
Case report: durable response of gliomatosis cerebri with concurrent tumor-treating fields (TTFields) and chemoradiotherapy treatment
Highlight box
Key findings
• Tumor-treating fields (TTFields) with chemoradiation led to rapid and durable response of gliomatosis cerebri in a patient with newly diagnosed glioblastoma (GBM).
What is known and what is new?
• TTFields is FDA approved newly diagnosed and recurrent GBM.
• The current case demonstrated its potential effect on gliomatosis cereberi in a patient with GBM.
What is the implication, and what should change now?
• Further evaluation is needed to better define the effect of TTFields on gliomatosis.
Introduction
Gliomatosis cerebri (GC) represents an unconventional and distinct pattern of glioma with widespread infiltration of tumor in at least three lobes of the brain. While no longer a formal diagnosis under Word Health Organization (WHO) guidelines, the term is still used to refer to its specific presentation. Two types of primary GC exist: with (type 1) and without (type 2) focal masses being involved (1,2). The disease is characteristically variable in its manifestation and progression (3,4). The prognosis of GC compared to equally graded gliomas is worse (3,4); with a median overall survival (OS) of grade 4 gliomas with GC change being 9 months and 5-year survival rate of 18% (5). Effective treatment modalities for GC are controversial and not well agreed upon due to the rarity of the disease and corresponding paucity of data describing GC (6). Treatment varies with presentation, but can involve partial tumor resection or biopsy, chemotherapeutic agents [temozolomide (TMZ) being the most common], and targeted or whole brain radiation therapy (3,6).
Tumor treating fields (TTFields) treatment is a more recent advancement in glioma treatment delivered through low energy, intermediate frequency (200 kHz) electromagnetic fields, causing mitotic cell death (7,8). It is approved for both recurrent and newly diagnosed glioblastoma (GBM) (9,10). In patients with newly diagnosed (GBM), the addition of TTFields to maintenance TMZ significantly improved OS and progression-free survival (PFS) (11). Preclinical studies suggested synergistic effect between TTFields treatment in combination with radiation treatment. The SPARE trial (NCT03477110) was a pilot study designed to investigate the feasibility and safety of concurrent radiation treatment and TTFields treatment (12). In addition, an international phase 3 trial (EF-32 trial; NCT04471844) is currently investigating the efficacy of concurrent radiation and TTFields treatment in newly diagnosed GBM. Here we report one patient with left parietal GBM, IDH wild type, WHO grade 4 with extensive gliomatosis change who recieved concurrent TTFields and chemoradiation on the SPARE trial (Figure 1). We present this case in accordance with the CARE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-114/rc).
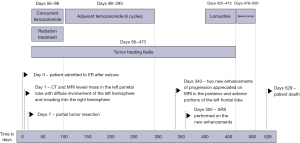
Case presentation
The study was approved by the institutional review board and followed the tenets set by the Declaration of Helsinki (as revised in 2013) and the Health Insurance Portability and Accountability Act. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The patient was a 64-year-old African American male with no significant past medical history who presented with dizziness and lightheadedness. A few days later, he further developed aphasia, left upper extremity weakness, and had two clonic-tonic seizures. He was taken to the emergency room (ER) for evaluation. Upon arriving at the ER, he suffered a second seizure and was placed on levetiracetam (1,000 mg daily) and dexamethasone (16 mg daily). Magnetic resonance imaging (MRI) of the brain was performed. This MRI revealed a ring enhancing mass on the left parietal lobe with additional small enhancing lesions anteriorly. There was a 6-mm rightward midline shift with compression of the left lateral ventricle. Broad areas T2/fluid-attenuated inversion recovery (FLAIR) hyperintense signals were noted throughout the left hemisphere of the brain and along the splenium of the corpus callosum with invasion into the right parietal, temporal, and occipital lobes resulting in gyral thickening and sulcal effacement, consistent with GC (Figure 2).
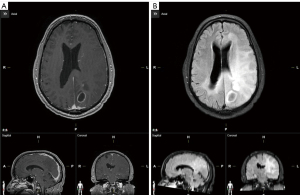
The patient then underwent a craniotomy resection of the enhancing mass in the left parietal lobe. The patient tolerated surgery well without any complication. The final comprehensive pathology diagnosis was GBM, IDH wild type, WHO grade 4. O6-methylguanine-DNA methyl-transferase (MGMT) promotor hypermethylation was positive, 15.4%. Further molecular evaluation showed p53 mutation and PI3KCA mutation. No other mutation was detected.
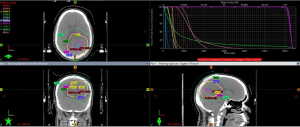
Postoperative MRI showed postsurgical change with unchanged diffuse T2/FLAIR abnormality. At this time the patient had mild anomic aphasia, and no other neurological abnormality. Karnofsky performance score (KPS) was 90. The patient enrolled in the SPARE trial (NCT03477110). The patient started radiation treatment with concurrent TMZ (155 mg daily; 80 mg/m2) and TTFields treatment 7 weeks from surgery. The radiation volume was based on EORTC guideline targeting the enhancing lesion and surgical cavity in the left parietal lobe (Figure 3) (13). The GC change area was not covered by radiation fields to limit radiation induced side effects.
The patient tolerated the 6-week radiation with concurrent TMZ and TTFields well with no toxicity higher than grade 1 (scalp rash). He had no delays or breaks in radiation treatment. During the second week of treatment, a scattered papular rash outside of the TTFields placement developed and was intermittently present throughout the course managed with hydrocortisone as needed. The patient’s compliance with TTFields usage so far had been excellent (93%). Three weeks after finishing radiation treatment MRI showed a similar small curvilinear focus of enhancement along the inferomedial aspect of the surgical cavity, and mildly more prominent small clustered ring enhancing lesions anteriorly (Figure 4). There was significant resolution of the T2/FLAIR signal abnormality, and interval resolution of the left lateral ventricle compression (Figure 4). The patient remained on TTFields treatment and started maintenance TMZ treatment 1 month after finishing radiation. He tolerated treatment well with no significant toxicity. Follow up MRI showed stable findings (Figure 4).
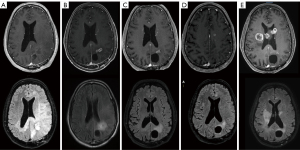
The patient continued to do well, with KPS of 90. However, 11 months after surgery, follow up MRI showed two small new enhancing lesions in the left anterior (6 mm) and posterior frontal lobe (8 mm), consistent with progression. The T2/FLAIR hyperintensity attributed to the GC changes remained resolved on MRI. TMZ treatment was discontinued. He received stereotactic radiosurgery (SRS, 21 Gy in 1 fraction) to these two new lesions. After the SRS treatment, he was started on lomustine treatment. Unfortunately, 2 months later, the patient experienced significant deterioration, becoming wheelchair bound, developing left hemiparesis with corresponding ataxic gait, slurred speech, and anomic aphasia. His KPS score at this time was 50. MRI (13 months after surgery) showed significant progression of disease with multiple new enhancing lesions in the splenium of the corpus callosum crossing the midline to the right, left ventricular margin of occipital horn, genu of corpus callosum, and left frontoparietal area, with local mass effect but no midline shift. Despite this deterioration clinically, there was no recurrence of GC changes (Figure 4). Due to the patients’ continually worsening condition, treatment with TTFields was discontinued. The patient overall received 11.5 months of TTFields treatment, with overall compliance of 19.8 h/day, 82.4%. Bevacizumab treatment was initiated. However, the patient only had limited improvement in symptoms for a short time. Two months after initiation of bevacizumab treatment, the patient’s condition declined rapidly, he was placed on hospice care and later passed away. The OS was 17 months from diagnosis.
Discussion
GC is a rare and aggressive form of glioma with diffuse brain involvement. Research on GC is sparse, despite the rise in recognized incidence in the preceding decades (5). GC was reclassified as a diffuse pattern of growth within glioma in 2016 because of multiple studies identifying a lack of molecular distinction between GC and glioma (14). Analysis of methylation patterns in GC revealed similar signature seen in multiple pre-defined glioma subgroups (1). This finding was corroborated in pediatric GC which corresponded to known glioma methylation profiles as well as both genetic and epigenetic characteristics (15).
Clinic management of GC is therefore often similar to glioma due to the lack of a standard treatment regime for GC (6). Due to GCs diffuse nature, surgery has little role. Radiation treatment and chemotherapy are the primary treatments. Historically, whole brain radiation treatment is the most common radiation approach. The doses range from 20 to 59 Gy (6). However, it has limited efficacy and significant neurotoxicity (16). The pattern of failure study suggested it may be treated with partial brain radiation with limited margin (17). Thus in this case, in a patient with GBM and GC pattern, the decision was made to treat the enhancing lesion and surgical cavity with margin only per the EORTC guideline (13). As a result, the GC involvement area was not covered by radiation (Figure 3). The rapid and durable response of GC, thus, was unlikely due to the benefit from radiation treatment.
Chemotherapy is often used in patients with GC, either alone or with radiation treatment. NOA-05 prospectively evaluated the efficacy of chemotherapy in GC (18). The median PFS was 14 months and median OS was 30 months, suggesting initial treatment with procarbazine and lomustine may have potential clinical benefit for patients with GC (18). TMZ is widely used for gliomas and is often used in GC. Retrospective studies indicating TMZ may have a PFS and OS ranging from 9–18 and 14–37.3 months, respectively (6). MGMT promotor methylation is a predictive factor for treatment response to TMZ (19). This patient had a methylated MGMT promotor, the observed the response of GC can be at least partially contributing to TMZ.
TTFields treatment is a new modality for the management of GBM. It is FDA approved for newly diagnosed and recurrent GBM, based on phase 3 randomized trials (EF14 and EF11) (11,20). The mechanism of action of TTFields is anti-mitosis (7,8). However, TTFields treatment has other complex functions, including anti-migration, inhibition of DNA damage repair, affects on cell membrane permeability, disruption of the blood brain barrier (BBB), immunological effects, and more (21). Besides GBM, TTFields should have similar biological effects on G3 and low-grade glioma, though the data is limited. Like radiation, as a field treatment, TTFields has a wide treatment distribution. Dosimetric studies showed TTFields distribute throughout large regions of the brain in a heterogeneous manner (22-24). However, the planning system for acute modeling the TTFields field strength through the brain is not yet commercially available (23). Nonetheless, the current findings do support TTFields can be effective at targeting larger volumes of gross and subclinical disease safely (24). Moreover, when TTFields treatment is combined with radiation and chemotherapy they may achieve synergistic effects (7,25). The patient in this case received concurrent TTFields with chemoradiation on the SPARE trial (NCT03477110) (12,26). The rapid and durable response of GC was likely a benefit from the combination therapy. Lastly, TTFields treatment is delivered over long periods of time. The compliance with TTFields therapy is directly associated with the dose of TTFields treatment. Evaluation of patients receiving TTFields treatment on the EF14 trial demonstrated a compliance threshold of 50% with TTFields/TMZ correlated with significantly improved OS and PFS versus TMZ alone (27). Patients with compliance >90% showed extended median and 5-year survival rate close to 30% (27). The patient presented in this study had excellent compliance during the concurrent TTFields with chemoradiation treatment (93%). This may have contributed to the rapid response of GC as observed 3 weeks after finishing radiation treatment. The patient continued to have a favorable compliance rate (>80%), and this may have further contributed to his durable response of GC.
Conclusions
In this GBM patient with extensive GC change, concurrent TTFields with chemoradiation induced a rapid and durable response of the GC. This finding suggests the benefits of TTFields treatment in patients with GC. Further evaluation is needed to better understand the role of TTFields in patients GC.
Acknowledgments
The authors would also like to thank Ms. Peggy Grove for philanthropic support for this study.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Chinese Clinical Oncology for the series “Recent Advances in Neuro-Oncology”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-114/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-114/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-114/coif). The series “Recent Advances in Neuro-Oncology” was commissioned by the editorial office without any funding or sponsorship. W.S. served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Chinese Clinical Oncology from October 2023 to September 2025. W.S. also received consulting fees from Brainlab, Novocure, Zai Lab, and Varian. J.G. serves as an unpaid editorial board member of Chinese Clinical Oncology from March 2023 to February 2025. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board and followed the tenets set by the Declaration of Helsinki (as revised in 2013) and the Health Insurance Portability and Accountability Act. Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herrlinger U, Jones DTW, Glas M, et al. Gliomatosis cerebri: no evidence for a separate brain tumor entity. Acta Neuropathol 2016;131:309-19. [Crossref] [PubMed]
- Divé I, Steidl E, Wagner M, et al. Gliomatosis Cerebri Growth Pattern: Association of Differential First-Line Treatment with Overall Survival in WHO Grade II and III Gliomas. Oncology 2021;99:215-24. [Crossref] [PubMed]
- Morales La Madrid A, Ranjan S, Warren KE. Gliomatosis cerebri: a consensus summary report from the Second International Gliomatosis cerebri Group Meeting, June 22-23, 2017, Bethesda, USA. J Neurooncol 2018;140:1-4. [Crossref] [PubMed]
- Anghileri E, Schettino C, Pollo B, et al. Gliomatosis cerebri (GC) or GC-like? A picture to be reconsidered in neuro-oncology based on large retrospective analysis of GC series. Neurol Sci 2020;41:2111-20. [Crossref] [PubMed]
- Georgakis MK, Tsivgoulis G, Spinos D, et al. Prognostic Factors and Survival of Gliomatosis Cerebri: A Systematic Review and Meta-Analysis. World Neurosurg 2018;120:e818-54. [Crossref] [PubMed]
- Ranjan S, Warren KE. Gliomatosis Cerebri: Current Understanding and Controversies. Front Oncol 2017;7:165. [Crossref] [PubMed]
- Giladi M, Munster M, Schneiderman RS, et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol 2017;12:206. [Crossref] [PubMed]
- Giladi M, Schneiderman RS, Porat Y, et al. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014;14:54-63. [Crossref] [PubMed]
- Stupp R, Taillibert S, Kanner A, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017;318:2306-16. [Crossref] [PubMed]
- Zhu P, Zhu JJ. Tumor treating fields: a novel and effective therapy for glioblastoma: mechanism, efficacy, safety and future perspectives. Chin Clin Oncol 2017;6:41. [Crossref] [PubMed]
- Stupp R, Taillibert S, Kanner A, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017;318:2306-16. [Crossref] [PubMed]
- Miller R, Song A, Ali A, et al. Scalp-Sparing Radiation With Concurrent Temozolomide and Tumor Treating Fields (SPARE) for Patients With Newly Diagnosed Glioblastoma. Front Oncol 2022;12:896246. [Crossref] [PubMed]
- Niyazi M, Brada M, Chalmers AJ, et al. ESTRO-ACROP guideline "target delineation of glioblastomas". Radiother Oncol 2016;118:35-42. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Broniscer A, Chamdine O, Hwang S, et al. Gliomatosis cerebri in children shares molecular characteristics with other pediatric gliomas. Acta Neuropathol 2016;131:299-307. [Crossref] [PubMed]
- Chen S, Tanaka S, Giannini C, et al. Gliomatosis cerebri: clinical characteristics, management, and outcomes. J Neurooncol 2013;112:267-75. [Crossref] [PubMed]
- Kandula S, Saindane AM, Prabhu RS, et al. Patterns of presentation and failure in patients with gliomatosis cerebri treated with partial-brain radiation therapy. Cancer 2014;120:2713-20. [Crossref] [PubMed]
- Glas M, Bähr O, Felsberg J, et al. NOA-05 phase 2 trial of procarbazine and lomustine therapy in gliomatosis cerebri. Ann Neurol 2011;70:445-53. [Crossref] [PubMed]
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997-1003. [Crossref] [PubMed]
- Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 2012;48:2192-202. [Crossref] [PubMed]
- Rominiyi O, Vanderlinden A, Clenton SJ, et al. Tumour treating fields therapy for glioblastoma: current advances and future directions. Br J Cancer 2021;124:697-709. [Crossref] [PubMed]
- Wenger C, Salvador R, Basser PJ, et al. Improving Tumor Treating Fields Treatment Efficacy in Patients With Glioblastoma Using Personalized Array Layouts. Int J Radiat Oncol Biol Phys 2016;94:1137-43. [Crossref] [PubMed]
- Makarov SNNoetscher GMNummenmaa A, et al. Tumor-Treating Fields at EMBC 2019: A Roadmap to Developing a Framework for TTFields Dosimetry and Treatment Planning. 2021.
- Wenger C, Miranda PC, Salvador R, et al. A Review on Tumor-Treating Fields (TTFields): Clinical Implications Inferred From Computational Modeling. IEEE Rev Biomed Eng 2018;11:195-207. [Crossref] [PubMed]
- Giladi M, Weinberg U, Schneiderman RS, et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin Oncol 2014;41:S35-41. [Crossref] [PubMed]
- Song A, Bar-Ad V, Martinez N, et al. Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma. J Neurooncol 2020;147:653-61. [Crossref] [PubMed]
- Toms SA, Kim CY, Nicholas G, et al. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol 2019;141:467-73. [Crossref] [PubMed]


