
The emerging perioperative treatment paradigm for non-small cell lung cancer: a narrative review
Introduction
The year 2023 has been a landmark for the treatment of early non-small cell lung cancer (NSCLC). All major developments that gradually transformed the management of metastatic disease over the past 10 years, namely use of tyrosine kinase inhibitors (TKI), frontline immunotherapy, and molecular profiling with next-generation sequencing (NGS) already at initial diagnosis (1-3), are now introduced simultaneously in the routine clinical practice for potentially resectable tumors. With the upcoming generalization of lung cancer screening in many European countries using low-dose computerized tomography (CT) based on NELSON, NLST and other pivotal studies (4,5), soon the great majority (>65%) of NSCLC patients will fall into this category and be cured according to the principles set forth today. Aim of the current review is to analyze and put them into their practical and historical context. This article is presented in accordance with the Narrative Review reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-137/rc).
Methods
A literature review was conducted for randomised phase 3 and phase 2 trials of immunotherapy and targeted drugs for resectable NSCLC with published results during the last 3 years based on PubMed and the content presented at major international oncology congresses, including the annual congress of the American Society for Clinical Oncology (ASCO), the annual congress of the European Society for Medical Oncology (ESMO), the annual World Congress for Lung Cancer (WCLC), the annual congress of the American Association for Cancer Research (AACR), and the annual European Lung Cancer Congress (ELCC). Identified studies are listed in Table 1 along with the main publication for each. Additional congress content and journal publications for each study were manually collected and analyzed, while references were also reviewed and used as additional sources, when appropriate. The search strategy is summarized in Table 2.
Table 1
| Trial | Intervention† | Control | pCR, % | EFS HR | 2-year EFS, % | OS HR | 2-year OS, % | Ref. (main) |
|---|---|---|---|---|---|---|---|---|
| IMpower010 | Adjuvant atezolizumab (n=507) | Placebo | – | 0.79 | 70 | 0.95 | NR | (6) |
| Keynote-091 | Adjuvant pembrolizumab (n=590) | Placebo | – | 0.74 | 67 | 0.87 | NR | (7) |
| Checkmate-816 | Neoadjuvant 3× nivolumab-CHT (n=179) | CHT | 24 | 0.63 | 64 | 0.62 | 83 | (8) |
| Checkmate-816 | Neoadjuvant 3× nivolumab +1× ipilimumab (n=113) | CHT | 20 | 0.77 | 60 | 0.73 | 82 | (9) |
| Checkmate-77T | Perioperative 4× nivolumab-CHT (n=229) | CHT | 25 | 0.58 | 63 | NR | NR | (10) |
| Keynote-671 | Perioperative 4× pembrolizumab-CHT (n=397) | CHT | 18 | 0.59 | 62 | 0.72 | 79 | (11) |
| AEGEAN | Perioperative 4× durvalumab-CHT (n=400) | CHT | 17 | 0.68 | 63 | NR | NR | (12) |
| Neotorch†,‡ | Perioperative 3× + 1× toripalimab-CHT (n=202) | CHT | 25 | 0.40 | 65 | NR | NR | (13) |
| Rationale-315 | Perioperative 3–4× tislelizumab-CHT (n=226) | CHT | 41 | NR | NR | NR | NR | (14) |
| NADIM-2 (phase 2) | Perioperative 3× nivolumab-CHT (n=57) | CHT | 37 | 0.46 | 67 | 0.56 | 85 | (15) |
†, in perioperative studies, preoperative treatment was with doublet chemotherapy combined with a PD-(L)1 inhibitor for up to 3–4 cycles, as indicated, while postoperative treatment was with the same PD-(L)1 inhibitor as monotherapy for 1 year. The sole exception was the Neotorch trial, in which the 4th cycle of chemoimmunotherapy was given postoperatively, followed by immunotherapy until the end of 1 year. ‡, only the stage III part of the Neotorch study has been reported so far. NSCLC, non-small cell lung cancer; pCR, pathologic complete remission; NR, not reported; EFS, event-free survival; OS, overall survival; HR, hazard ratio; CHT, platinum-based chemotherapy (doublet); PD-(L)1, programmed cell death (ligand) 1.
Table 2
| Items | Specification |
|---|---|
| Date of search | Jan 11, 2023 |
| Databases and other sources searched | PubMed, ASCO website, ESMO/ELCC website, IASLC/WCLC website, AACR website |
| Search terms used | Perioperative immunotherapy, adjuvant immunotherapy, neoadjuvant immunotherapy, NSCLC |
| Timeframe | 2020–2023 |
| Inclusion criteria | Results from randomized studies |
| Selection process | The selection was conducted by the author of this article |
ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology; ELCC, European Lung Cancer Congress; IASLC, International Association for the Study of Lung Cancer; WCLC, World Conference on Lung Cancer; AACR, American Association for Lung Research; NSCLC, non-small cell lung cancer.
Resectability according to tumor node metastasis (TNM) 8 vs. TNM7
One first difficulty in the interpretation of results from recent immunotherapy trials for potentially resectable NSCLC is caused by the change of TNM staging system in 2018. As a result, some studies designed earlier have been conducted according to TNM7 (16), like IMpower010, Keynote-091, Checkmate-816, ADAURA and ALINA, while later studies followed the TNM8 system (17), e.g., Keynote-671, Aegean, Checkmate-77T, Neotorch, NADIM-2, and Rationale-315. Therefore, the designation of tumors eligible for each trial differs according to the TNM system used by the investigators and comprises “stage IB ≥4 cm large up to stage IIIA tumors” according to TNM7 vs. “stage IIA up to stage IIIB N2 tumors” according to TNM8. However, it is important to appreciate that despite the different nomenclature, both descriptions largely refer to the same tumors, as shown in Figure 1: the spectrum begins with tumors at least 4 cm large without lymph nodes, which correspond exactly to both the TNM7 “stage IB ≥4 cm” and TNM8 “stage IIA” designations, and reaches up to large tumors with ipsilateral mediastinal lymph nodes (N2), which were called “stage IIIA” according to TNM7, but “stage IIIB up to N2” by TNM8. One minor discrepancy at this upper end of the spectrum concerns N2 tumors with T4 for reasons other than size (>7 cm) or invasion of the diaphragm, which includes T4 due to a separate nodule in a different ipsilateral lobe, or invasion of the mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body and carina: these tumors are stage IIIB according to both the TNM7 and TNM8, and were therefore excluded from TNM7-based studies, but could be potentially included in newer studies which follow TNM8 (18). However, such tumors are rare and have generally poor resectability, therefore they have been explicitly excluded by the protocol of several TNM8-based studies, like AEGEAN and NADIM-2 (12,15). Thus, despite altered terminology, the TNM shift has not per se affected which tumors are considered candidates for perioperative concepts. The realm of resectability is nonetheless expanding due to the higher efficacy of novel systemic therapies, as exemplified by tumors with multilevel N2 involvement: these have generally been viewed as unresectable in the past (19), but were included in both the TNM7-based and TNM8-based pre-/postoperative immunotherapy studies (Figure 1). Moreover, the randomized NADIM-2 study showed an impressive benefit of perioperative (chemo-)immunotherapy for multi-level N2 tumors with a progression-free survival (PFS) hazard ratio (HR) of 0.39 compared to plain chemotherapy, which was even more pronounced that that observed for single-level N2 tumors in the same trial, and highlights the need to redefine resectability in the new era of perioperative NSCLC management with novel drugs.

The special merit of perioperative concepts
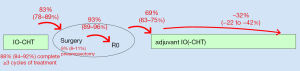
The establishment of perioperative concepts for resectable NSCLC was the single most important development in thoracic oncology for 2023 and facilitated by the positive results of 4 large randomized phase 3 trials successively announced during this year: Keynote-671 (first presented at ASCO 2023) (11), AEGEAN (first presented at AACR 2023) (12), Checkmate-77T (first presented at ESMO 2023) (10), and Neotorch (first presented at the ASCO Virtual Plenary of April 2023) (13) (Table 1). All these studies had similar designs, with use of programmed cell death (ligand) 1 PD-(L)1 blockade together with chemotherapy for 3–4 cycles preoperatively, followed by surgery and postoperative administration of the same PD-(L)1 inhibitor for 1 year (Table 1); one notable exception was the Neotorch study, which administered one single (fourth) chemotherapy cycle after surgery (Table 1). With their concordant findings, all studies showed that this complex treatment plan has a good feasibility, with >90% of patients able to complete at least 3 cycles of neoadjuvant treatment, >80% of patients able to undergo surgery, >90% of surgeries being complete resections (R0), and approximately 70% of patients starting adjuvant therapy (Figure 2, Table S1). At the same time, it also offers several key advantages compared to older approaches.
The first one is universal applicability, which means that perioperative therapies combine the benefits of purely adjuvant and purely neoadjuvant strategies. Purely adjuvant therapies, like the postoperative administration of atezolizumab for 1 year in the IMpower010 study and the postoperative administration of pembrolizumab for 1 year in the Keynote-091 study, had shown similar EFS benefit for both stage II and III tumors with a HR of approximately 0.70 (marked in italics in Table 3). On the other hand, the purely neoadjuvant chemoimmunotherapy according to Checkmate-816 had shown an even better EFS HR of approximately 0.55, but its benefit was restricted only to stage III and/or PD-L1 positive [tumor proportion score (TPS) ≥1%] tumors (marked in bold in Table 3). Perioperative therapies now abolish this duality, combining both the significant EFS HR of approximately 0.55 for stage III and/or PD-L1 positive tumors (similar to Checkmate-816), with the significant EFS HR of approximately 0.70 for stage II and PD-L1 negative tumors (similar to IMpower010 and Keynote-091) under one single strategy (Table 3). Thus, these regimens are inherently suitable for broad utilization.
Table 3
| Clinical trials | EFS HR by stage (95% CI) | EFS HR by PD-L1 expression (95% CI) | Ref. | |||
|---|---|---|---|---|---|---|
| Stage II (IIA; IIB) | Stage III (IIIA; IIIB) | PD-L1 TPS <1% | PD-L1 TPS ≥1% (1–49%; ≥50%) | |||
| IMpower010 (adjuvant)† | 0.73–0.77 (0.43–1.24; 0.35–1.69) | 0.62 (0.42–0.90) | – | 0.66 (0.49–0.87) | (6) | |
| Keynote-091 (adjuvant) | 0.70 (0.55–0.91) | 0.92 (0.69–1.24) | 0.78 (0.58–1.03) | 0.67–0.82 (0.48–0.92; 0.57–1.18) | (7) | |
| Checkmate-816 (neoadjuvant) | 0.87 (0.48–1.56) | 0.54 (0.37–0.80) | 0.85 (0.54–1.32) | 0.41 (0.24–0.70) | (8) | |
| Keynote-671 (perioperative) | 0.68 (0.51–0.90)‡ | 0.54–0.52 (0.41–0.72; 0.31–0.88) | 0.72 (0.58–0.89)§ | 0.51–0.42 (0.34–0.75; 0.28–0.65) | (11) | |
| AEGEAN (perioperative) | 0.68 (0.51–0.90)‡ | 0.57–0.83 (0.39–0.83; 0.52–1.32)¶ | 0.72 (0.58–0.89)§ | 0.70–0.60 (0.46–1.05; 0.35–1.01)¶ | (12) | |
| Checkmate-77T (perioperative) | 0.68 (0.51–0.90)‡ | 0.51 (0.36–0.72) | 0.72 (0.58–0.89)§ | 0.52 (0.35–0.78) | (10) | |
| Neotorch (perioperative) | 0.68 (0.51–0.90)‡ | 0.40 (0.28–0.57) | 0.72 (0.58–0.89)§ | 0.31–0.31 (0.18–0.55; 0.15–0.62) | (13) | |
Results of benefit with moderate magnitude (HR ≈0.70) are shown in italics, while results of greater magnitude (HR ≈0.50) are shown in bold. Perioperative therapies combine the benefit of purely adjuvant therapies (HR ≈0.70 across stages and levels of PD-L1 expression) with that of purely neoadjuvant therapy as analyzed in the Checkmate-816 trial (a better HR ≈0.50, but only for stage III and/or PD-L1 positive tumors). †, IMpower010 data only for tumors with PD-L1 TPS ≥1% (ITT population of the IMpower010 study). ‡, pooled analysis of the all phase 3 perioperative (chemo-)immunotherapy studies for stage II NSCLC with published EFS results (Keynote-671, AEGEAN, Checkmate-77T for stage II): total events 80/303 vs. 115/312 for chemoimmunotherapy vs. chemotherapy; heterogeneity Chi2=1.1, df =2 (P=0.60), I2=0%; test for overall effect Z=2.70 (P=0.007) (20). §, pooled analysis of the all phase 3 perioperative (chemo-)immunotherapy studies for potentially resectable NSCLC with published EFS results (Keynote-671, AEGEAN, Checkmate-77T, Neotorch patients with PD-L1 <1%): total events 143/422 vs. 197/439 for chemoimmunotherapy vs. chemotherapy; heterogeneity Chi2=0.59, df =3 (P=0.90), I2=0%; test for overall effect Z=2.99 (P=0.003) (20). ¶, the slightly worse outcome in AEGEAN is thought to result mainly from the higher number of (mainly stage IIIB) patients who did not complete surgery, and the protocol was later amended to exclude stage IIIB tumors with reason for T4 other than tumor size. NSCLC, non-small cell lung cancer; EFS, event-free survival; HR, hazard ratio; CI, confidence interval; TPS, tumor-proportion score; PD-L1, programmed death ligand 1; ITT, intention to treat.
At the same time, they offer additional merits over current alternatives, which will probably make them the dominant choice for all resectable NSCLC once approved: first, compared to purely adjuvant strategies, they permit evaluation of response in the surgical specimen, and particularly the assessment of whether or not pathologic complete remission (pCR) could be achieved, which has emerged as an excellent surrogate of favorable long-term outcome in all studies so far (21); second, compared to purely neoadjuvant approaches, the postoperative component of perioperative therapies seems to improve the outcome of patients who fail to achieve a pCR: for these patients, the EFS HR was 0.84 (95% CI: 0.61–1.17) in the Checkmate-816 study without any postoperative treatment, 0.69 (95% CI: 0.55–0.85) in the Keynote-671 study with postoperative administration of the PD-1 inhibitor pembrolizumab as monotherapy for 1 year, and approximately 0.50 in the Neotorch study with additional postoperative administration of 1 chemoimmunotherapy cycle within the 1-year long adjuvant toripalimab treatment (8,11,13). Of note, the Neotorch study, which was the only one to offer postoperative chemoimmunotherapy, showed a uniquely low EFS HR also for other “difficult” patient subsets, including PD-L1 negative tumors (0.59; 95% CI: 0.33–1.03), stage IIIB tumors (0.30; 95% CI: 0.15–0.56), and squamous cell carcinomas (0.35; 95% CI: 0.23–0.52), supporting the hypothesis that postoperative chemoimmunotherapy may be particularly beneficial in the high-risk setting (13). However, it should be noted that HR comparisons among studies are dangerous because of the differences in the patient populations and control arms among studies, while comparisons based on the more robust indexes of 2-year EFS and 2-year OS rates show that all phase 3 results were very similar and within the narrow corridors of 64–67% and 81–85%, respectively (Table 1). It is also important to keep in mind that a direct comparison of endpoints between adjuvant and neoadjuvant/perioperative studies is impossible, because randomization in adjuvant studies occurs later, after surgery and adjuvant chemotherapy, and results in a selected patient population with more favorable features compared to the all-comer populations of studies that randomize already at initial diagnosis. In terms of tolerability, all phase 3 perioperative trials have shown rates of adverse events similar to these observed under chemoimmunotherapy in the metastatic setting, while first phase 2 results additionally suggest that immune-related adverse events may be associated with improved prognosis for surgical patients, similar to what has already been described for patients with advanced disease (22,23).
Which chemotherapy?
From a practical standpoint, an important question is whether a specific chemotherapy should be preferred as the partner of immunotherapy in the neoadjuvant setting. Regarding the choice between cisplatin and carboplatin, we know already from the metastatic setting that the difference between them is small. For example, in the Keynote-189 study, in which either cisplatin or carboplatin was used with the same cytotoxic partner (pemetrexed) and pembrolizumab, there was a broad overlap of EFS HR vs. plain chemotherapy with 0.41 (95% CI: 0.24–0.69) for cisplatin and 0.52 (95% CI: 0.39–0.71) for carboplatin (Table 4). A similar small numerical difference in favor of cisplatin was also noted in the AEGEAN trial in terms of EFS HR, which was 0.59 (95% CI: 0.35–1.00) for cisplatin vs. 0.73 (95% CI: 0.54–0.98) for carboplatin, but this trend was reversed when the association with pCR was analyzed, with an odds ratio of 14.1 (95% CI: 8.9–19.8) for carboplatin and 9.9 (95% CI: 3.1–18.0) for cisplatin (Table 4). On the other hand, in the Checkmate-816 trial, where carboplatin was used together with paclitaxel, its EFS HR of 0.31 (95% CI: 0.14–0.67) was better than the 0.71 (95% CI: 0.49–1.03) of cisplatin, which was administered together with pemetrexed (for non-squamous) and gemcitabine (for squamous tumors) instead of a taxane. Taken together, these observations suggest that the choice of the platinum partner may be more important than that of the platinum compound in the context of neoadjuvant chemoimmunotherapy. Along the same lines, all perioperative/neoadjuvant trials with higher pCR rates have utilized predominantly paclitaxel (highlighted in bold in Table 4). Of particular importance in this respect is the NADIM-2 trial, which had a rather balanced distribution of histological subtypes (44% adenocarcinomas vs. 37% squamous carcinomas in the experimental arm) and showed clearly that carboplatin-paclitaxel alongside immunotherapy causes high pCR rates in both non-squamous (pCR, 38.9%; 95% CI: 23.0–54.8%) and squamous tumors (pCR, 33.3%; 95% CI: 13.2–53.5%) (15), while the Neotorch and Rationale-315 studies had mainly included squamous tumors, and their high pCR rates could theoretically have been confounded by histology. Results from the adjuvant (IMpower010) and metastatic setting (POSEIDON) also suggest that taxanes are the most active platinum partner for immunotherapy, followed by pemetrexed and gemcitabine (Table 4).
Table 4
| Clinical trial, endpoint | Platinum drug | Platinum partner [pCR% by main platinum partner (95% CI)]‡ | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Cisplatin | Carboplatin | Taxane | Vinorelbine | Pemetrexed | Gemcitabine | |||
| Keynote-189, PFS | 0.41 (0.24–0.69) | 0.52 (0.39–0.71) | NA | NA | NA | NA | (24) | |
| POSEIDON, OS | NA | NA | 0.64 (0.34–1.24) | NA | 0.81 (0.65–1.01) | 0.93 (0.70–1.23) | (25) | |
| IMpower010, EFS | NA | NA | 0.72 (0.42–1.23) | 0.67 (0.46–0.99) | 0.84 (0.61–1.16) | 0.94 (0.56–1.57) | (6) | |
| AEGEAN, EFS† | 0.59 (0.35–1.00) | 0.73 (0.54–0.98) | NA | NA | NA | NA | (12) | |
| AEGEAN, pCR† | 12.0 (6.4–20.0) | 19.2 (14.36–24.4) | NA | NA | 17.2% (13.5–21.5%)‡ | NA | (12) | |
| Checkmate-816, EFS† | 0.71 (0.49–1.03) | 0.31 (0.14–0.67) | NA | NA | – | NA | (8) | |
| Checkmate-816, pCR† | 21.8 (14.9–30.1) | 30.8 (17.0–47.6) | NA | NA | [24.0% (18.0–31.0%)]‡ | NA | (8) | |
| Keynote-671, pCR | NA | NA | NA | NA | [18.1% (14.5–22.3%)]‡ | NA | (11) | |
| NADIM-2, pCR | NA | NA | [37.0% (24.0–51.0%)]‡ | NA | NA | NA | (15) | |
| Neotorch, pCR | NA | NA | [24.8% (19.0–31.3%)]‡ | NA | NA | NA | (13,26) | |
| Rationale-315, pCR | NA | NA | [40.7% (34.2–47.4%)]‡ | NA | NA | NA | (14) | |
Survival HR and rates of pCR (95% CI) are given for immunotherapy trials with available outcome data according to the type of accompanying chemotherapy. The use of taxanes is consistently associated with higher rates of pCR (highlighted in bold), while the difference in efficacy between carboplatin and cisplatin is marginal and influenced by the choice of chemotherapy partner. †, carboplatin could only be combined with paclitaxel in the Checkmate-816 trial (for both squamous and non-squamous tumors), while it could be combined with pemetrexed for non-squamous tumors, and with either gemcitabine or paclitaxel for squamous tumors in the AEGEAN trial. ‡, most frequently used platinum partner was pemetrexed in AEGEAN (55.1% of patients in the experimental arm), pemetrexed in Checkmate-816 (exact percentage of patients not provided), pemetrexed in Keynote-671 (56% of patients in the experimental arm), paclitaxel in NADIM-2 (100% of patients in the experimental arm), paclitaxel/docetaxel in Neotorch (>80% of patients in the experimental arm, i.e., all patients with squamous histology), and paclitaxel in Rationale-315 (>79% of patients in the experimental arm, i.e., all patients with squamous histology). NSCLC, non-small cell lung cancer; PFS, progression-free survival; OS, overall survival; EFS, event-free survival; HR, hazard ratio; pCR, pathologic complete remission; CI, confidence interval; NA, not available/not applicable.
NGS is now necessary for potentially resectable NSCLC already at initial diagnosis
From a regulatory perspective, cardinal developments this year were the EMA approval for the Checkmate-816 regimen in June 2023, which thus became the first neoadjuvant chemoimmunotherapy for NSCLC in Europe, and the FDA approval of the Keynote-671 regimen in October 2023 as the first perioperative (chemo-)immunotherapy routinely available worldwide (NB; the FDA had already approved the Checkmate-816 regimen in March 2022, while the EMA is expected to approve the Keynote-671 regimen in early 2024). One important practical consequence is that molecular profiling now becomes necessary already at initial diagnosis for potentially resectable tumors needing additional systemic therapy, i.e., for all stage II–IIIB tumors according to TNM8. While neither the EMA nor the FDA have required EGFR/ALK-testing within the approval label of the Checkmate-816 and Keynote-671 regimens, probably because preoperative testing has not been widely implemented yet, and a formal requirement for this would cause logistic problems, molecular profiling is necessary from a scientific view for several reasons: no benefit from the treatment of EGFR/ALK-positive tumors was noted in the adjuvant IMpower010 and the perioperative AEGEAN trials (6,12), while the putative benefit reported by the adjuvant Keynote-091 and the perioperative Keynote-671 trials for EGFR-mutated NSCLC remains unclear (7,11), since testing was performed only for a subset of patients at the discretion of the investigator, the exact types of EGFR mutations have not been disclosed, and patients with classic EGFR alterations (i.e., del19 or L858R) in TKI-refractory tumors did not derive any benefit from additional immunotherapy compared to plain chemotherapy in the recently published Keynote-789 and Checkmate-722 trials (27,28). Identifying tumors with classic EGFR mutations and ALK fusions upfront in order to spare them from neoadjuvant chemoimmunotherapy became even more imperative since the significant benefit for adjuvant osimertinib (EFS HR =0.20, OS HR =0.49, TKI duration 3 years) and adjuvant alectinib (EFS HR =0.24, TKI duration 2 years) vs. placebo was reported for EGFR (del19, L858R) and ALK-positive tumors, respectively (29-31). Based on the consistent results observed in ADAURA and ALINA, further adjuvant TKI trials, like the LIBRETTO-432 (NCT04819100) with selpercatinib over 3 years after resection of tumors with RET fusions (32), are expected to achieve similar success and further expand the scope of molecular testing for newly diagnosed resectable NSCLC in the near future. One important question is whether postoperative chemotherapy should be offered between surgery and adjuvant TKI for these tumors, inasmuch as adjuvant TKI studies have taken different stances on that: in ADAURA, chemotherapy was given to approximately 60% of patients in the experimental arm (n=203/339) before randomization (33), ALINA precluded use of chemotherapy for the alectinib arm (30), while LIBRETTO-432 mandates it for all eligible patients (32). The benefit from osimertinib in ADAURA was comparable regardless of whether adjuvant chemotherapy had been previously given {EFS HR 0.16 [0.10–0.26] with previous chemotherapy vs. 0.23 [0.13–0.40] without (31); OS HR 0.49 [0.30–0.79] with previous chemotherapy vs. 0.47 [0.25–0.83] without (29)}, which may indicate that adjuvant osimertinib and adjuvant chemotherapy have independent effects. On the other hand, EFS rates so far have also been very similar regardless of chemotherapy, e.g., 91–89% at 2 years for stage II–III patients with adjuvant chemotherapy vs. 89–86% for those without, as the benefit from adjuvant osimertinib is much larger that from adjuvant chemotherapy (2-year EFS rate 91–89% for stage II–III patients with adjuvant chemotherapy and osimertinib vs. 59–33% for patients with adjuvant chemotherapy only) (33,34). Moreover, a retrospective analysis from 4 randomized phase 3 trials failed to show a significant effect of adjuvant chemotherapy for EGFR-mutated NSCLC, however this result is limited by the small patient numbers (overall n=64 with adjuvant chemotherapy) (35). Besides statistical uncertainties, the most important caveat is probably that the EFS effect of adjuvant osimertinib appears to wane after its discontinuation at 3 years in ADAURA (36), so that chemotherapy may matter more in the long run, as also suggested by a substantial difference in 5-year OS with 87% (95% CI: 80–91%) after previous adjuvant chemotherapy vs. 80% (95% CI: 66–89%) without (29). Overall, the data remain inconclusive at present and most experts would offer adjuvant chemotherapy besides TKI to most eligible patients. Another unclear point is the potential importance of TP53 mutations, which are a recognized risk factor for TKI-treated metastatic EGFR-mutated NSCLC (37), but had no effect in the aforementioned retrospective analysis of phase 3 adjuvant chemotherapy data from EGFR-positive tumors (35). For resectable tumors with other IO-resistant drivers, like ROS1 fusions and EGFR exon 20 insertions (38,39), management remains problematic at present. Also, it is unclear how we should handle resectable tumors with rare activating EGFR mutations, which have lower TKI sensitivity (37) and are not eligible for adjuvant osimertinib (31). Based on the benefit noted in Keynote-091 and Keynote-671 trials (7,11), perioperative immunotherapy could be considered for some of these patients, especially if there is a smoking history, PD-L1 positivity, or coexistence of further oncogenic drivers, like KRAS/NRAS mutations (40). The tumor mutational burden (TMB) could be an additional potentially helpful biomarker, as it has shown association with pathologic responses in neoadjuvant nivolumab and atezolizumab trials (41,42), however the difference in pCR and EFS rates between TMB-high (≥12.3 mut/Mb) and TMB-low tumors for chemoimmunotherapy vs. chemotherapy was not very large in Checkmate-816 [pCR 28.1% (95% CI: 11.6–43.9%) vs. 20.6% (95% CI: 8.2–34.1%); and EFS HR 0.69 (95% CI: 0.33–1.46) vs. 0.86 (95% CI: 0.4–1.57), respectively] (8), while TMB estimation from panel-based NSC also poses several technical challenges (43). Peripheral blood biomarkers, like the neutrophil-to-lymphocyte ratio (NLR), the advanced lung inflammation index (ALI), and activation markers of various immune cells have shown promising associations with patient outcome in the metastatic setting (44) as well as the single-arm phase 2 perioperative NADIM trial (45), but no data from randomized studies have been published yet.
The evolving role of MRD assays
The lack of strong and reliable baseline predictors increases the need for novel tools of disease monitoring that could facilitate earlier detection of tumor responses or relapse. Most promising in this regard are circulating tumor DNA (ctDNA) assays to quantify the minimal residual disease (MRD) after surgery and systemic therapies. By definition, the required sensitivity of MRD assays goes well below the 0.1% minimal detectable variant allelic frequency (VAF) achieved by standard ctDNA NGS (46), which becomes possible only through the simultaneous measurement of multiple genetic alterations along with special methods for error suppression (47). Currently, several commercial ctDNA assays with sensitivities <0.01% are available (48), but their application for the treatment of early NSCLC remains to be defined.
Measurement of samples from the adjuvant IMpower010 trial with the Signatera method (minimal detectable VAF <0.01%) showed that patients with detectable ctDNA after surgery had a worse prognosis with a median EFS <2 years vs. not reached at 4 years for patients with negative ctDNA assays, but they also benefited from adjuvant atezolizumab more with an EFS HR of 0.61 (95% CI: 0.39–0.94) vs. 0.72 (95% CI: 0.52–1.00, Table 4) (49). Similarly, for patients with detectable ctDNA after surgery, repeat measurements after adjuvant chemotherapy showed benefit for patients with both subsequent clearance [EFS HR =0.67 (95% CI: 0.34–1.32)] and persistence of ctDNA [EFS HR =0.70 (95% CI: 0.37–1.34)], but the latter had a much worse prognosis with a median EFS of approximately 4 months vs. >1 year (50). Along the same lines, measurement of samples from the neoadjuvant Checkmate-816 trial with the very sensitive Archer MRD assay (minimum detectable AF 0.003%) showed that ctDNA clearance after two neoadjuvant courses was necessary, but not sufficient for the achievement of pCR (8), and that many ctDNA negative patients would experience relapse afterwards, albeit it at a lower rate than ctDNA-positive cases (9/24 vs. 9/19) (8). These results were recently echoed by the analysis of samples from the perioperative AEGEAN trial reported at the ESMO 2023 using the Invitae method (minimum detectable VAF <0.01%), which also showed that ctDNA clearance under neoadjuvant treatment is necessary (negative predictive value 100% after 3 cycles), but not sufficient (positive predictive value 40.5%) for the achievement of pCR (51). Taken together, all presented results suggest that MRD assays can already identify patients with inadequate responses and worse prognosis who would be good candidates for escalation trials of more aggressive management, but their sensitivity is still insufficient for deescalation trials. For example, the omission of postoperative treatment and maybe also surgery for patients with ctDNA clearance under neoadjuvant therapy is dangerous, because many patients appearing as MRD-negative actually harbor residual disease and have indeed suffered disease relapses in the aforementioned studies (Table 5). The achievement of pCR is currently a more reliable predictor than ctDNA clearance under neoadjuvant treatment and showed a lower EFS HR of 0.13 (95% CI: 0.05–0.37) vs. 0.60 (95% CI: 0.20–1.82) in the Checkmate-816 trial (Table 5) (8). One way to improve ctDNA-based predictions would be the serial measurement of longitudinal samples from the same patient after surgery, aka MRD surveillance, which can increase the detection of cases destined to relapse (52). An alternative could be the use of more sensitive and specific assays for single time-point, aka landmark, measurements, before or after surgery, which could reveal more cases with poor prognosis at an even earlier time-point (52). The most promising methods for this application currently are whole-genome sequencing (WGS)-based quantifications of the tumor fraction (TF), like MRDetect with a minimal detectable TF ≈0.0003% (53), or the custom PhasedSeq assay, which can achieve an even lower sensitivity <0.00001%, but requires the construction of individualized assays with multiple phased variants based on WGS of the tumor tissue and blood for each patient (54). Of note, despite the 2× higher yield of ctDNA (+) patients compared to other MRD assays, relapses of ctDNA (−) cases did also occur in a pilot study of PhasedSeq for resectable NSCLC reported at the AACR 2023, which highlights the need for further progress (55). The additional consideration of ctDNA features beyond mutation calling, like fragmentomic and epigenomic characteristics, could further improve performance (56). Besides technical challenges, another obstacle for the wide adoption of MRD assays are high costs associated with deep sequencing (57). Recognizing the difficulties, but also the importance of this field, the FDA has published detailed guidance about the setup of trials to generate robust evidence for the future approval and reimbursement of MRD assays in the routine setting (58).
Table 5
| Clinical trial | EFS HR | EFS HR | PPV for pCR | NPV for pCR | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| ctDNA (+) | ctDNA (−) | pCR (+) | ctDNA (−) | ctDNA (−) | |||||
| IMpower010 (adjuvant), post surgery† | 0.61 (0.39–0.94)‡ | 0.72 (0.52–1.00)‡ | NA | NA | NA | (49) | |||
| IMpower010 (adjuvant), post chemotherapy† | 0.67 (0.34–1.32)‡ | 0.70 (0.37–1.34)‡ | NA | NA | NA | (50) | |||
| Checkmate-816 (neoadjuvant)† | 0.60 (0.20–1.82)§ | NA | 0.13 (0.05–0.37)¶ | NA | NA | (41) | |||
| AEGEAN (perioperative)† | NA | NA | NA | 40.5% | 100.0% | (51) | |||
Whenever available, pCR results are shown for comparison. The NADIM-2 trial has also published results of ctDNA analyses, but these were performed with the TSO-500 ctDNA assay, which is not sensitive enough to qualify as an MRD assay (nominal minimal detectable VAF of 0.1%) (10). †, landmark ctDNA assays were performed directly after surgery before the first chemotherapy cycle, as well as after the last chemotherapy cycle (for patients with detectable ctDNA after surgery) in the IMpower010 trial (Signatera assay with nominal minimal detectable VAF <0.01%), after 2 cycles of neoadjuvant chemoimmunotherapy in the Checkmate-816 trial (Archer assay with nominal minimal detectable VAF ≈0.003%), and after 3 cycles of neoadjuvant chemoimmunotherapy in the AEGEAN trial (Invitae assay with nominal minimal detectable VAF <0.01%). ‡, vs. the control arm of best supportive care. §, in the experimental arm vs. ctDNA (−) patients. ¶, in the experimental arm vs. pCR (−) patients. MRD, minimal residual disease; ctDNA, circulating tumor DNA; NSCLC, non-small cell lung cancer; EFS, event-free survival; HR, hazard ratio; PPV, positive predictive value; pCR, pathologic complete remission; NPV, negative predictive value; NA, not applicable/not available; VAF, variant allelic frequency.
Discussion and future steps
Overall, 2023 was a landmark year that established both the perioperative treatment paradigm for resectable NSCLC, and the need for routine molecular profiling of these tumors already at initial diagnosis. This progress is based on highest-quality evidence from several randomized phase 3 trials showing consistent results, eliminating any concerns about technical drawbacks. At the same time, other exciting developments are unfolding in various directions to further transform our practice in the near future.
First, the use of systemic therapy will probably be extended to even smaller tumors with a size <4 cm, i.e., stage I according to TNM8: for stage IA2 and IA3 (1 up to <4 cm large according to TNM8) NSCLC with classical activating EGFR mutations (del19 or L858R), which do not have an indication for adjuvant chemotherapy currently, the randomized ADAURA-2 trial (NCT05120349) is prospectively examining the value of adjuvant osimertinib over 3 years vs. placebo (59). Second, the duration of treatment with adjuvant TKI will likely also increase, as for example the single-arm phase 2 trial TARGET is already exploring the feasibility and efficacy of 5 instead of 3 years adjuvant osimertinib for tumors with classic EGFR mutations (60). Furthermore, for stage I–II, up to 5 cm large, N0 tumors in medically inoperable patients, the first randomized phase 2 trial ISABR showed significant advantage for the administration of 4 cycles of the PD-1 inhibitor nivolumab along with stereotactic radiotherapy (SBRT) with an EFS HR of 0.42 (95% CI: 0.22–0.80) compared to SBRT alone (61). Of note, the benefit showed similar magnitude in both smaller [up to 2 cm, EFS HR 0.35 (95% CI: 0.14–0.86)] and larger [2–5 cm, EFS HR 0.40 (95% CI: 0.14–1.20)] tumors. Moreover, for potentially resectable tumors with classic EGFR mutations and ALK fusions, the efficacy of perioperative osimertinib or alectinib is being tested within the phase 3 neoADAURA (NCT04351555) (62) and the phase 2 NAUTIKA1 (NCT04302025) and ALNEO (NCT05015010) trials (63,64). At the other end of the spectrum, the higher efficacy of perioperative chemoimmunotherapy is expanding the realm of resectability to include multilevel N2 disease (15), while the ongoing LAURA (NCT03521154) and HORIZON-01 (NCT05170204) trials are investigating the clinical benefit from consolidative osimertinib, alectinib and entrectinib for 3 years instead of durvalumab after curative-intent chemoradiation for unresectable stage III tumors with classical EGFR mutations or ALK/ROS1 fusions, respectively (65,66). Taken together, these studies will result in all NSCLC being molecularly tested for actionable alterations at initial diagnosis in the next few years regardless of stage. It should also be noted that the previous recommendation of the ESMO to test only non-squamous tumors with NGS in 2020 (67) has already been superseded by the ASCO and various national recommendations to test NSCLC regardless of histology (68,69), so that the histologic subtype is not a boundary to molecular testing anymore. The ESMO recommendation is also under revision with a new version expected in the near future (70).
Another important direction will be the further improvement of preoperative therapy and the fine adjustment of postoperative management according to the success of neoadjuvant treatment. The current generation of trials (Keynote-671, AEGEAN, Checkmate-77T, Neotorch) have all used neoadjuvant chemoimmunotherapy and stipulated 1 year of adjuvant PD-(L)1 blockade with the same agent that was already given preoperatively, regardless of pathologic remission status, but this cannot be the optimal choice for all patients. As experience from all these trials shows, patients achieving a pCR have an excellent prognosis, and deescalation, for example, a shorter adjuvant IO course of only 6 months, has already shown very promising results in a pivotal phase 2 study (15). On the other hand, patients failing to achieve a pCR will need more aggressive postoperative therapy, which could include chemotherapy, as nicely demonstrated by the very good results of the Neotorch trial, as already described in a previous section (13). Evolving concepts for these patients include the administration of next-generation immunotherapeutics without cross-resistance to PD-(L)1 inhibitors, like inhibitors of TIGIT, LAG3 and other ICI, antibody drug conjugates (ADC), multispecific antibodies, cell therapies, or even personalized neoantigen-directed vaccines, as the recent success of the Keynote-942 trial in patients with resectable melanoma showed (71,72). There is also strong interest in improving preoperative therapy by replacing chemotherapy with novel immunotherapeutic agents, but it is unclear at present which ones should be prioritized. The recently announced results of the ipilimumab-nivolumab arm from the Checkmate-816 trial showed a decent 2-year EFS rate of 60% (Table 1), but an early crossing of the curves with the control (chemotherapy-only) arm, which is problematic and lead to premature abandonment of this concept within the trial (9). Growing understanding of the immunologic tumor microenvironment for oncogene-driven, e.g., EGFR/ALK/HER2-positive NSCLC will likely facilitate development of effective perioperative immunotherapies which could be used in combination with targeted drugs in order to achieve even higher cure rates for these tumors in the future (73).
Conclusions
Perioperative immunotherapy is becoming the dominant paradigm of treatment for potentially resectable NSCLC, abolishing purely adjuvant and neoadjuvant regimens. NGS already at initial diagnosis is the new standard-of-care for these tumors and essential for the decision between immunotherapy and targeted drugs, whose indications are rapidly expanding.
Acknowledgments
Funding: This work was supported in part by an unrestricted grant from the German Center for Lung Research (DZL).
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-137/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-137/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-137/coif). P.C. serves as an unpaid editorial board member of Chinese Clinical Oncology from September 2023 to August 2025. P.C. has received research funding from AstraZeneca, Amgen, Boehringer Ingelheim, Merck, Novartis, Roche, and Takeda; speaker’s honoraria from AstraZeneca, Gilead, Janssen, Novartis, Roche, Pfizer, Thermo Fisher, Takeda; support for attending meetings from AstraZeneca, Eli Lilly, Daiichi Sankyo, Janssen, Gilead, Novartis, Pfizer, Takeda; personal fees for participating to advisory boards from AstraZeneca, Boehringer Ingelheim, Chugai, Pfizer, Novartis, MSD, Takeda and Roche; all outside the submitted work.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tan AC, Tan DSW. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J Clin Oncol 2022;40:611-25. [Crossref] [PubMed]
- Lahiri A, Maji A, Potdar PD, et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer 2023;22:40. [Crossref] [PubMed]
- Volckmar AL, Leichsenring J, Kirchner M, et al. Combined targeted DNA and RNA sequencing of advanced NSCLC in routine molecular diagnostics: Analysis of the first 3,000 Heidelberg cases. Int J Cancer 2019;145:649-61. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022;23:1274-86. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Awad MM, Forde PM, Girard N, et al. 1261O Neoadjuvant nivolumab (N) + ipilimumab (I) vs chemotherapy (C) in the phase III CheckMate 816 trial. Ann Oncol 2023;34:S731. [Crossref]
- Cascone T, Awad MM, Spicer JD, et al. LBA1 CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. Ann Oncol 2023;34:S1295. [Crossref]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:1672-84. [Crossref] [PubMed]
- Lu S, Wu L, Zhang W, et al. Perioperative toripalimab + platinum-doublet chemotherapy vs chemotherapy in resectable stage II/III non-small cell lung cancer (NSCLC): Interim event-free survival (EFS) analysis of the phase III Neotorch study. J Clin Oncol 2023;41:425126. [Crossref]
- Yue D, Wang W, Liu H, et al. LBA58 Pathological response to neoadjuvant tislelizumab (TIS) plus platinum-doublet (PtDb) chemotherapy (CT) in resectable stage II-IIIA NSCLC patients (pts) in the phase III (Ph3) RATIONALE-315 trial. Ann Oncol 2023;34:S1299. [Crossref]
- Provencio M, Nadal E, González-Larriba JL, et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:504-13. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Lim W, Ridge CA, Nicholson AG, et al. The 8(th) lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg 2018;8:709-18. [Crossref] [PubMed]
- Putora PM, Leskow P, McDonald F, et al. International guidelines on stage III N2 nonsmall cell lung cancer: surgery or radiotherapy? ERJ Open Res 2020;6:00159-2019. [Crossref] [PubMed]
- Garassino MC. Annual ESMO 2023 congress; Presidential session 1; Invited Discussion of LBA1 and LBA2. Available online: https://esmocongress.esmo.org/esmo/esmo2023/en-GB/presentation/639637 (last accessed on November 11 2023).
- Deutsch JS, Cimino-Mathews A, Thompson E, et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat Med 2024;30:218-28. [Crossref] [PubMed]
- Tao Y, Li X, Liu B, et al. Association of early immune-related adverse events with treatment efficacy of neoadjuvant Toripalimab in resectable advanced non-small cell lung cancer. Front Oncol 2023;13:1135140. [Crossref] [PubMed]
- Daniello L, Elshiaty M, Bozorgmehr F, et al. Therapeutic and Prognostic Implications of Immune-Related Adverse Events in Advanced Non-Small-Cell Lung Cancer. Front Oncol 2021;11:703893. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Johnson ML, Cho BC, Luft A, et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol 2023;41:1213-27. [Crossref] [PubMed]
- Neotorch investigators. Synopsis of the Neotorch study; Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04158440. Accessed on 05.11.2023.
- Mok T, Nakagawa K, Park K, et al. LBA8 Nivolumab (NIVO) + chemotherapy (chemo) vs chemo in patients (pts) with EGFR-mutated metastatic non-small cell lung cancer (mNSCLC) with disease progression after EGFR tyrosine kinase inhibitors (TKIs) in CheckMate 722. Ann Oncol 2022;33:S1561-2. [Crossref]
- Yang JCH, Lee DH, Lee JS, et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR-mutant, metastatic nonsquamous NSCLC: Phase 3 KEYNOTE-789 study. J Clin Oncol 2023;41:LBA9000. [Crossref]
- Tsuboi M, Herbst RS, John T, et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 2023;389:137-47. [Crossref] [PubMed]
- Solomon BJ, Ahn JS, Dziadziuszko R, et al. LBA2 ALINA: Efficacy and safety of adjuvant alectinib versus chemotherapy in patients with early-stage ALK+ non-small cell lung cancer (NSCLC). Ann Oncol 2023;34:S1295-6. [Crossref]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Tsuboi M, Goldman JW, Wu YL, et al. LIBRETTO-432, a phase III study of adjuvant selpercatinib or placebo in stage IB-IIIA RET fusion-positive non-small-cell lung cancer. Future Oncol 2022;18:3133-41. [Crossref] [PubMed]
- Wu YL, John T, Grohe C, et al. Postoperative Chemotherapy Use and Outcomes From ADAURA: Osimertinib as Adjuvant Therapy for Resected EGFR-Mutated NSCLC. J Thorac Oncol 2022;17:423-33. [Crossref] [PubMed]
- Zhang SS, Ou SI. Deconstructing ADAURA: It is Time to Forgo Adjuvant Platinum-Based Chemotherapy in Resected IB-IIIA EGFR+ NSCLC (Except with RB Alterations?) When Adopting Adjuvant Osimertinib. Lung Cancer (Auckl) 2022;13:23-31. [Crossref] [PubMed]
- Shepherd FA, Lacas B, Le Teuff G, et al. Pooled Analysis of the Prognostic and Predictive Effects of TP53 Comutation Status Combined With KRAS or EGFR Mutation in Early-Stage Resected Non-Small-Cell Lung Cancer in Four Trials of Adjuvant Chemotherapy. J Clin Oncol 2017;35:2018-27. [Crossref] [PubMed]
- Herbst RS, Wu YL, John T, et al. Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non-Small-Cell Lung Cancer: Updated Results From the Phase III Randomized ADAURA Trial. J Clin Oncol 2023;41:1830-40. [Crossref] [PubMed]
- Christopoulos P, Kirchner M, Roeper J, et al. Risk stratification of EGFR(+) lung cancer diagnosed with panel-based next-generation sequencing. Lung Cancer 2020;148:105-12. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Christopoulos P, Kluck K, Kirchner M, et al. The impact of TP53 co-mutations and immunologic microenvironment on outcome of lung cancer with EGFR exon 20 insertions. Eur J Cancer 2022;170:106-18. [Crossref] [PubMed]
- Marino FZ, Ronchi A, Accardo M, et al. Concomitant ALK/KRAS and ALK/EGFR mutations in non small cell lung cancer: different profile of response to target therapies. Transl Cancer Res 2017;6:S457-60. [Crossref]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Chaft JE, Oezkan F, Kris MG, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med 2022;28:2155-61. [Crossref] [PubMed]
- Budczies J, Kazdal D, Allgäuer M, et al. Quantifying potential confounders of panel-based tumor mutational burden (TMB) measurement. Lung Cancer 2020;142:114-9. [Crossref] [PubMed]
- Mountzios G, Samantas E, Senghas K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open 2021;6:100254. [Crossref] [PubMed]
- Laza-Briviesca R, Cruz-Bermúdez A, Nadal E, et al. Blood biomarkers associated to complete pathological response on NSCLC patients treated with neoadjuvant chemoimmunotherapy included in NADIM clinical trial. Clin Transl Med 2021;11:e491. [Crossref] [PubMed]
- Elazezy M, Joosse SA. Techniques of using circulating tumor DNA as a liquid biopsy component in cancer management. Comput Struct Biotechnol J 2018;16:370-8. [Crossref] [PubMed]
- Newman AM, Lovejoy AF, Klass DM, et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol 2016;34:547-55. [Crossref] [PubMed]
- Chen K, Shields MD, Chauhan PS, et al. Commercial ctDNA Assays for Minimal Residual Disease Detection of Solid Tumors. Mol Diagn Ther 2021;25:757-74. [Crossref] [PubMed]
- Zhou C, Das Thakur M, Srivastava MK, et al. 2O IMpower010: Biomarkers of disease-free survival (DFS) in a phase III study of atezolizumab (atezo) vs best supportive care (BSC) after adjuvant chemotherapy in stage IB-IIIA NSCLC. Ann Oncol 2021;32:S1374. [Crossref]
- Felip E, Srivastava M, Reck M, et al. 1O IMpower010: ctDNA status in patients (pts) with resected NSCLC who received adjuvant chemotherapy (chemo) followed by atezolizumab (atezo) or best supportive care (BSC). Immunooncol Technol 2022;16:100106. [Crossref]
- Reck M, Gale D, Harpole D, et al. LBA59 - Associations of ctDNA clearance and pathological response with neoadjuvant treatment in patients with resectable NSCLC from the phase III AEGEAN trial. Ann Oncol 2023;34:S1300. [Crossref]
- Moding EJ, Nabet BY, Alizadeh AA, et al. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov 2021;11:2968-86. [Crossref] [PubMed]
- Zviran A, Schulman RC, Shah M, et al. Genome-wide cell-free DNA mutational integration enables ultra-sensitive cancer monitoring. Nat Med 2020;26:1114-24. [Crossref] [PubMed]
- Kurtz DM, Soo J. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat Biotechnol 2021;39:1537-47. [Crossref] [PubMed]
- Isbell JM, Li BT, Razavi P, et al. Abstract 3375: Ultrasensitive ctDNA minimal residual disease monitoring in early NSCLC with PhasED-Seq. Cancer Res 2023;83:3375. [Crossref]
- Angeles AK, Janke F, Bauer S, et al. Liquid Biopsies beyond Mutation Calling: Genomic and Epigenomic Features of Cell-Free DNA in Cancer. Cancers (Basel) 2021;13:5615. [Crossref] [PubMed]
- Christopoulos P. Liquid biopsies come of age in lung cancer. Transl Lung Cancer Res 2022;11:706-10. [Crossref] [PubMed]
- U.S. Food and Drug Administration. Use of Circulating Tumor Deoxyribonucleic Acid for Early-Stage Solid Tumor Drug Development; Draft Guidance for Industry. May 2022. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-circulating-tumor-deoxyribonucleic-acid-early-stage-solid-tumor-drug-development-draft-guidance. Accessed 9 Nov 2023.
- Tsutani Y, Goldman JW, Dacic S, et al. Adjuvant Osimertinib vs. Placebo in Completely Resected Stage IA2-IA3 EGFR-Mutated NSCLC: ADAURA2. Clin Lung Cancer 2023;24:376-80. [Crossref] [PubMed]
- Soo RA, de Marinis F, Han JY, et al. TARGET: A Phase II, Open-Label, Single-Arm Study of 5-Year Adjuvant Osimertinib in Completely Resected EGFR-Mutated Stage II to IIIB NSCLC Post Complete Surgical Resection. Clin Lung Cancer 2024;25:80-4. [Crossref] [PubMed]
- Chang JY, Lin SH, Dong W, et al. Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. Lancet 2023;402:871-81. [Crossref] [PubMed]
- Tsuboi M, Weder W, Escriu C, et al. Neoadjuvant osimertinib with/without chemotherapy versus chemotherapy alone for EGFR-mutated resectable non-small-cell lung cancer: NeoADAURA. Future Oncol 2021;17:4045-55. [Crossref] [PubMed]
- Lee JM, Sepesi B, Toloza EM, et al. EP02.04-005 Phase II NAUTIKA1 Study of Targeted Therapies in Stage II-III NSCLC: Preliminary Data of Neoadjuvant Alectinib for ALK+ NSCLC. J Thor Oncol 2022;17:S233-4. [Crossref]
- Leonetti A, Minari R, Boni L, et al. EP02.04-001 Alectinib as Neoadjuvant Treatment in Surgically Resectable Stage III ALK-Positive NSCLC: ALNEO Phase II Trial (GOIRC-01-2020). J Thor Oncol 2022;17:S231. [Crossref]
- Lu S, Casarini I, Kato T, et al. Osimertinib Maintenance After Definitive Chemoradiation in Patients With Unresectable EGFR Mutation Positive Stage III Non-small-cell Lung Cancer: LAURA Trial in Progress. Clin Lung Cancer 2021;22:371-5. [Crossref] [PubMed]
- Paz-Ares L, Gay CM, Zhou C, et al. 131TiP A phase I–III platform study evaluating the safety and efficacy of multiple therapies in patients (pts) with biomarker-defined locally advanced, unresectable stage III non-small cell lung cancer (NSCLC). J Thor Oncol 2023;18:S114. [Crossref]
- Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol 2020;31:1491-505. [Crossref] [PubMed]
- Chakravarty D, Johnson A, Sklar J, et al. Somatic Genomic Testing in Patients With Metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. J Clin Oncol 2022;40:1231-58. [Crossref] [PubMed]
- Schütte W, Gütz S, Nehls W. S3 Leitlinie: Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/lungenkarzinom/. Accessed 29 Dec 2022.
- André F, Mosele F, Westphalen Benedikt. Precision Oncology: Genomics Guided Care – Update of the Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancer. Available online: https://www.esmo.org/meeting-calendar/precision-oncology-genomics-guided-care-update-of-the-recommendations-for-the-use-of-next-generation-sequencing-ngs-for-patients-with-metastatic-cancer. Accessed 9 Nov 2023.
- Gaissmaier L, Christopoulos P. Immune Modulation in Lung Cancer: Current Concepts and Future Strategies. Respiration 2020; Epub ahead of print. [Crossref] [PubMed]
- Khattak A, Weber JS, Meniawy T, et al. Distant metastasis-free survival results from the randomized, phase 2 mRNA-4157-P201/KEYNOTE-942 trial. J Clin Oncol 2023;41:LBA9503. [Crossref]
- Budczies J, Kirchner M, Kluck K, et al. Deciphering the immunosuppressive tumor microenvironment in ALK- and EGFR-positive lung adenocarcinoma. Cancer Immunol Immunother 2022;71:251-65. [Crossref] [PubMed]


