
A systematic review of undifferentiated pleomorphic sarcoma of the chest wall
Highlight box
Key findings
• There are more reported cases of undifferentiated pleomorphic sarcoma (UPS) in the chest for women than men.
• Recurrence/metastasis for these patients was not different for patients treated with radiotherapy and/or chemotherapy.
• Secondary development of UPS was associated with radiation in 5/22 patients.
What is known and what is new?
• 1.5 cm margin distance is likely sufficient in preventing recurrence.
• Relapse-free survival is better in patients receiving some forms of radiotherapy, whether adjuvant or neoadjuvant.
• Surgical resection is the primary mode of treatment and is often supplemented with radiotherapy and chemotherapy.
• Prognosis is worse in patients with deeper and larger tumors of the chest wall.
What is the implication, and what should change now?
• Future case reports need to provide information on margin distance to limit complications without compromising patient outcomes.
• Future research needs to elucidate the necessity of other therapies in the context of margin distance, tumor size, and tumor location.
Introduction
Sarcomas are a heterogenous group of malignancies that originate from mesenchymal stem cells with a yearly incidence of 5 cases per 100 thousand individuals (1,2). They may be broadly grouped into osteosarcomas, originating from the bone, and soft-tissue sarcomas (STS).
STS account for around 1% of all adult malignancies and encompass over 70 subtypes (3,4). The specific subtype defines treatment options and prognosis (5,6). Subtype diagnosis, however, may be challenging, and undifferentiated pleomorphic sarcoma (UPS) accounts for around 11–17% of STS (7). UPS commonly originates in the lower extremity muscles and deep fascia, so breast and chest wall presentation are atypical (1,8). Originally termed “malignant fibrous histiocytomas”, UPS is staged using tumor, node, and metastasis (TNM) criteria and histologic grade criteria—as determined by a tumor’s mitotic count, necrosis extension, and differentiation—from the French Federation of Cancer Centers Sarcoma Group (FNCLCC) (9,10). During the period of time in which UPS were classified as malignant fibrous histiocytomas, attempts were made at subclassifying these cases into distinct histological subtypes: pleomorphic/storiform, giant cell, inflammatory, angiomatoid, and myxoid (11). However, this system is no longer utilized due to the reclassification of these lesions to UPS and the relative difficulty in defining a lesion as a specific subtype. Given the changes in diagnostic classification and the growing number of reports on UPS involving the chest wall and breast, this review aims to characterize the outcomes, treatment modalities, prognoses, and histology of the current case reports.
The following review has several objectives that we want to address: (I) patient characteristics with the lesion; (II) patient outcomes following surgical resection or resection attempt; (III) best treatment modalities outside of surgical resection; (IV) common histological characteristics of these lesions; (V) current recommendations for UPS resection when considering localization to the thorax; (VI) classification of lesions as primary or secondary and association with radiation. We present this article in accordance with the PRISMA reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-71/rc).
Methods
Literature search and search criteria
A literature search was conducted using the PubMed database to identify case reports of UPS localized to the chest wall or breast tissue. Studies were considered within the date range of 01/01/2003 to 05/21/2023. Search criteria was placed into PubMed as follows: (undifferentiated pleomorphic sarcoma) AND ((breast) OR (trunk) OR (chest) or (chest wall)).
Of note, search terms did not include “malignant fibrous histiocytoma” because of possible over-classification and misclassification prior to 2002 (12). Increasing adoption by pathologists of tumor classification using immunohistochemistry and electron microscopy resulted in the World Health Organization’s reclassification of malignant fibrous histiocytoma to UPS in 2002 (13).
Inclusion and exclusion criteria
Studies were initially excluded if they did not contain at least one of the following terms in their title or abstract: “undifferentiated pleomorphic sarcoma”, “breast”, “chest wall”, or “trunk”. Included case reports were screened for relevance, if they contained “undifferentiated pleomorphic sarcoma” in the text, and full-text accessibility. Further, only case reports that considered or used surgical resection as a treatment modality were analyzed. All case reports were screened by one reviewer, and no automation tools were used. The PRISMA framework was utilized to identify possible studies to be included in the final tabulation.
Data synthesis and analysis
All data was collected manually by one reviewer. Information from the case reports were compiled into a table for greater understanding of current treatment practices and treatment effectiveness. The topics that were extracted from these cases are as follows: authors, year published, patient age, patient sex, histology of tumor, tumor location, lesion size, if neoadjuvant or adjuvant therapy was given, surgical treatment protocol, patient follow-up, if tumor was primary or secondary, association with radiation, tumor grade, and margin distance following surgery. Any information that was not given in these studies were indicated as not specified, except in the case of adjuvant and neoadjuvant therapy. For this, it was assumed that the patients did not receive this treatment as this would likely be reported in the case.
Data synthesis occurred in two parts. Our current understanding of UPS of the chest and its treatment modalities were synthesized from guidelines, studies, or other forms of literature with information, such as margin distance, patient characteristics, or treatment modality. Furthermore, the case reports were used to compile information into an accessible table for clinicians and researchers to identify areas of improvement in future research.
Meta-analysis and other statistical methods analyzing heterogeneity and outcome certainty were not performed with this study for a few key reasons. First, the rarity of non-extremity UPS and the lack of controlled trials comparing treatment modalities make it difficult to draw conclusions and assess heterogeneity accurately. Secondly, this study compiles information from unique case reports, so there is no control patient cohort to compare patient presentations, making it difficult to draw conclusive statistical metrics. Third, an intended goal of this review was not to draw statistical conclusions but to provide researchers with cogent topics to analyze by systematically selecting literature.
A Chi-squared test of independence was performed comparing patients who received therapy versus patients who did not receive therapy to see if there was any association with recurrence or metastasis. The programming language, R, was utilized to perform these assessments.
Bias assessment
Given that the studies analyzed were all unique case reports or series, there is risk of inherent bias in each report. However, it is difficult to quantify this bias without a control cohort of patients. Moreover, this study is intended to supplement current surgical practices by providing an overview of current treatment approaches and identify unique characteristics that may further our current understanding of this non-extremity UPS.
Results
Included studies
Initial screening identified 433 records in the PubMed database (Figure 1). Of these, 88 reports were further screened, resulting in 24 case reports with 32 cases total and 22 other studies being included in the final review. The cases are summarized in Table 1 along with further details on tumor origin/grade and treatment modalities employed.
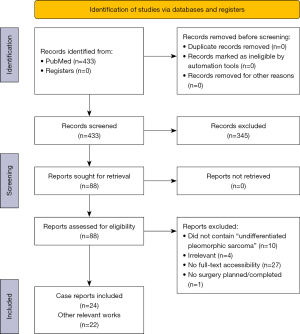
Table 1
| Authors | Year | Age, year | Sex | Histologic type | Location | Size of lesion | Adjuvant or neoadjuvant therapy? | Surgical treatment | Follow-up | Primary or secondary? | Radiation-induced? | Tumor grade | Margin distance? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cariboni et al. (14) | 2021 | 62 | Male | NS | Paravertebral, infiltrating the aortic wall and the 9th thoracic vertebra | 25 mm × 19 mm | No therapy | Aortic resection with bypass grafting and triple en bloc vertebrectomy with tumor excision | No evidence of disease (22 months) | Primary | NA | Grade 3 (FNCLCC standards) | NS |
| Cozzolino et al. (15) | 2018 | 65 | Female | NS | Left breast | >5 cm | Adjuvant radiotherapy | Mastectomy | NS | Primary | NA | High-grade (NCI criteria) | NS |
| Hoshi et al. (16) | 2020 | 44 | Male | NS | Right chest wall | 10 cm × 7 cm × 9 cm | Neoadjuvant chemotherapy and Mohs’ chemosurgery | Wide resection | Lung metastasis (6 months), death (18 months) | NS | NS | High-grade (NCI criteria) | NS |
| Kong et al. (17) | 2020 | 75 | Female | NS | Right breast | 6 cm | Adjuvant radiotherapy | Wide local resection without invasion of the chest wall | No evidence of disease (15 months) | Secondary | Yes | Grade 2 | 0.1 cm posterior margin distance |
| Patel et al. (18) | 2019 | 58 | Male | Spindle cells | Left chest wall/lung mass protruding through first and second rib | 9 cm × 7 cm × 4 cm | Neoadjuvant chemotherapy and Mohs’ chemosurgery | Left chest wall resection and left upper lobectomy | NS | Secondary | Yes | Grade 2 (FNCLCC criteria) | NS |
| Qorbani and Nelson (19) | 2019 | 66 | Male | Epithelioid | Right chest wall/lung mass protruding through the 8th and 9th intercostal spaces | 15 cm × 13.6 cm × 6.2 cm | Adjuvant radiotherapy and chemotherapy | Wedge resection of right lower and middle lobes and adjacent right chest wall | No evidence of disease (4 months) | Primary | NA | High-grade | NS |
| Sahu et al. (20) | 2021 | 31 | Male | Primarily spindle with giant cells | Superior, anterior aspect of right chest wall | 5.1 cm × 2.4 cm × 4.2 cm | Adjuvant radiotherapy | Wide local excision | No evidence of disease (6 months) | Primary | NA | Grade 2 (FNCLCC criteria) | NS |
| Singh et al. (21) | 2021 | 52 | Male | Spindle cells | Right chest wall infiltrating underlying skeletal muscle | 4.7 cm × 3.6 cm × 3.1 cm | No therapy | Right mastectomy | Systemic metastasis (6 months) | Secondary | No | NS | NS |
| Komaei et al. (22) | 2019 | 63 | Female | Spindle-shaped, fibroblast-like cells; multinucleated giant cells | Left breast | 3.0 cm diameter | No therapy | Wide local excision | NS | Secondary | Yes | NS | NS |
| Chakrabarti et al. (23) | 2013 | 60 | Female | Spindle cells with multinucleated giant cells | Left breast | 6 cm × 4 cm | No therapy | Wide local excision | DOD (3 weeks) | Primary | NA | High-grade | NS |
| Kocama et al. (24) | 2021 | 71 | Male | Spindle cell with focal myxoid change; high cellularity with nuclear pleomorphism | Right scapula | 16 cm diameter | No therapy | Wide local excision | Local recurrence (6 months); non-progression of local recurrence (15 months) | Primary | NA | High-grade | 3 cm skin margin |
| Prakash et al. (25) | 2022 | 77 | Female | Osteoblastic-like, multinucleated giant cells and spindle cells | Right posterior shoulder | 11.2 cm × 14.2 cm × 8.8 cm | No therapy | En bloc, wide local excision | NS | Primary | NA | High-grade (FNCLCC criteria) | NS |
| Srinivasamurthy et al. (26) | 2016 | 29 | Female | Spindle cells with hyperchromatic nuclei and eosinophilic cytoplasm, bizarre cells, osteoclast-like giant cells | Left breast | 7 cm × 4 cm × 3 cm | No therapy | Total mastectomy | NS | Primary | NA | High-grade | NS |
| Qiu et al. (27) | 2013 | 68 | Female | It should be noted that they did not provide which patients had the specific types | Left breast | 7.9 cm diameter | No therapy | Modified radical mastectomy | Alive, NED | Primary | NA | NS | NS |
| 58 | Female | Left breast | 5 cm diameter | No therapy | Modified radical mastectomy | Lung metastasis, DOD (6 months) | Primary | NA | NS | NS | |||
| 63 | Female | 1 case had xanthoma cells, atypical spindle cells, and inflammatory cells | Left breast | 15 cm diameter | Adjuvant chemotherapy | Modified radical mastectomy | Liver metastasis, DOD (7 months) | Primary | NA | NS | NS | ||
| 24 | Female | Left breast | 5 cm diameter | Adjuvant chemotherapy | Modified radical mastectomy | Alive, NED | Primary | NA | NS | NS | |||
| 52 | Female | 1 case had osteoclast-like giant cells | Right breast | 13 cm diameter | Adjuvant chemotherapy | Modified radical mastectomy | Alive, NED | Primary | NA | NS | NS | ||
| 20 | Female | Left breast | 3 cm diameter | No therapy | Lumpectomy | Alive, NED | Primary | NA | NS | NS | |||
| 73 | Female | 7 cases had mixed heteromorphic giant cells, spindle cells, and histiocytic-like cells | Left breast | 4 cm diameter | No therapy | Modified radical mastectomy | Chest wall recurrence (19 months), DOD (26 months) | Primary | NA | NS | NS | ||
| 51 | Female | Left breast | 4 cm diameter | Adjuvant chemotherapy | Modified radical mastectomy | Chest wall recurrence (3 months), DOD (8 months) | Primary | NA | NS | NS | |||
| 48 | Female | Left breast | 17 cm diameter | Adjuvant chemotherapy | Radical mastectomy | Dead, NED | Primary | NA | NS | NS | |||
| Quadros et al. (28) | 2006 | 44 | Female | Pleomorphic bizarre giant tumor cells with multinuclear spindle cells | Left breast | 9.5 cm × 9.0 cm × 8.5 cm | Neoadjuvant chemotherapy | Total radical mastectomy with chest wall resection | NED (44 months) | Secondary | Yes | High-grade | >2 cm |
| Yam (29) | 2022 | 53 | Female | Spindle cells | Right breast | 17.4 cm × 10.2 cm × 18 cm | Neoadjuvant and adjuvant CRT | Total radical mastectomy | NED (12 months) | Primary | NA | High-grade | NS |
| Sang et al. (30) | 2021 | 51 | Female | Atypical spindle cells | Left breast | 8 cm × 4 cm × 9 cm | Neoadjuvant and adjuvant CRT | Radical mastectomy | Brain and lung metastasis (8 months) | Primary | NA | High-grade | NS |
| Bertucci et al. (31) | 2015 | 61 | Female | Fibroblast-like spindle cells | Right breast | 2 cm diameter | None | Radical mastectomy with wide chest wall en bloc resection | Local recurrence (4 months), DOD (25 months) | Secondary | Yes | High-grade | NS |
| Noh et al. (32) | 2012 | 70 | Female | NS | Left axillary region | 8 cm diameter | None | Wide local excision | NS | Secondary | Yes | Grade 3 (FNCLCC criteria) | NS |
| Balbi et al. (33) | 2013 | 50 | Female | Atypical spindle-shaped and ovoid cells with multinuclear giant cells and epithelioid cells | Right breast | 10 cm diameter | None | Total radical mastectomy | NED (15 months) | Primary | NA | NS | NS |
| Yamazaki et al. (34) | 2018 | 55 | Female | Spindle-shaped cells with heteromorphic strong nuclei | Right breast | >5 cm diameter | Neoadjuvant chemotherapy | Simple mastectomy | Lung metastasis, DOD (4 months) | Primary | NA | NS | NS |
| Gambichler et al. (35) | 2023 | 58 | Female | Giant and atypical spindle-shaped tumor cells with nuclear pleomorphism | Left breast | NS | Adjuvant immunotherapy and radiotherapy | Total radical mastectomy | Local recurrence (3 months), distal metastasis, DOD (15 months) | Primary | NA | High-grade | NS |
| Jeong et al. (36) | 2011 | 76 | Male | Spindle cells with eosinophilic infiltrates and lymphoplasma cells. Atypical cells were noted | Left breast | 3.8 cm diameter | None | Wide local en bloc resection | NS | Primary | No | High-grade | NS |
| Anzali et al. (37) | 2023 | 58 | Female | NS | Left breast | Extremely large based on image (>5 cm, size not given) | Neoadjuvant chemotherapy and radiotherapy | None | DOD (time not specified) | Primary | No | High-grade | NA |
NS, not specified; NA, not Applicable; NCI, National Cancer Institute; FNCLCC, Fédération Nationale des Centres de Lutte Contre le Cancer; DOD, died of disease; NED, no evidence of disease; CRT, chemoradiotherapy.
Findings
Table 1 reveals some metrics that may be interesting to consider given our current understanding of UPS. First, 8/32 (25%) of the patients identified in our review of trunk UPS were male. Additionally, 24/32 patients (75%) had tumor diameters greater than 5 cm in one dimension.
In terms of treatment, 17/32 (53.13%) received some form of adjuvant or neoadjuvant therapy. Of these 17 individuals, 6 (35.3%) still developed distal metastasis or local recurrence. Comparatively, 6/10 (60%) that had follow-up information and no adjuvant therapy reported metastasis or recurrence. The Chi-squared test results revealed that there is no connection between therapy application and the occurrence of metastasis or local recurrence (χ2=1.56, df=1, P=0.2122).
For patients who received adjuvant or neoadjuvant chemotherapy, 5/13 (38.5%) experienced some form of recurrence or distal metastasis, with the liver and lungs being the primary locations. For patients who received neoadjuvant or adjuvant radiotherapy (RT), 2/8 (25%) had local recurrence or metastasis. Furthermore, 6/16 (37.5%) of surviving patients with tumor diameter greater than 5 cm in one dimension had local recurrence or metastasis, and 2 were noted as dying of their disease; 5/22 (22.7%) with a UPS of the breast had a radiation-associated malignancy as well.
Discussion
Historically termed malignant fibrous histiocytoma by Ozzello et al. in 1963, UPS accounts for approximately 15% of all adult soft tissue sarcomas and primarily arises in the limbs (38-42). UPS lesions are found less frequently in the trunk (~15% of all cases), specifically near the chest wall and breasts (24,40). The five-year overall survival rate typically ranges from 55% to 65%, and patients with higher grades of UPS exhibit worse long-term survival as compared with other STS counterparts (1).
In the breast, UPS is characterized by a local recurrence rate of 44% and distal metastasis of 42% (43). A retrospective review of 192 patients with resection of STS of the chest wall found that 32/192 (17%) patients’ sarcomas were classified as UPS, second to desmoid tumors (44). The most common histological subtype of recurrence in this cohort was UPS as well (44). Multiple studies have demonstrated that high tumor grade and large maximal tumor diameter, typically >5 cm, is associated with higher risk for recurrence (2,27,45,46).
Histologic characteristics for undifferentiated STS are remarkably diverse. These sarcomas are commonly divided into pleomorphic, spindle cell, round cell, epithelioid, and unspecified types (1,40,47). Pleomorphic lesions are often patternless but are defined by variation in nuclear size, hyperchromasia, and necrosis surrounding the lesion (1,40). These lesions involve a variety of cells, including fibroblast-like spindle and giant cells with multiple nuclei (20,22,23,48).
Treatment depends on staging. Metastatic UPS is typically treated with systemic therapy. Treatment of localized non-metastatic UPS involves surgical resection, when technically feasible, occasionally coupled with neoadjuvant or adjuvant therapies, which may include radiation therapy and chemotherapy. With regards to surgical resection, adequate margin distance is a crucial to limit, and studies typically indicate that 4 cm is an adequate size (27,49,50). However, a recent retrospective study of 41 patients demonstrated increased rates of recurrence when margin distance was less than 1.5 cm as compared with patients with margin distances greater than 1.5 cm (46). Larger tumor size as well as proximity to important structures may limit accessible margin distances, leading to poorer prognoses and outcomes (46). Inadequacy of margins with large tumors are often secondary to proximity to deeper, vital structures located within the thoracic cavity and mediastinum. Furthermore, deeper, and larger (>5 cm diameter) tumors are more likely to have local recurrence, metastasis, and mortality (27,51).
Neoadjuvant or adjuvant chemotherapy and RT, though debated, are often used to supplement surgical resection of UPS. The National Cancer Comprehensive indicates that neoadjuvant RT is more effective than its counterpart in treating UPS in the trunk. Some studies have demonstrated that RT reduces local recurrence, and Issakov et al. [2005] found that patients who received adjuvant RT had a 10-year relapse free survival of 62% (15,50,52,53). Comparatively, a 5-year relapse free survival rate of 55% was determined for 100 UPS patients who did not receive RT in a long-term follow-up (54). Cozzolino et al. [2018] described a necessary dosage of at least 60 GyRBE to adequately treat the tumor bed, but it will ultimately need to consider margin distance, tumor size, and grade to best balance risks and benefits (15). According to the National Comprehensive Cancer Network (NCCN), adjuvant RT should only be applied with R1 and R2 resections, since R1/2 resections have demonstrated worse outcomes compared to R0 (55,56). Since margin distance is crucial for limiting recurrence, RT benefits may outweigh the risks when patients do not achieve a margin distance of at least 1.5 cm. As always, a multidisciplinary approach should be applied to determine ideal treatment options for each case.
Broadly speaking, chemotherapy is commonly applied to help treat higher grade (intermediate and high grade) UPS (10). The typical chemotherapy regimens for UPS include anthracycline-based medications, such epirubicin, and ifosfamide (9,40). In comparison to treatment with gemcitabine plus docetaxel, multiple studies have demonstrated greater efficacy with the standard chemotherapy than the histotype-specific regimen with gemcitabine (57-59). Like RT, the use of chemotherapy is debated, but growing evidence suggests that adjuvant chemotherapy may have some benefit in reducing distant recurrence (48).
In regards to the results, there are a few limitations that are important when considering these results. As stated prior, these are case reports and case series of a rare phenomenon, so there is likely some selection bias. As such, there were more reported cases of females with UPS of the breast than males. This outcome could be explained by a few factors with greater selection of female patients, sex-related differences in tumor location, or difficulty identifying tumors early in women due to the proximity to breast tissue. Furthermore, 75% of the patients had a tumor size greater than 5 cm in 1 dimension. Given that 24 of the patients were female, difficulty in noticing small masses in the chest and a lack of notable symptoms could allow tumors to grow >5 cm without being detected.
The Chi-squared test of independence noted no association with recurrence or metastasis and whether the patient received other therapies. However, these are case reports and not random controlled trials, so it is difficult to draw conclusions relative to current literature without proper experimental methods.
Current literature suggests that radiation-associated UPS occurs in 5.2% of UPS cases, but these case reports demonstrate an occurrence of 5/22 for secondary malignancies (22.7%) (60). Consequently, this may further support current evidence that radiation-associated UPS is more commonly found in the chest than other parts of the body, but this data needs to be taken likely given the nature of the reports.
For the purpose of this study, a systematic review was performed with the intention of qualitatively selecting literature for the purpose of providing a cogent direction for future researchers to focus on when considering this entity. Given its rarity and the minimal empirical work done to draw strong conclusions regarding the surgical treatment of this type of tumor, this work was supplemented with information from case reports and series to help fill in some of these gaps. We elected to follow a systematic approach to help keep cases relatively consistent in terms of the presented information in those studies.
It is important to note our review demonstrates characteristics of a limited number of patients. Additionally, we note a possible bias towards primary UPS in our review as many reports only documented primary malignancies, such as Qiu et al. [2013] (27). Beyond the limited number of patients, we utilized case reports given the lack of other study types assessing surgical treatment for these cases. As such, it should be noted there was likely bias and heterogeneity within these publications. We do not intend to draw significant conclusions on better treatment modalities or outcomes, but we do intend to provide possible areas of interest for future research and some of the characteristics we are seeing in patients from our current understanding.
UPS of the trunk and chest wall is increasingly reported in the literature. Margin size, use of chemoradiation, and prior radiation exposure are all areas of ongoing study. These areas should be explored in greater depth in future research to optimize patient outcomes. We also suggest that case reports provide greater details on this information to help researchers draw more effective conclusions. Consultation with a multidisciplinary team is crucial in the care of patients with UPS to review all treatment options and provide individualized care to each patient (61).
Conclusions
Overall, the study aims to enlighten researchers on the current best practices for UPS, specifically involving the chest wall. This study illustrates that are more case reports of women with UPS of the chest wall despite men tending to develop UPS more often in general. Further, there is some discrepancy in proper margin distance, but 1.5 cm may be sufficient in preventing recurrence. However, this study was unable to acquire sufficient information on margin size in these cases to make a definitive conclusion. Additionally, RT and chemotherapy are often recommended in the treatment UPS, but this study found no difference in the recurrence or metastasis of UPS in the chest wall between patients receiving therapy and those who did not. A final point to note is that this study also corroborates previous reports that radiation-associated UPS is more commonly found in the trunk as well.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-71/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-71/coif). A.C. serves as an unpaid editorial board member of Chinese Clinical Oncology from April 2022 to March 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sbaraglia M, Dei Tos AP. The pathology of soft tissue sarcomas. Radiol Med 2019;124:266-81. [Crossref] [PubMed]
- Adem C, Reynolds C, Ingle JN, et al. Primary breast sarcoma: clinicopathologic series from the Mayo Clinic and review of the literature. Br J Cancer 2004;91:237-41. [Crossref] [PubMed]
- Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology 2014;64:2-11. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Brennan MF, Antonescu CR, Moraco N, et al. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg 2014;260:416-21; discussion 421-2. [Crossref] [PubMed]
- Du XH, Wei H, Zhang P, et al. Heterogeneity of Soft Tissue Sarcomas and Its Implications in Targeted Therapy. Front Oncol 2020;10:564852. [Crossref] [PubMed]
- Blay JY, Honoré C, Stoeckle E, et al. Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol 2019;30:1143-53. [Crossref] [PubMed]
- Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol 2021;28:44-58. [Crossref] [PubMed]
- Robles-Tenorio A, Solis-Ledesma G. Undifferentiated Pleomorphic Sarcoma. In: StatPearls. Treasure Island (FL): StatPearls Publishing; April 10, 2023.
- von Mehren M, Kane JM, Bui MM, et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J Natl Compr Canc Netw 2020;18:1604-12. [Crossref] [PubMed]
- Seomangal K, Mahmoud N, McGrath JP. Malignant fibrous histiocytoma, now referred to as Undifferentiated Pleomorphic Sarcoma: A Case Report of an unexpected histology of a subcutaneous lesion. Int J Surg Case Rep 2019;60:299-302. [Crossref] [PubMed]
- Daugaard S. Current soft-tissue sarcoma classifications. Eur J Cancer 2004;40:543-8. [Crossref] [PubMed]
- Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology 2006;48:3-12. [Crossref] [PubMed]
- Cariboni U, Gennaro N, Costa F, et al. Multi-Step Combined Upfront Surgery for Locally Advanced Paravertebral Sarcoma: A Case Report. Front Surg 2021;8:664089. [Crossref] [PubMed]
- Cozzolino M, Oliviero C, D'Andrea B, et al. The Role of Adjuvant Radiotherapy for a Case of Primary Breast Sarcoma: A Plan Comparison between Three Modern Techniques and a Review of the Literature. Case Rep Med 2018;2018:4137943. [Crossref] [PubMed]
- Hoshi M, Iwai T, Oebisu N, et al. Successful pre-operative local control of skin exposure by sarcoma using combination of systemic chemotherapy and Mohs' chemosurgery. World J Surg Oncol 2020;18:36. [Crossref] [PubMed]
- Kong J, Shahait AD, Kim S, et al. Radiation-induced undifferentiated pleomorphic sarcoma of the breast. BMJ Case Rep 2020;13:e232616. [Crossref] [PubMed]
- Patel R, Hu J, Chopra S, et al. Neoadjuvant chemotherapy for radiation-associated soft-tissue sarcoma: A case report. Rare Tumors 2019;11:2036361318821763. [Crossref] [PubMed]
- Qorbani A, Nelson SD. Primary pulmonary undifferentiated pleomorphic sarcoma (PPUPS). Autops Case Rep 2019;9:e2019110. [Crossref] [PubMed]
- Sahu S, Halder S, Jain S, et al. Undifferentiated pleomorphic sarcoma of the chest wall: a rare diagnosis. BMJ Case Rep 2021;14:e245366. [Crossref] [PubMed]
- Singh BK, Pol MM. Pleomorphic liposarcoma of the male breast: lessons from a rare malignancy during COVID-19 pandemic. BMJ Case Rep 2021;14:e244056. [Crossref] [PubMed]
- Komaei I, Guccione F, Sarra F, et al. Radiation-induced undifferentiated pleomorphic sarcoma of the breast: a rare but serious complication following breast-conserving therapy. A case report and literature review. G Chir 2019;40:544-50. [PubMed]
- Chakrabarti I, Ghosh N, Giri A. Cytologic diagnosis of undifferentiated high grade pleomorphic sarcoma of breast presenting with brain metastasis. J Neurosci Rural Pract 2013;4:188-90. [Crossref] [PubMed]
- Kocaman G, Yenigün MB, Kaya B, et al. A rare giant sarcoma of the chest wall: Undifferentiated pleomorphic sarcoma. Turk Gogus Kalp Damar Cerrahisi Derg 2021;29:552-5. [Crossref] [PubMed]
- Prakash S, Luis Rayas J, Rojas Murguia A, et al. Undifferentiated Pleomorphic Sarcoma Presenting With Cardiac Tamponade: A Case Report and Review. J Investig Med High Impact Case Rep 2022;10:23247096221141190. [Crossref] [PubMed]
- Srinivasamurthy BC, Kulandaivelu AR, Saha K, et al. Primary undifferentiated pleomorphic sarcoma of the breast in a young female: a case report. World J Surg Oncol 2016;14:186. [Crossref] [PubMed]
- Qiu SQ, Wei XL, Huang WH, et al. Diagnostic and therapeutic strategy and the most efficient prognostic factors of breast malignant fibrous histiocytoma. Sci Rep 2013;3:2529. [Crossref] [PubMed]
- Quadros CA, Vasconcelos A, Andrade R, et al. Good outcome after neoadjuvant chemotherapy and extended surgical resection for a large radiation-induced high-grade breast sarcoma. Int Semin Surg Oncol 2006;3:18. [Crossref] [PubMed]
- Yam MKH A. 53-year-old female with a large breast sarcoma: A case report from Hong Kong. Radiol Case Rep 2022;17:3055-8. [Crossref] [PubMed]
- Sang NV, Duc NM, My TT, et al. A rare case report of breast sarcoma. Radiol Case Rep 2021;16:1047-50. [Crossref] [PubMed]
- Bertucci F, Faure M, Ghigna MR, et al. High-grade soft tissue sarcoma arising in a desmoid tumor: case report and review of the literature. Clin Sarcoma Res 2015;5:25. [Crossref] [PubMed]
- Noh JM, Huh SJ, Choi DH, et al. Two cases of post-radiation sarcoma after breast cancer treatment. J Breast Cancer 2012;15:364-70. [Crossref] [PubMed]
- Balbi G, Di Martino L, Pitruzzella G, et al. Undifferentiated pleomorphic sarcoma with osteoclast-like giant cells of the female breast. World J Surg Oncol 2013;11:21. [Crossref] [PubMed]
- Yamazaki H, Shimizu S, Yoshida T, et al. A case of undifferentiated pleomorphic sarcoma of the breast with lung and bone metastases. Int J Surg Case Rep 2018;51:143-6. [Crossref] [PubMed]
- Gambichler T, Horny K, Mentzel T, et al. Undifferentiated pleomorphic sarcoma of the breast with neoplastic fever: case report and genomic characterization. J Cancer Res Clin Oncol 2023;149:1465-71. [Crossref] [PubMed]
- Jeong YJ, Oh HK, Bong JG. Undifferentiated pleomorphic sarcoma of the male breast causing diagnostic challenges. J Breast Cancer 2011;14:241-6. [Crossref] [PubMed]
- Anzali BC, Goli R, Faraji N, et al. Invasion of undifferentiated pleomorphic sarcoma (UPS) in breast tissue; a case report study. Int J Surg Case Rep 2023;107:108307. [Crossref] [PubMed]
- Brčić I, Rosenberg AE. Pathology of pleomorphic/undifferentiated and dedifferentiated bone neoplasms. Semin Diagn Pathol 2021;38:163-9. [Crossref] [PubMed]
- Henderson MT, Hollmig ST. Malignant fibrous histiocytoma: changing perceptions and management challenges. J Am Acad Dermatol 2012;67:1335-41. [Crossref] [PubMed]
- Mocellin S. Undifferentiated Pleomorphic Sarcoma. In: Mocellin S, editor. Soft Tissue Tumors: A Practical and Comprehensive Guide to Sarcomas and Benign Neoplasms. Cham: Springer International Publishing; 2021:779-83.
- Nascimento AF, Raut CP. Diagnosis and management of pleomorphic sarcomas (so-called "MFH") in adults. J Surg Oncol 2008;97:330-9. [Crossref] [PubMed]
- Ozzello L, Stout AP, Murray MR. Cultural characteristics of malignant histiocytomas and fibrous xanthomas. Cancer 1963;16:331-44. [Crossref] [PubMed]
- Miliaras D, Konstantinides E. Malignant fibrous histiocytoma of the breast: a case report. Case Rep Pathol 2012;2012:579245. [Crossref] [PubMed]
- McMillan RR, Sima CS, Moraco NH, et al. Recurrence patterns after resection of soft tissue sarcomas of the chest wall. Ann Thorac Surg 2013;96:1223-8. [Crossref] [PubMed]
- Rubino C, Shamsaldin A, Lê MG, et al. Radiation dose and risk of soft tissue and bone sarcoma after breast cancer treatment. Breast Cancer Res Treat 2005;89:277-88. [Crossref] [PubMed]
- Park I, Shin S, Kim HK, et al. Primary Chest Wall Sarcoma: Surgical Outcomes and Prognostic Factors. Korean J Thorac Cardiovasc Surg 2019;52:360-7. [Crossref] [PubMed]
- Matushansky I, Charytonowicz E, Mills J, et al. MFH classification: differentiating undifferentiated pleomorphic sarcoma in the 21st Century. Expert Rev Anticancer Ther 2009;9:1135-44. [Crossref] [PubMed]
- Brennan MF, Antonescu CR, Maki RG. Undifferentiated Pleomorphic Sarcoma (UPS; Malignant Fibrous Histiocytoma: MFH) and Myxofibrosarcoma. In: Brennan MF, Antonescu CR, Maki RG, editors. Management of Soft Tissue Sarcoma. New York, NY: Springer; 2013:129-36.
- Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer 2001;92:172-80. [Crossref] [PubMed]
- Pollard SG, Marks PV, Temple LN, et al. Breast sarcoma. A clinicopathologic review of 25 cases. Cancer 1990;66:941-4. [Crossref] [PubMed]
- De Cesare A, Fiori E, Burza A, et al. Malignant fibrous histiocytoma of the breast. Report of two cases and review of the literature. Anticancer Res 2005;25:505-8. [PubMed]
- Issakov J, Kollender Y, Soyfer V, et al. A single-team experience of limb sparing approach in adults with high-grade malignant fibrous histiocytoma. Oncol Rep 2005;14:1071-6. [Crossref] [PubMed]
- Mancino AT. Diseases of the Breast. Philadelphia, PA: Lippincott Williams & Wilkins, Inc. Ann Surg 2001;233:594. [Crossref]
- Chen S, Huang W, Luo P, et al. Undifferentiated Pleomorphic Sarcoma: Long-Term Follow-Up from a Large Institution. Cancer Manag Res 2019;11:10001-9. [Crossref] [PubMed]
- von Mehren M, Kane JM, Armstrong SA, et al. Soft Tissue Sarcoma: NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network; 2023.
- Zhang S, Zhang X, Zhao Z, et al. Undifferentiated pleomorphic sarcoma of the extremity and trunk: a retrospective cohort study of 166 cases in a large institution. Transl Cancer Res 2022;11:678-88. [Crossref] [PubMed]
- Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812-22. [Crossref] [PubMed]
- Gronchi A, Palmerini E, Quagliuolo V, et al. Neoadjuvant Chemotherapy in High-Risk Soft Tissue Sarcomas: Final Results of a Randomized Trial From Italian (ISG), Spanish (GEIS), French (FSG), and Polish (PSG) Sarcoma Groups. J Clin Oncol 2020;38:2178-86. [Crossref] [PubMed]
- Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 J Clin Oncol 2007;25:2755-63. [corrected]. [Crossref] [PubMed]
- Dineen SP, Roland CL, Feig R, et al. Radiation-Associated Undifferentiated Pleomorphic Sarcoma is Associated with Worse Clinical Outcomes than Sporadic Lesions. Ann Surg Oncol 2015;22:3913-20. [Crossref] [PubMed]
- Gómez J, Tsagozis P. Multidisciplinary treatment of soft tissue sarcomas: An update. World J Clin Oncol 2020;11:180-9. [Crossref] [PubMed]


