in treating 3 to 10 brain metastases: A comparison of Gamma Knife, single-isocenter VMAT, CyberKnife, and TomoTherapy stereotactic radiosurgery. Pract Radiat Oncol 2017;7:183-9. [Crossref] [PubMed]
- Haldbo-Classen L, Amidi A, Lukacova S, et al. Cognitive impairment following radiation to hippocampus and other brain structures in adults with primary brain tumours. Radiother Oncol 2020;148:1-7. [Crossref] [PubMed]
- Noël G, Antoni D. Organs at risk radiation dose constraints. Cancer Radiother 2022;26:59-75. [Crossref] [PubMed]
- Chandarana H, Wang H, Tijssen RHN, et al. Emerging role of MRI in radiation therapy. J Magn Reson Imaging 2018;48:1468-78. [Crossref] [PubMed]
- Brock KK, Mutic S, McNutt TR, et al. Use of image registration and fusion algorithms and techniques in radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132. Med Phys 2017;44:e43-76. [Crossref] [PubMed]
- Olch AJ, Gerig L, Li H, et al. Dosimetric effects caused by couch tops and immobilization devices: report of AAPM Task Group 176. Med Phys 2014;41:061501. [Crossref] [PubMed]
- Bissonnette JP, Balter PA, Dong L, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: a report of the AAPM TG-179. Med Phys 2012;39:1946-63. [Crossref] [PubMed]
- Popp I, Rau A, Kellner E, et al. Hippocampus-Avoidance Whole-Brain Radiation Therapy Is Efficient in the Long-Term Preservation of Hippocampal Volume. Front Oncol 2021;11:714709. [Crossref] [PubMed]
- Oh SA, Yea JW, Park JW, et al. Use of a head-tilting baseplate during volumetric-modulated arc therapy (VMAT) to better protect organs at risk in hippocampal sparing whole brain radiotherapy (HS-WBRT). PloS One 2020;15:e0232430. [Crossref] [PubMed]
- Miura K, Kurosaki H, Utsumi N, et al. Use of a Head-Tilting Baseplate During Tomotherapy to Shorten the Irradiation Time and Protect the Hippocampus and Lens in Hippocampal Sparing-Whole Brain Radiotherapy. Technol Cancer Res Treat 2021;20:1533033820986824. [Crossref] [PubMed]
- Petti PL, Rivard MJ, Alvarez PE, et al. Recommendations on the practice of calibration, dosimetry, and quality assurance for gamma stereotactic radiosurgery: Report of AAPM Task Group 178. Med Phys 2021;48:e733-70. [Crossref] [PubMed]
- Grimm MA, Köppen U, Stieler F, et al. Prospective assessment of mask versus frame fixation during Gamma Knife treatment for brain metastases. Radiother Oncol 2020;147:195-9. [Crossref] [PubMed]
- Régis J, Merly L, Balossier A, et al. Mask-Based versus Frame-Based Gamma Knife ICON Radiosurgery in Brain Metastases: A Prospective Randomized Trial. Stereotact Funct Neurosurg 2022;100:86-94. [Crossref] [PubMed]
- Kutuk T, Kotecha R, Tolakanahalli R, et al. Zero Setup Margin Mask versus Frame Immobilization during Gamma Knife® Icon™ Stereotactic Radiosurgery for Brain Metastases. Cancers (Basel) 2022;14:3392. [Crossref] [PubMed]
- Gutiérrez AN, Westerly DC, Tomé WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys 2007;69:589-97. [Crossref] [PubMed]
- Jiang A, Sun W, Zhao F, et al. Dosimetric evaluation of four whole brain radiation therapy approaches with hippocampus and inner ear avoidance and simultaneous integrated boost for limited brain metastases. Radiat Oncol 2019;14:46. [Crossref] [PubMed]
- Wang D, Ma X, Fu L, et al. The Capabilities and Characteristics of Helical Tomotherapy and Co-Planar Dual Arcs Volumetric-Modulated arc Therapy Associated with Hippocampal Sparing During Prophylactic Cranial Irradiation. Technol Cancer Res Treat 2021;20:15330338211043975. [Crossref] [PubMed]
- Yokoyama K, Kurosaki H, Oyoshi H, et al. Plan Quality Comparison Between Hippocampus-Sparing Whole-Brain Radiotherapy Treated With Halcyon and Tomotherapy Intensity-Modulated Radiotherapy. Technol Cancer Res Treat 2022;21:15330338221108529. [Crossref] [PubMed]
- Chang JS, Perez-Andujar A, Barani IJ, et al. Estimating the probability of underdosing microscopic brain metastases with hippocampal-sparing whole-brain radiation. Radiother Oncol 2016;120:248-52. [Crossref] [PubMed]
- Nevelsky A, Ieumwananonthachai N, Kaidar-Person O, et al. Hippocampal-sparing whole-brain radiotherapy using Elekta equipment. J Appl Clin Med Phys 2013;14:4205. [Crossref] [PubMed]
- Andreas JJM, Kundapur V. Hippocampus Avoidance Whole-brain Radiation Therapy: A Practical Intensity-modulated Radiation Therapy Planning and Delivery Approach to RTOG 0933. J Med Imaging Radiat Sci 2015;46:78-84. [Crossref] [PubMed]
- Huang L, Qi P, Chao S, et al. A treatment planning class solution for hippocampal avoidance whole brain irradiation using volumetric modulated arc radiotherapy. Appl Rad Oncol 2013:8-11.
- Pokhrel D, Sood S, Lominska C, et al. Potential for reduced radiation-induced toxicity using intensity-modulated arc therapy for whole-brain radiotherapy with hippocampal sparing. J Appl Clin Med Phys 2015;16:131-41. [Crossref] [PubMed]
- Shen J, Bender E, Yaparpalvi R, et al. An efficient Volumetric Arc Therapy treatment planning approach for hippocampal-avoidance whole-brain radiation therapy (HA-WBRT). Med Dosim 2015;40:205-9. [Crossref] [PubMed]
- Yuen AHL, Wu PM, Li AKL, et al. Volumetric modulated arc therapy (VMAT) for hippocampal-avoidance whole brain radiation therapy: planning comparison with Dual-arc and Split-arc partial-field techniques. Radiat Oncol 2020;15:42. [Crossref] [PubMed]
- Kazda T, Vrzal M, Prochazka T, et al. Left hippocampus sparing whole brain radiotherapy (WBRT): A planning study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:397-402. [Crossref] [PubMed]
- Hsu F, Carolan H, Nichol A, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases: a feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2010;76:1480-5. [Crossref] [PubMed]
- Pokhrel D, Sood S, McClinton C, et al. Treatment planning strategy for whole-brain radiotherapy with hippocampal sparing and simultaneous integrated boost for multiple brain metastases using intensity-modulated arc therapy. Med Dosim 2016;41:315-22. [Crossref] [PubMed]
- Popp I, Grosu AL, Fennell JT, et al. Optimization of hippocampus sparing during whole brain radiation therapy with simultaneous integrated boost-tutorial and efficacy of complete directional hippocampal blocking. Strahlenther Onkol 2022;198:537-46. [Crossref] [PubMed]
- Wang S, Zheng D, Zhang C, et al. Automatic planning on hippocampal avoidance whole-brain radiotherapy. Med Dosim 2017;42:63-8. [Crossref] [PubMed]
- Zieminski S, Khandekar M, Wang Y. Assessment of multi-criteria optimization (MCO) for volumetric modulated arc therapy (VMAT) in hippocampal avoidance whole brain radiation therapy (HA-WBRT). J Appl Clin Med Phys 2018;19:184-90. [Crossref] [PubMed]
- Okada H, Ito M, Minami Y, et al. Automatic one-click planning for hippocampal-avoidance whole-brain irradiation in RayStation. Med Dosim 2022;47:98-102. [Crossref] [PubMed]
- Feng CH, Cornell M, Moore KL, et al. Automated contouring and planning pipeline for hippocampal-avoidant whole-brain radiotherapy. Radiat Oncol 2020;15:251. [Crossref] [PubMed]
- Liu H, Clark R, Magliari A, et al. RapidPlan hippocampal sparing whole brain model version 2-how far can we reduce the dose? Med Dosim 2022;47:258-63. [Crossref] [PubMed]
- Sprowls CJ, Shah AP, Kelly P, et al. Whole brain radiotherapy with hippocampal sparing using Varian HyperArc. Med Dosim 2021;46:264-8. [Crossref] [PubMed]
- Rusu I, Roeske J, Solanki A, et al. Fully automated planning and delivery of hippocampal-sparing whole brain irradiation. Med Dosim 2022;47:8-13. [Crossref] [PubMed]
- Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 1951;102:316-9. [PubMed]
- Chang JS, Ma L, Barani IJ, et al. Hippocampal Dose With Radiosurgery for Multiple Intracranial Targets: The Rationale for Proactive Beam Shaping. Technol Cancer Res Treat 2016;15:555-9. [Crossref] [PubMed]
- Nguyen TK, Sahgal A, Detsky J, et al. Single-Fraction Stereotactic Radiosurgery Versus Hippocampal-Avoidance Whole Brain Radiation Therapy for Patients With 10 to 30 Brain Metastases: A Dosimetric Analysis. Int J Radiat Oncol Biol Phys 2019;105:394-9. [Crossref] [PubMed]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [Crossref] [PubMed]
- Yamamoto M, Serizawa T, Higuchi Y, et al. A Multi-institutional Prospective Observational Study of Stereotactic Radiosurgery for Patients With Multiple Brain Metastases (JLGK0901 Study Update): Irradiation-related Complications and Long-term Maintenance of Mini-Mental State Examination Scores. Int J Radiat Oncol Biol Phys 2017;99:31-40. [Crossref] [PubMed]
- Sahgal A, Ruschin M, Ma L, et al. Stereotactic radiosurgery alone for multiple brain metastases? A review of clinical and technical issues. Neuro Oncol 2017;19:ii2-ii15. [Crossref] [PubMed]
- Susko MS, Garcia MA, Ma L, et al. Stereotactic Radiosurgery to More Than 10 Brain Metastases: Evidence to Support the Role of Radiosurgery for Ideal Hippocampal Sparing in the Treatment of Multiple Brain Metastases. World Neurosurg 2020;135:e174-80. [Crossref] [PubMed]
- Yuan J, Lee R, Dusenbery KE, et al. Cumulative Doses to Brain and Other Critical Structures After Multisession Gamma Knife Stereotactic Radiosurgery for Treatment of Multiple Metastatic Tumors. Front Oncol 2018;8:65. [Crossref] [PubMed]
- Scorsetti M, Navarria P, Cozzi L, et al. Radiosurgery of limited brain metastases from primary solid tumor: results of the randomized phase III trial (NCT02355613) comparing treatments executed with a specialized or a C-arm linac-based platform. Radiat Oncol 2023;18:28. [Crossref] [PubMed]
- Di Carlo C, Trignani M, Caravatta L, et al. Hippocampal sparing in stereotactic radiotherapy for brain metastases: To contour or not contour the hippocampus? Cancer Radiother 2018;22:120-5. [Crossref] [PubMed]
- Daniela Falco M, Giancaterino S, D’Andrea M, et al. Hippocampal sparing approach in fractionated stereotactic brain VMAT radio therapy: A retrospective feasibility analysis. J Appl Clin Med Phys 2018;19:86-93. [Crossref] [PubMed]
- Gude Z, Adamson J, Kirkpatrick JP, et al. Hippocampal Avoidance in Multitarget Radiosurgery. Cureus 2021;13:e15399. [PubMed]
- Hahn C, Kavanagh B, Bhatnagar A, et al. Choosing wisely: the American Society for Radiation Oncology’s top 5 list. Pract Radiat Oncol 2014;4:349-55. [Crossref] [PubMed]
- Harth S, Abo-Madyan Y, Zheng L, et al. Estimation of intracranial failure risk following hippocampal-sparing whole brain radiotherapy. Radiother Oncol 2013;109:152-8. [Crossref] [PubMed]
- Ghia A, Tomé WA, Thomas S, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys 2007;68:971-7. [Crossref] [PubMed]


