
Boosted abscopal effect from radiotherapy and pembrolizumab in anaplastic thyroid cancer: a mini-review and case report
Highlight box
Key findings
• This case report then describes a patient with anaplastic thyroid cancer (ATC) who demonstrated exceptional response to combination radiotherapy and anti-programmed cell death protein 1 (anti-PD-1) immunotherapy. In addition, a mini-review is added to describe the abscopal effect and summarise its proposed underlying mechanisms.
What is known and what is new?
• The abscopal effect has been described in other solid cancers, but its role in ATC is unknown.
• Our patient’s dramatic response to treatment supports the effectiveness of anti-PD-1 immunotherapy and may also demonstrate the boosted abscopal effect of combination radiotherapy and immunotherapy in ATC.
What is the implication, and what should change now?
• Future trials should consider combining radiotherapy and anti-PD-1 immunotherapy in the treatment of ATC.
• Studies should be done to understand the underlying mechanisms of the abscopal effect and uncover predictive biomarkers for response to treatment.
Introduction
Role and mechanisms of radiotherapy in cancer treatment
Cancer is responsible for the second highest burden among noncommunicable diseases, with 200 million disease-associated life years attributed to malignant neoplasms in the World Health Organisation’s latest global health estimate (1). A frequently employed treatment modality for cancer is radiotherapy. Up to 50% of cancer patients receive radiotherapy, which is employed locally for cure and for palliation, to reduce tumour burden and improve survival (2).
As part of radiotherapy, ionising radiation is delivered to target sites. This radiation directly or indirectly leads to deoxyribonucleic acid (DNA) damage by inducing double stranded breaks in DNA. Unrepaired double stranded DNA (dsDNA) breaks form micronuclei that readily degrade, exposing dsDNA to cytosolic sensors which activate the immune response as part of danger-associated molecular patterns (DAMPs). Radiotherapy results in other DAMPs, such as the exposure of adenosine triphosphate (ATP), calreticulin and non-coding ribonucleic acids (RNAs) to the external environment, which promote immunogenic cell death. Ionising radiation can also facilitate immune cell infiltration into the tumour through priming immune cells and upregulating adhesion molecule expression. Hence, irradiated cells that survive ultimately become targets for eradication by immune cluster of differentiation 8 (CD8+) T cells and natural killer (NK) cells (3).
Observations of the abscopal effect in radiotherapy
Although largely utilised as a form of local cancer control, radiotherapy has also been occasionally observed to produce systemic anticancer effects away from the site of irradiation. The abscopal effect, first described more than 50 years ago, refers to the clinical phenomenon wherein radiation induces a systemic anti-tumour immune response resulting in regression of non-irradiated tumours distant from the primary irradiated site (4).
The abscopal effect from radiotherapy is a rare phenomenon. In a systematic review of the literature between 1969 and 2014, only 46 clinical cases of the abscopal effect were reported (5). Amongst the reported cases, patients had a median age of 64 years (range, 28–83 years), with a median radiation dose of 31 Gy (range, 0.45–60.75 Gy), and a median noted time to abscopal effect of 6 months (range, 0–24 months). Ohba et al. report the abscopal effect in primary hepatocellular carcinoma after external beam radiotherapy (EBRT) of metastatic vertebral bony metastases, with a dramatic reduction in size of hepatic lesions along with an accompanying fall in alpha-fetoprotein levels from 429,998 ng/mL to less than 10 ng/mL (5). Meanwhile, Rees and Ross describe the abscopal effect in lung metastases of a patient after EBRT of the primary oesophageal adenocarcinoma, with near-complete regression of lung metastases, including lesions outside the irradiated field (5). The abscopal effect may have also been observed in metastatic medullary thyroid carcinoma, in which irradiation of a distant metastatic lymph node led to partial regression of a separate minimally irradiated lymph node (5).
Immune bases of the abscopal effect
The abscopal effect likely arises from the immunogenic effect of radiotherapy. Even at a sublethal level, radiation of cancer cells can result in a change in cell phenotype, upregulating immunogenic genes such as FAS, intercellular adhesion molecule 1 (ICAM-1) and major histocompatibility complex (MHC) class 1 to increase CD8+ T cell directed killing of cancer cells (6). Ionising radiation results in the release of DAMPs enhances the expression of tumour-associated antigens (TAAs), and ultimately activates the adaptive immune response which may facilitate the systemic eradication of cancer cells through immunogenic cell death (7). Once primed by TAAs at the irradiated site, CD8+ T cells may require lower antigen levels to mediate cancer cell death in other non-irradiated areas of the body, resulting in the abscopal effect (8).
The release of inflammatory cytokines and chemokines in response to radiotherapy may have a part to play in the abscopal effect. Cytokines augment the immune response to mediate tumour surveillance, inhibit tumour growth and participate in tumour destruction (9). Radiotherapy induces the production of pro-inflammatory and immunomodulatory cytokines such as interleukin (IL)-1β, tumour necrosis factor alpha (TNFα) and type 1 and 2 interferons (10). A study in a rat animal model has found that cytokines IL-1α, IL-1β, IL-6, TNFα and transforming growth factor beta (TGFβ) are produced for up to four months after radiation (9). Pro-inflammatory cytokines facilitate the production of reactive oxygen species through oxidative stress, promoting the maturation of dendritic cells and subsequent priming of CD8+ T cells (10). The cytokine release after radiotherapy may also promote CD8+ T cell migration and function, resulting in a systemic anti-cancer response. Radiotherapy also induces the production of chemokines such as chemokine (C-X-C motif) ligand (CXCL) 9, CXCL10, and CXCL16, which promote recruitment of effector cluster of differentiation 4 (CD4+) and CD8+ T cells (11). By promoting the priming and effector phases of the immune response, radiotherapy enhances cancer-directed T cell activation.
Immune bases of the boosted abscopal effect in combination radiotherapy and immunotherapy
While radiotherapy alone may be sufficient to produce the abscopal effect in a small minority of patients, the addition of immunotherapy boosts the abscopal effect. With immune evasion a hallmark of carcinogenesis, the degree by which radiotherapy alone can overcome the immunosuppressive state to effectively induce systemic immune activation is limited. The tumour microenvironment (TME) is recognised as a driver of carcinogenesis. Characterised by immunosuppressive interactions between cancer cells and their surrounding stroma, the TME allows for continued malignant cell survival and proliferation (12). Combination immunotherapy potentially overcomes the immunosuppressive TME and enhances subsequent immune responses.
For example, the programmed cell death protein 1 and programmed cell death ligand 1 (PD-1/PD-L1) pathway plays an important role in immune tolerance within the TME. PD-1 is highly expressed by tumour-specific T cells and inhibits the immune response, while PD-L1 expressed by tumour cells promotes proliferation and survival, contributing to tumour progression (13). By targeting the immunosuppressive TME, PD-1/PD-L1 immunotherapy has led to progress in cancer treatment. Anti-PD-1 agent pembrolizumab has been approved by the United States Food and Drug Administration for treatment of cancers including metastatic melanoma, small cell lung cancer and head and neck squamous cell cancer (14). While radiotherapy triggers T cells to induce immunogenic death of cancer cells, anti-PD-1 immunotherapy improves T cell-mediated immune response through blocking the PD-1/PD-L1 inhibition of T cells (15). Through their respective roles in immunomodulation, combination radiotherapy and immunotherapy may have a synergistic systemic anti-cancer effect.
Although radiotherapy produces a desirable immunogenic anti-cancer effect, it also contributes to immunosuppression by activating immunosuppressive cytokine TGFβ, increasing regulatory T cells and promoting tumorigenic M2 macrophages (11). The addition of targeted immunotherapy may shift the balance of radiotherapy towards tumour rejection. In the case of the PD-1/PD-L1 pathway, radiotherapy upregulates local PD-L1 expression, and PD-L1 blockade is necessary to overcome resistance to radiotherapy in a pre-clinical model (16). The irradiation of cancer cells also promotes CD8+ T cell activity, which may complement the role of immunotherapy to ultimately target different facets of the immune response for enhanced immunogenic cell death (17). Additionally, radiotherapy drastically reduces the cancer cell burden, decreasing immune tolerance arising from the persistent presence of cancer antigens, and facilitating the effect of immunotherapy (18).
Observations of the boosted abscopal effect with current combination therapies
With the rise of immunotherapy in the last decade, the once rare abscopal effect has become increasingly reported in the literature. The boosted abscopal effect has been described in patients with metastatic solid cancers treated with focal radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF). Although GM-CSF has no therapeutic impact in solid cancers, 27% of recruited patients demonstrated an abscopal effect and had improved survival (8). A retrospective study on patients with advanced melanoma has found that patients who received both radiotherapy and anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA4) immunotherapy ipilimumab have a higher rate of complete response (CR) (25.7% vs. 6.5% for ipilimumab monotherapy; P=0.04) and improved survival (median 19 vs. 10 months for ipilimumab monotherapy, P=0.01) compared to patients who received immunotherapy alone (3). In a separate study, advanced melanoma patients receiving radiotherapy after ipilimumab have shown a 52% abscopal effect rate despite previous disease progression with ipilimumab alone (19).
The boosted abscopal effect has also been reported with PD-1/PD-L1 immunotherapy. In metastatic non-small cell lung cancer, patients treated with radiotherapy and pembrolizumab demonstrated a response rate of 41.7%, compared to 19.7% in patients treated with pembrolizumab alone (20). The abscopal effect with radiotherapy and pembrolizumab has also been reported in other cancers such as malignant pleural mesothelioma (21), uterine carcinosarcoma (22), and lung squamous cell carcinoma (23).
Although these results are promising, many reports of dramatic responses to combination therapy may be attributable to systemic immunotherapy. The boosted abscopal effect remains difficult to predict and ascertain. Understanding the underlying mechanisms behind the boosted abscopal effect and identifying patients who are most likely to respond will improve the utility of combination therapy in treatment of advanced cancers, or cancers which are otherwise challenging to treat.
Potential boosted abscopal effect in anaplastic thyroid cancer (ATC)
ATC is notoriously difficult to treat. Although thyroid cancer is the commonest endocrine cancer (24), with a vast majority (>95%) being well-differentiated papillary thyroid cancer or follicular thyroid cancer (25), if left untreated, up to 5% of differentiated thyroid cancers can undergo de-differentiation into ATC (26), a highly lethal cancer with a median overall survival of less than 6-months after diagnosis (27). While surgery is the standard of care in treating well-differentiated thyroid cancer (28), only a minority of patients with ATC are deemed suitable for surgical resection while most patients have unresectable disease at presentation (29). ATC only constitutes 2% of all thyroid cancers, yet accounts for more than half of the thyroid cancer-related deaths each year (30).
In addition to this mini-review, we report a patient with metastatic, widely invasive follicular thyroid cancer with significant anaplastic transformation who was successfully treated with radiotherapy and pembrolizumab with palliative intent. She achieved CR at the primary site, allowing laryngeal preservation. Additionally, she showed CR at secondary distant lung and peri-hilar metastases, illustrating the boosted abscopal effect of radiotherapy and PD-1 blockade therapy in ATC for the first time. Her high combined positive score (CPS) of more than 70% may be a potential biomarker for good response to this multimodal treatment. We present this case in accordance with the CARE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-55/rc).
Case presentation
A 51-year-old lady with no known thyroid disease presented with a 3-week history of left thyroid swelling with hoarseness. On physical examination, there was a 3-cm large and hard left thyroid mass without associated lymphadenopathy. Flexible direct laryngoscopy demonstrated concomitant left vocal cord palsy. Computed tomography (CT) imaging showed a large solid cystic thyroid mass compressing the left trachea-oesophageal groove. An ultrasound-guided fine needle aspiration (FNA) of the thyroid mass yielded a Bethesda V category (suspicious for malignancy). Accordingly, the patient was counselled for total thyroidectomy.
Intraoperatively, her left sided thyroid tumour was closely adherent to the cricoid cartilage and inferior constrictor muscle. The left recurrent laryngeal nerve was completely encased by the tumour and sacrificed en-bloc with the tumour. Total thyroidectomy was completed with shave excision of the cricoid and laryngopharyngeal framework cartilage. Further resection of the cricoid cartilage was not performed as it would have necessitated a total laryngectomy.
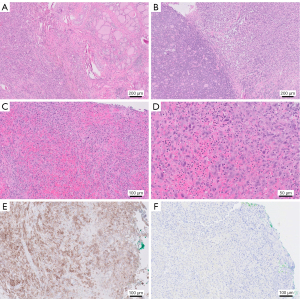
The resected tissue was sent for histopathological analysis. PD-L1 22C3 immunohistochemical assay (Agilent Dako) was performed on formalin-fixed paraffin embedded tissue and interpreted as per manufacturer’s instructions. The final histopathology confirmed a pT4aN1a widely invasive follicular thyroid cancer with significant (50%) anaplastic component (Figure 1A-1D). There was also extensive lympho-vascular, peri-neural, angioinvasion and extrathyroidal extension into the cricothyroid and inferior constrictor musculature. Mutational profiling using a panel of mutation screen showed positive NRAS mutation [NM_002524.5(NRAS):c.181C>A (p.Gln61Lys)] only. The p53 gene was not part of this screening panel. Notably, BRAF V600E mutation was negative but PD-L1 expression was exceedingly high, with a CPS of more than 70% (Figure 1E,1F).
Instead of a post-operative neck ultrasound, a positron emission tomography-CT (PET-CT) scan was performed 2-weeks after surgery with anatomical correlation. This PET-CT demonstrated fluorodeoxyglucose (FDG) avidity of the cricoid cartilage (Figure 2A), single lung metastasis, suprahilar node (Figure 2B) and several intramuscular metastases, with no residual soft tissue in the thyroid bed. Due to the high CPS score, our institution’s multi-disciplinary tumour board recommended QUADshot hypofractionated radiotherapy comprising 30 Gy and 4-weekly pembrolizumab (anti-PD-1 antibody) with palliative intent.
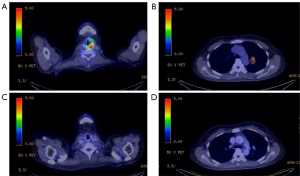
After two cycles of radiotherapy and pembrolizumab, repeat PET-CT imaging demonstrated complete tumour regression of both primary (Figure 2C) and lung metastasis (Figure 2D) using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (31). The patient continued to receive pembrolizumab for 2-years and remained clinically and biochemically euthyroid. There were no long-term adverse side effects to pembrolizumab. She remained well for 32 months, before surveillance magnetic resonance imaging (MRI) found a lytic lesion in the right mandibular condyle infiltrating the adjacent right lateral pterygoid suspicious for bone metastasis. PET-CT demonstrated FDG avidity of the right mandible only. CT-guided FNA showed follicular thyroid cells without anaplastic features. The patient is planned for radiotherapy to the bony lesion without systemic therapy. A timeline of events is shown (Figure 3).

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). This case report has no identifiable information. Publication of this case report and accompanying images was waived from patient consent according to our institutional review board [SingHealth Centralised Institutional Review Board (SingHealth CIRB)].
Discussion
Since the majority of ATC patients have unresectable disease at presentation (29), other treatment strategies for ATC are being explored, with neoadjuvant radiotherapy and BRAF-directed combination therapy showing promise in rendering unresectable ATC resectable (32,33). However, our patient’s ATC was negative for BRAF V600E mutation, which limited the utility of tyrosine kinase inhibitors such as Dabrafenib and Trametinib (34). While NRAS mutation was identified, this mutation is currently not actionable with no effective targeted therapy presently available (35).
Anti-PD-1 immunotherapy is emerging as a potential therapy for ATC given the high PD-L1/PD-1 expression in ATC (36). The addition of pembrolizumab (anti-PD-1 antibody) with a tyrosine kinase inhibitor such as dabrafenib potentially enables surgical resection of previously unresectable disease (37). Treatment of ATC with spartalizumab (anti-PD-1 antibody) has shown a CR in 7% of patients and 1-year survival of 40% (38). Additionally, combining pembrolizumab and lenvatinib in ATC has prolonged the median overall survival to 18.5 months, from the historic figure of 6 months (27,39). With these studies describing good clinical response of ATC to pembrolizumab in patients with high PD-1/PD-L1 expression (38-43), high PD-L1 expression quantified using CPS may be a potential biomarker for predicting response to anti-PD-1 antibody. The results of a recently concluded clinical trial investigating if ATC PD-1/PD-L1 expression is predictive of response to pembrolizumab in ATC [National Library of Medicine (NLM), NCT02688608] may strengthen this hypothesis (44).
In view of the high expression of PD-L1 (CPS >70%) in the ATC sample, our patient was treated with pembrolizumab, with radiotherapy administered locally for local control. With this combination, complete resolution of residual cancer at the cricoid cartilage was achieved, allowing for laryngeal preservation. Notably, CR of distant pulmonary metastases was also observed. The exceptional response of our patient’s distant disease to treatment supports the effectiveness of anti-PD-1 immunotherapy and may also demonstrate the boosted abscopal effect of combination radiotherapy and pembrolizumab.
Conclusions
It is unclear if our patient’s CR is attributable to pembrolizumab alone or in combination with radiotherapy. Based on previous reports demonstrating the abscopal effect with radiotherapy and pembrolizumab in other solid cancers (20-23), we are inclined to attribute this exceptional response to the combination therapy. Although our case study cannot conclusively demonstrate the boosted abscopal effect, our patient’s exceptional response to combination treatment suggests that future trials should consider combining radiotherapy and pembrolizumab in ATCs with high CPS to reproduce the exceptional response seen in our patient. Trials with a control arm of immunotherapy alone and an experimental arm combining immunotherapy and radiotherapy may assess for the abscopal response. Further trials may also generate deeper insights on the optimal dosage, timing and sequence of therapy.
Acknowledgments
Funding: This work did not receive direct funding. Melvin L.K. Chua is supported by the National Medical Research Council Singapore Clinician Scientist Award (NMRC/CSAINV20nov-0021), the Duke-NUS Oncology Academic Program Goh Foundation Proton Research Programme, NCCS Cancer Fund, and the Kua Hong Pak Head and Neck Cancer Research Programme.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-55/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-55/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). This case report has no identifiable information. Publication of this case report and accompanying images was waived from patient consent according to our institutional review board [SingHealth Centralised Institutional Review Board (SingHealth CIRB)].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organisation. Global health estimates: Leading causes of DALYs. 2019. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys
- Citrin DE. Recent Developments in Radiotherapy. N Engl J Med 2017;377:1065-75. [Crossref] [PubMed]
- Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol 2018;39:644-55. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25-37. [Crossref] [PubMed]
- Craig DJ, Nanavaty NS, Devanaboyina M, et al. The abscopal effect of radiation therapy. Future Oncol 2021;17:1683-94. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Demaria S, Formenti SC. The abscopal effect 67 years later: from a side story to center stage. Br J Radiol 2020;93:20200042. [Crossref] [PubMed]
- Siva S, MacManus MP, Martin RF, et al. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett 2015;356:82-90. [Crossref] [PubMed]
- Pouget JP, Georgakilas AG, Ravanat JL. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid Redox Signal 2018;29:1447-87. [Crossref] [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [Crossref] [PubMed]
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov 2022;12:31-46. [Crossref] [PubMed]
- Barcellos-Hoff MH, Lyden D, Wang TC. The evolution of the cancer niche during multistage carcinogenesis. Nat Rev Cancer 2013;13:511-8. [Crossref] [PubMed]
- U.S. Food and Drug Administration. KEYTRUDA® (pembrolizumab) injection, for intravenous use. Highlights of Prescribing Information 2023. Available online: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- Pevzner AM, Tsyganov MM, Ibragimova MK, et al. Abscopal effect in the radio and immunotherapy. Radiat Oncol J 2021;39:247-53. [Crossref] [PubMed]
- Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer 2016;40:10-24. [Crossref] [PubMed]
- Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer 2018;18:313-22. [Crossref] [PubMed]
- Drake CG. Combination immunotherapy approaches. Ann Oncol 2012;23:viii41-6. [Crossref] [PubMed]
- Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014;3:e28780. [Crossref] [PubMed]
- Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467-75. [Crossref] [PubMed]
- Mampuya WA, Bouchaab H, Schaefer N, et al. Abscopal effect in a patient with malignant pleural mesothelioma treated with palliative radiotherapy and pembrolizumab. Clin Transl Radiat Oncol 2021;27:85-8. [Crossref] [PubMed]
- Yano M, Aso S, Sato M, et al. Pembrolizumab and Radiotherapy for Platinum-refractory Recurrent Uterine Carcinosarcoma With an Abscopal Effect: A Case Report. Anticancer Res 2020;40:4131-5. [Crossref] [PubMed]
- Wang W, Huang C, Wu S, et al. Abscopal effect induced by modulated radiation therapy and pembrolizumab in a patient with pancreatic metastatic lung squamous cell carcinoma. Thorac Cancer 2020;11:2014-7. [Crossref] [PubMed]
- Wells SA Jr. Progress in Endocrine Neoplasia. Clin Cancer Res 2016;22:4981-8. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2017. National Cancer Institute 2017. Available online: https://seer.cancer.gov/archive/csr/1975_2017/
- Antonelli A, Fallahi P, Ferrari SM, et al. Dedifferentiated thyroid cancer: a therapeutic challenge. Biomed Pharmacother 2008;62:559-63. [Crossref] [PubMed]
- Saini S, Tulla K, Maker AV, et al. Therapeutic advances in anaplastic thyroid cancer: a current perspective. Mol Cancer 2018;17:154. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol 2006;13:453-64. [Crossref] [PubMed]
- Cornett WR, Sharma AK, Day TA, et al. Anaplastic thyroid carcinoma: an overview. Curr Oncol Rep 2007;9:152-8. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg 2015;4:44-51. [PubMed]
- Maniakas A, Dadu R, Busaidy NL, et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol 2020;6:1397-404. [Crossref] [PubMed]
- Food U, Administration D. FDA approves dabrafenib plus trametinib for anaplastic thyroid cancer with BRAF V600E mutation. Published May 2018;4. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-anaplastic-thyroid-cancer-braf-v600e-mutation
- Boespflug A, Caramel J, Dalle S, et al. Treatment of NRAS-mutated advanced or metastatic melanoma: rationale, current trials and evidence to date. Ther Adv Med Oncol 2017;9:481-92. [Crossref] [PubMed]
- Ma M, Lin B, Wang M, et al. Immunotherapy in anaplastic thyroid cancer. Am J Transl Res 2020;12:974-88. [PubMed]
- Cabanillas ME, Ferrarotto R, Garden AS, et al. Neoadjuvant BRAF- and Immune-Directed Therapy for Anaplastic Thyroid Carcinoma. Thyroid 2018;28:945-51. [Crossref] [PubMed]
- Capdevila J, Wirth LJ, Ernst T, et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J Clin Oncol 2020;38:2620-7. [Crossref] [PubMed]
- Dierks C, Seufert J, Aumann K, et al. Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid 2021;31:1076-85. [Crossref] [PubMed]
- Spalart V, Legius B, Segers K, et al. Dramatic Response to First Line Single Agent Pembrolizumab in Anaplastic Thyroid Carcinoma. Case Rep Endocrinol 2019;2019:9095753. [Crossref] [PubMed]
- Nabhan F, Kander E, Shen R, et al. Pembrolizumab in a Patient with Treatment-Naïve Unresectable BRAF-Mutation Negative Anaplastic Thyroid Cancer. Case Rep Endocrinol 2021;2021:5521649. [Crossref] [PubMed]
- Yang SR, Tsai MH, Hung CJ, et al. Anaplastic Thyroid Cancer Successfully Treated With Radiation and Immunotherapy: A Case Report. AACE Clin Case Rep 2021;7:299-302. [Crossref] [PubMed]
- Giordano SMA, Scaldaferri M, Caiazza E, et al. 5PSQ-158 Off-label use of pembrolizumab in PD-L1 positive metastatic anaplastic thyroid carcinoma: a case report. Eur J Hosp Pharm 2021;28:A134.
- Khan SA. Pembrolizumab in Anaplastic/Undifferentiated Thyroid Cancer. US National Library of Medicine 2022. NCT02688608.


