
Left total pneumonectomy performed after alectinib treatment for anaplastic lymphoma kinase-positive lung adenocarcinoma: a case report
Highlight box
Key findings
• This case report describes a patient with anaplastic lymphoma kinase-positive (ALK-positive) lung adenocarcinoma who underwent a left total lung resection after significant tumor shrinkage following neoadjuvant therapy with alectinib. These results indicate that alectinib neoadjuvant therapy is very effective.
What is known and what is new?
• Alectinib has been previously studied as a neoadjuvant therapy in patients with ALK-positive lung adenocarcinoma, but left total pneumonectomy after neoadjuvant therapy is rare.
• Our patient underwent neoadjuvant therapy with alectinib and underwent left total pneumonectomy. The patient still had a good quality of life after one year of follow-up. This case proves that alectinib is safe and reliable as a neoadjuvant therapy.
What is the implication, and what should change now?
• Future studies should focus on the timing of alectinib neoadjuvant therapy and the most appropriate time of use after surgery.
• At the same time, we should also focus on the problem of pulmonary fibrosis in the course of alectinib neoadjuvant therapy, explore its mechanism and find out solutions.
Introduction
Neoadjuvant therapy has provided more surgical opportunities to patients with lung cancer. Chemotherapy is the main mode of neoadjuvant therapy, but its effect is limited, and preoperative chemotherapy alone can improve the 5-year survival rate by only 5% (1). The use of novel therapeutic agents in neoadjuvant therapy for non-small cell lung cancer (NSCLC) is an emerging area of research aimed at achieving higher cure rates, and it has shown good results in NSCLC patients without gene mutations (2). Although neoadjuvant therapy may sometimes delay surgery and carry the risk of disease progression, its advantages are well known. Alectinib is a potent and highly selective second-generation anaplastic lymphoma kinase (ALK) inhibitor for patients with ALK-positive NSCLC. ALNEO trial is a phase II, open-label, single-arm, multicenter study to assess the activity and safety of alectinib as neo-adjuvant therapy in patients with ALK-positive locally advanced stage III NSCLC is currently on going (3). There are no mature clinical data on the perioperative treatment of alectinib for ALK-positive NSCLC. Most data are derived from case reports or small-sample studies. However, all have shown good results. Therefore, in this case, we chose to use alectinib as the targeted drug for neoadjuvant therapy. We present this article in accordance with the CARE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-111/rc).
Case presentation
A 52-year-old Asian woman presented with wheezing that was aggravated after activity had had no obvious inducement 6 months before admission. She had no fever, coughing, phlegm production, or chest pain. Ten days before presentation, she developed sudden hemoptysis. The patient remained in good health with no history of major diseases, smoking, or drinking or a family history of tumors. Chest computed tomography (CT) showed mass shadows in the hilum of the left lung and the soft tissue of the left mediastinum with a maximum cross-sectional area of 46 mm × 50 mm, as well as lymph node shadows beside the aortic arch. Lung cancer was considered to have invaded the mediastinum. The carcinoembryonic antigen concentration was 81.30 ng/mL (reference range, 0.00–5.00 ng/mL). Pathological biopsy by tracheoscopy showed invasive mucinous adenocarcinoma in the lower lobe of the left lung. According to the 8th tumor node metastasis (TNM) stage, the patient was cT4N2M0, IIIB.
Based on National Comprehensive Cancer Network (NCCN) guidelines, patients are recommended to undergo radical concurrent chemoradiotherapy. ALK [echinoderm microtubule-associated protein-like 4 (EML4)-ALK fusion positive] was detected by next-generation sequencing, with no other genetic variants. Alectinib is recommended as a first-line targeted drug for ALK-positive patients. According to some previous case reports, alectinib is better than traditional radiotherapy or chemotherapy in patients with locally advanced ALK-positive NSCLC. So after full disclosure and consultation with the patient and her family, we decided to give the patient the targeted drug alectinib, 600 mg twice daily for two and a half months. The carcinoembryonic antigen concentration was 5.63 ng/mL (reference range, 0.00–5.00 ng/mL), which was significantly lower than that before targeted therapy. After targeted therapy, pulmonary function testing revealed small airway dysfunction, slightly reduced diffusion function, and a normal ratio of residual volume to total lung capacity. Lung function was thus improved compared with that before treatment (Table 1).
Table 1
| Lung function items | Before targeted therapy | After targeted therapy | Six months after surgery |
|---|---|---|---|
| VC (L) | 1.82 | 2.72 | 1.64 |
| FVC (L) | 1.82 | 2.72 | 1.64 |
| FEV1 (L) | 1.41 | 2.05 | 1.28 |
| FEV1/FVC (%) | 77.63 | 75.53 | 78.2 |
| MEF50 (L/s) | 1.4 | 2.26 | 1.27 |
| PEF (L/s) | 3.96 | 5.72 | 3.7 |
VC, vital capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; MEF50, maximal expiratory flow at 50% of forced vital capacity; PEF, peak expiratory flow; L, liter; s, second.
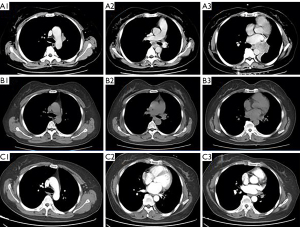
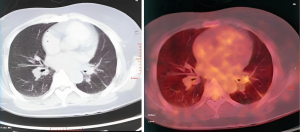
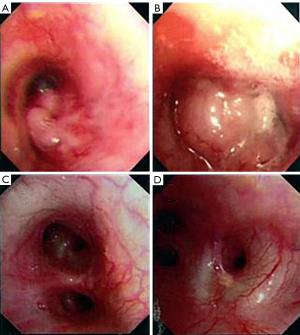
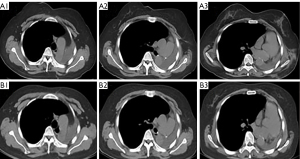
Chest CT showed that the maximum cross-sectional area of the left lung tumor before targeted therapy was 46 mm × 50 mm. After treatment, the soft tissue mass in the left hilar area and the left mediastinum was significantly reduced, and the enlarged lymph nodes around the aortic arch and in the mediastinum were also significantly reduced (Figure 1). Two months after targeted therapy, positron emission tomography (PET) revealed a standardized uptake value of 3.9 for the tumor in the left hilar region, with no enlarged hilar or mediastinal lymph nodes or tracer accumulation. The maximum cross-sectional area of the left lung tumor after targeted therapy was 4 mm × 7 mm (Figure 2). The comparison of bronchoscopy before and after the administration of targeted drugs is shown in Figure 3.
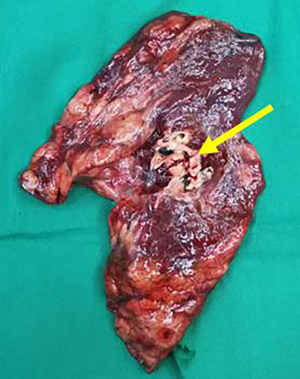
The patient’s TNM stage was reassessed to ycT2aN0M0, stage IB. No abnormalities were found on brain magnetic resonance imaging (MRI) and abdominal CT. There were no contraindications to surgery. Subsequently, surgical treatment was performed, and the planned surgical modalities were left inferior lobectomy. Thoracoscopic exploration showed that the left hilum of the lung showed dense fibrotic changes, the lower lobe trachea and pulmonary veins could not be separated by thoracoscopy. So the patient was converted to thoracotomy for left pneumonectomy. After opening the pericardium, the upper and lower pulmonary veins were removed and excised in the pericardium. The para-arch and subcarinal lymph nodes were fibrosed and fused and had reasonably clear boundaries with the surrounding tissue (Figure 4). Postoperative pathology showed foam cells, multinucleated giant cells, and scattered atypical cells in the whole lobe of the left lung, consistent with the changes after treatment of adenocarcinoma. There was no lymph node metastasis. Pathological results showed that the patient had achieved pathologic complete response (pCR).
The patient continued to take alectinib after discharge and has been followed up for more than one year. She has returned to her normal daily life. Table 1 shows the pulmonary function re-examination findings, and Figure 5 shows the CT re-examination findings. The carcinoembryonic antigen concentration decreased to 2.22 ng/mL. No wheezing occurred during light physical labor, and the dyspnea was relieved.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are the only currently approved type of targeted adjuvant therapy for NSCLC (4). The breakthrough results of the EVAN study, which explored erlotinib versus vinorelbine plus cisplatin as adjuvant therapy for EGFR-positive stage IIIA NSCLC, showed a significantly better 5-year overall survival rate with erlotinib than with chemotherapy (84.8% vs. 51.1%). Patients in the chemotherapy arm either reached the end point or were censored at the end of the follow-up; thus, the 5-year disease-free survival could not be calculated, the disease-free survival curve in the erlotinib group remained superior to that in the chemotherapy group throughout the trial, consistent with the overall survival curve (5). Osimertinib also achieved a breakthrough in the ADAURA study (6,7), becoming the first EGFR-TKI to receive an indication for adjuvant therapy. This reflects the efficacy of osimertinib regardless of previous chemotherapy. For evaluation of neoadjuvant therapy, the NeoADAURA study is currently ongoing; however, the phase III data are not yet available (8).
ALK rearrangement is a poor prognostic factor for patients with resectable NSCLC. In a previous study, ALK-fusion was detected in 29 (3.7%) of 764 patients with resectable stage I to III NSCLC, and ALK rearrangement was associated with a poor prognosis in patients with resectable NSCLC compared with other driver mutations (9). For these ALK-positive patients, the fusion rate is low and the prognosis is poor. However, based on the large number of patients with lung cancer, approximately 75,000 patients are diagnosed with ALK-positive NSCLC each year, and mature perioperative data are not available (10-13). The ALK-positive population has also been excluded from clinical trials of neoadjuvant or adjuvant therapy with immune checkpoint inhibitors for lung cancer (14,15). There are no controlled clinical studies to support the advantage of TKI treatment in patients with ALK-positive NSCLC.
Alectinib has been reported in several cases as neoadjuvant therapy for lung cancer. Yue et al. (16) reported a clinically successful case of alectinib in stage IIIB ALK-positive NSCLC downgrading to ypT1aN0M0 IB. Gu et al. (17) also reported a similar case of ALK-positive NSCLC with stage IIIB reduced to stage T1aN0M0, IA after treatment with alectinib. Lococo et al. (18) demonstrated the safety and feasibility of salvage surgery after treatment of advanced lung adenocarcinoma with alectinib in 10 cases. In this case, two and a half months after the patient received adjuvant alectinib, radiologic and pathological evidence of a substantially reduced tumor was obtained without any adverse events. The TNM stage was reduced from cT4N2M0, IIIb to ycT2aN0M0, IB. Clearly, the therapeutic efficacy of alectinib is obvious. In the ALEX study, the median progression-free survival of patients with advanced lung cancer treated with alectinib reached 34.8 months, and the 5-year overall survival was 62.5% (19). However, this patient chose surgical treatment in pursuit of long-term survival. Longer follow-up is needed to determine whether patients benefit from the surgery.
This case demonstrates that alectinib is feasible and has a good safety profile as a neoadjuvant therapy for ALK-positive NSCLC. However, there are still some unanswered questions, such as whether this patient should continue to use alectinib despite achieving pCR and how long the postoperative adjuvant therapy should be continued. According to the ADAURA study, osimertinib requires 3 years of oral administration after surgery for EGFR-positive NSCLC patients. But patients with advanced ALK-positive NSCLC have longer progression-free survival and overall survival. Does this necessitate longer adjuvant therapy after surgery? And with alectinib’s effective neoadjuvant therapy, was is really necessary to perform pneumonectomy or lobectomy or stereotactic body radiotherapy? These questions still need to be answered by controlled clinical studies or other studies with large sample sizes.
Conclusions
Alectinib is a potent second-generation ALK-TKI. In this case, two and a half months after the patient received adjuvant alectinib, radiologic and pathological evidence of a substantially reduced tumor was obtained without any adverse events. This case demonstrates that alectinib is feasible and has a good safety profile as a neoadjuvant therapy for ALK-positive NSCLC. It can be very effective downstaging and made the definitive treatment possible. Whether patients continued to use alectinib after neoadjuvant therapy; if patients continue to receive alectinib after surgery, the most reasonable duration of treatment and whether patients can achieve long-term remission or cure still need further investigation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-111/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-111/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-111/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- John AO, Ramnath N. Neoadjuvant Versus Adjuvant Systemic Therapy for Early-Stage Non-Small Cell Lung Cancer: The Changing Landscape Due to Immunotherapy. Oncologist 2023;28:752-64. [Crossref] [PubMed]
- Leonetti A, Minari R, Boni L, et al. Phase II, Open-label, Single-arm, Multicenter Study to Assess the Activity and Safety of Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-positive NSCLC: ALNEO Trial. Clin Lung Cancer 2021;22:473-7. [Crossref] [PubMed]
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013;24:2371-6. [Crossref] [PubMed]
- Yue D, Xu S, Wang Q, et al. Updated Overall Survival and Exploratory Analysis From Randomized, Phase II EVAN Study of Erlotinib Versus Vinorelbine Plus Cisplatin Adjuvant Therapy in Stage IIIA Epidermal Growth Factor Receptor+ Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:3912-7. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Tsuboi M, Herbst RS, John T, et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 2023;389:137-47. [Crossref] [PubMed]
- Tsuboi M, Weder W, Escriu C, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol 2021;17:4045-55. [Crossref] [PubMed]
- Hudson A, Chan C, Woolf D, et al. Is heterogeneity in stage 3 non-small cell lung cancer obscuring the potential benefits of dose-escalated concurrent chemo-radiotherapy in clinical trials? Lung Cancer 2018;118:139-47. [Crossref] [PubMed]
- Gridelli C, Peters S, Sgambato A, et al. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev 2014;40:300-6. [Crossref] [PubMed]
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer 2013;13:685-700. [Crossref] [PubMed]
- García-Campelo R, Bernabé R, Cobo M, et al. SEOM clinical guidelines for the treatment of non-small cell lung cancer (NSCLC) 2015. Clin Transl Oncol 2015;17:1020-9. [Crossref] [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Eichhorn F, Klotz LV, Bischoff H, et al. Neoadjuvant anti-programmed Death-1 immunotherapy by Pembrolizumab in resectable nodal positive stage II/IIIa non-small-cell lung cancer (NSCLC): the NEOMUN trial. BMC Cancer 2019;19:413. [Crossref] [PubMed]
- Yue P, Zhang S, Zhou L, et al. Perioperative alectinib in a patient with locally advanced anaplastic lymphoma kinase positive non-small cell lung cancer (NSCLC): a case report. Transl Cancer Res 2021;10:3856-63. [Crossref] [PubMed]
- Gu R, Shi Z, Duan T, et al. Feasibility and Safety of Neoadjuvant Alectinib in Pulmonary Invasive Mucinous Adenocarcinoma with ALK Rearrangement: Case Report and Literature Review. Onco Targets Ther 2021;14:5107-13. [Crossref] [PubMed]
- Lococo F, Cancellieri A, Chiappetta M, et al. Salvage Surgery After First-Line Alectinib for Locally-Advanced/Metastatic ALK-Rearranged NSCLC: Pathological Response and Perioperative Results. Clin Lung Cancer 2023;24:467-73. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]


