
Perioperative or neoadjuvant chemotherapy for locally advanced gastric or gastroesophageal junction cancer: from independent evidence in the West, the East, and Japan to global collaboration
Introduction
Gastric or gastroesophageal junction (G/GEJ) cancer is a major concern worldwide, and improvements in the treatment of patients with G/GEJ cancer are urgently warranted. G/GEJ cancer is the fifth most frequent malignancy and third leading cause of cancer-related mortality worldwide (1). In 2018, more than 1,000,000 new cases were diagnosed and there were 782,000 estimated deaths.
Early-stage G/GEJ cancer remaining within the mucosa is highly curable with endoscopic resection (2). Locoregional G/GEJ cancer is curatively resectable via surgery with adequate margins and lymph node dissection (3). Before 2000, the 5-year survival rate of patients with advanced G/GEJ cancer who underwent curative resection was approximately 65–70% in Japan and Korea (4,5). However, the 5-year survival rate of patients with locally advanced G/GEJ cancer who underwent surgery alone was 20–30% in clinical trials conducted in Europe and the United States (6-8). Locally advanced G/GEJ cancer without distant metastasis has been recognized as curable by complete resection in Eastern Asia but not by surgery alone in Western countries. The different perceptions of experts in the East and the West have affected the directions of therapeutic development in the treatment of locally advanced G/GEJ cancer.
In Western countries, perioperative chemotherapy for G/GEJ cancer among clinical T ≥2 and/or N+ is strongly recommended as the standard of care (9). On the basis of the results of the FLOT4 trial, fluorouracil plus leucovorin, oxaliplatin, and docetaxel (FLOT) has become the standard regimen (10). The survival outcome of G/GEJ cancer is similar in the United States and Europe, and the necessity for perioperative multimodal treatment has been acknowledged (11).
In Eastern countries, based on the successful results of several trials, upfront surgery followed by adjuvant chemotherapy has been the standard of care for pathological non-pT1 and stage II/III (12-17). Recently, neoadjuvant, or perioperative, chemotherapy has shown clinically meaningful improvements in disease progression compared with adjuvant chemotherapy in Korea and China (18,19). Consequently, preoperative chemotherapy for clinical T3–4aN+ or T4b tumors is one of the standard options (20).
Several clinical trials have made great progress in the development of minimally invasive surgery and organ preservation for the treatment of resectable G/GEJ cancer (21-28). However, further investigations of minimally invasive surgery are less likely to improve the survival outcomes of locally advanced G/GEJ cancer. Effective perioperative treatment is essential to achieve a better prognosis for locally advanced G/GEJ cancer. The indication of perioperative or neoadjuvant chemotherapy varies greatly between the West and the East or Japan and outside of Japan and remains a debatable issue in patient selection for minimizing overtreatment. To explore the optimal candidates for perioperative or neoadjuvant chemotherapy, this review highlights the clinical trials of perioperative or neoadjuvant chemotherapy for locally advanced G/GEJ cancer, with a main focus on the indication and oncological outcomes.
Review of clinical trials
Concept of review
The efficacy and safety of perioperative chemotherapy were established based on the results of the MAGIC and FNCLCC/FFCD trials in Western countries (6,8). Thus, we reviewed clinical trials conducted after 2005. The pivotal phase III trials of perioperative or neoadjuvant chemotherapy for locally advanced G/GEJ cancer are summarized in Figure 1 and Table 1. Herein, we assessed the indication and survival outcomes of perioperative or neoadjuvant chemotherapy mainly based on the phase III trials, with the inclusion of phase II trials in the categories where phase III trials were limitedly available. In addition, adenocarcinoma is the dominant histological type of esophageal cancer in Western countries (29). Adenocarcinoma of the lower esophagus was included with G/GEJ cancer in a clinical trial of pharmacological therapy. Based on the CROSS trial, preoperative chemoradiotherapy (CRT) is one of the standard treatment options for patients with GEJ adenocarcinoma. However, in Asian countries, squamous cell carcinoma is the dominant histological type of esophageal cancer, and the therapeutic strategy for esophageal squamous cell carcinoma differs from that for G/GEJ adenocarcinoma (30). Therefore, we excluded clinical trials that predominantly included adenocarcinomas of the lower esophagus or GEJ.
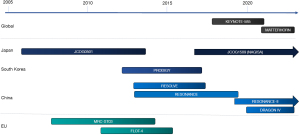
Table 1
| Country | Trial name | Phase | Trial period | Indication | Superiority or non-inferiority | Control arm (No. of pts) | Exp. arm (No. of pts) | Primary endpoint | Control vs. Exp. | HR (95% CI) | P value | Result |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Japan | JCOG0501 | III | Oct 2005, Jul 2013 | Type 4, type 3 (≥8 cm) | Superiority | Up-front surgery (n=158) | NAC SP (n=158) | OS at 3 yr | 62.4% vs. 60.9% | 0.92 (0.68–1.24) | 0.280 | Negative |
| Germany | FLOT4 | II/III | Aug 2010, Feb 2015 | cT2–4 or N+ | Superiority | Perioperative ECF/ECX (n=360) | Perioperative FLOT (n=356) | OS | 35 vs. 50 m | 0.77 (0.63–0.94) | 0.012 | Positive |
| Korea | PRODIGY | III | Jan 2012, Jan 2017 | cT2–3N+ or cT4 | Superiority | Up-front surgery (n=264) | NAC DOS (n=266) | PFS at 3 yr | 66.3% vs. 60.2% | 0.70 (0.52–0.95) | 0.023 | Positive |
| China | FOCUS | III | Jun 2011, Aug 2016 | cT4 | Non-inferiority | Perioperative SOX (n=293) | Perioperative FOLFOX (n=290) | OS at 3 yr | 75.2% vs. 67.8% | 0.84 (0.63–1.13) | – | Positive |
| China | RESOLVE (perioperative) | III | Aug 2012, Feb 2017 | cT4aN+ or cT4b | Superiority | Adjuvant CAPOX (n=345) | Perioperative SOX (n=337) | DFS at 3 yr | 51.1% vs. 59.4% | 0.77 (0.61–0.97) | 0.028 | Positive |
| RESOLVE (adjuvant) | Non-inferiority | Adjuvant CAPOX (n=345) | Adjuvant SOX (n=340) | DFS at 3 yr | 51.1% vs. 56.5% | 0.86 (0.68–1.07) | 0.170 | Positive | ||||
| China | DRAGON IV | II/III | Dec 2019, Dec 2022 | cT3–4a or N+ | Superiority | Perioperative SOX (n=180) | Perioperative SOX + C + R (n=180) | pCR and EFS | 5.0% vs. 18.3% | 4.5 (2.1–9.9) | <0.0001 | Not yet available |
| Global | KEYNOTE-585 | III | Oct 2017, Jan 2021 | cT3–4 or N+ | Superiority | Perioperative XP/FP (n=402) | Perioperative XP/FP + Pembro (n=402) | pCR and EFS | 2.0%/25.3 m vs. 12.9%/44.4 m | 0.81 (0.67–0.99) | 0.0198 | Negative |
| Global | MATTERHORN | III | Nov 2020, Dec 2022 | cT3–4 or N+ | Superiority | Perioperative FLOT (n=293) | Perioperative FLOT + Durva (n=293) | EFS | Not yet available | Not yet available | Not yet available | Not yet available |
No., number; pts, patients; Exp., experimental; HR, hazard ratio; CI, confidence interval; NAC, neoadjuvant chemotherapy; SP, S-1 plus cisplatin; OS, overall survival; ECF/ECX, epirubicin, cisplatin, and infused fluorouracil/epirubicin, cisplatin, and capecitabine; FLOT, fluorouracil plus leucovorin, oxaliplatin and docetaxel; yr, years; m, months; DOS, docetaxel, oxaliplatin, and S-1; PFS, progression-free survival; SOX, S-1 plus oxaliplatin; FOLFOX, 5-fluorouracil, leucovorin and oxaliplatin; CAPOX, capecitabine plus oxaliplatin; DFS, disease-free survival; C, camrelizumab; R, rivoceranib; pCR, pathological complete response; EFS, event-free survival; XP, capecitabine plus cisplatin; FP, 5-fluorouracil plus cisplatin; Pembro, pembrolizumab; Durva, durvalumab.
The prognosis of patients with tumors is clearly stratified by the tumor-lymph node-metastasis (TNM) staging system, combined with the depth of tumor invasion (T), number of lymph node metastases (N), and presence or absence of distant metastasis (M) (5,31). The TNM staging system released by the Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC) is widely available, clearly stratifying patient prognosis. It seemed reasonable to determine the indications for perioperative chemotherapy according to TNM staging. However, major revisions of the 7th and 8th editions of the UICC/AJCC have been made over the last 20 years (32,33). Furthermore, the Japanese Classification of Gastric Carcinoma from the Japanese Gastric Cancer Association (JGCA) is the standard reference for Japanese clinicians (34). Although depth of tumor invasion could be interchangeable among the different classifications, there was a large difference in the classification of lymph node metastasis that was not interchangeable between the JGCA classification 13th edition and the UICC/AJCC 7th or 8th edition. Considering the variations in TNM stage classification based on the timing or region in clinical trials, this review described the lymph node classification as the presence (N+) or absence (N−) of lymph node involvement.
Perioperative chemotherapy: the West vs. the East other than Japan vs. Japan
The West
The MAGIC trial was a randomized phase III trial to evaluate the efficacy of perioperative epirubicin, cisplatin, and infused fluorouracil (ECF); the FNCLCC/FFCD trial was another randomized phase III trial to compare surgery with or without perioperative chemotherapy using cisplatin and fluorouracil (CF) in the treatment of locally advanced G/GEJ cancer (6,8). Patients with stage IB–IVM0 disease according to the 1988 AJCC staging criteria were eligible for the MAGIC trial, and only those with gastroesophageal adenocarcinoma in situ were excluded from the FNCLCC/FFCD trial. Five-year overall survival (OS) rates of surgery alone in the MAGIC and the FNCLCC/FFCD trials were 23.0% [95% confidence interval (CI): 16.6–29.4%] and 24% (95% CI: 17–33%), respectively. Improved survival after the addition of perioperative chemotherapy was consistently observed in both trials. Based on these results, perioperative chemotherapy is strongly recommended for G/GEJ cancer with cStage ≥ IB in the AJCC/UICC TNM 8th edition in Western countries (9).
In the United States, the INT-0116 trial was conducted to evaluate the efficacy of postoperative CRT using fluorouracil and leucovorin with 45 Gy radiotherapy compared with surgery alone (7). The inclusion criteria for clinical stage were the same as those in the MAGIC trial (cStage IB to IVM0). The 3-year survival rate was 41% in the surgery alone group. Combination therapy with chemo(radio)therapy and surgery is a well-accepted therapeutic approach in the United States. The National Comprehensive Cancer Network guideline recommends perioperative chemotherapy as category 1 for clinically T2–4 or N+ patients (35).
The mortality rate of patients with locally advanced G/GEJ cancer who received perioperative epirubicin, cisplatin, and capecitabine (ECX) remains high, and the 5-year OS rate is <50% (6,36). Clinical investigations for a more effective chemotherapy regimen than ECF/ECX have been conducted in Western countries. The triplet regimen with docetaxel was the most promising, but because of its severe toxicities, docetaxel, cisplatin, and fluorouracil (DCF) was found to be intolerable and abandoned to develop as perioperative setting (37). While perioperative FLOT demonstrated the improved survival with manageable toxic profile in the FLOT4 trial, FLOT has therefore become the current standard therapy for perioperative chemotherapy (10). The FLOT4 trial was a randomized phase II/III trial that compared the efficacy and safety of FLOT and ECX/ECF for locally advanced G/GEJ cancer. Patients with cT ≥2 and/or N+M0 were included in the FLOT4 trial. The median OS of FLOT was 50 months (95% CI: 38.33–not reached) and showed superior efficacy over ECX/ECF [hazard ratio (HR) =0.77; 95% CI: 0.63–0.94; P=0.012].
The East
Because gastrectomy with D2 lymph node dissection has been standardized in East Asia, particularly Japan and Korea, extended lymphadenectomy is sufficient to eradicate locoregional disease in most patients. Chemotherapy or CRT based on pathological staging after curative-intent resection has been developed to improve survival in East Asia (12-16,38). Surgery followed by adjuvant chemotherapy is a reasonable approach to eliminate the patients with early-stage disease (pT1b-2N0M0) who could achieved a cure by surgery alone. While, because of weight loss or complications after surgery, intensive chemotherapy is available only for selected patients who have undergone gastrectomy. The primary objective of the discussion was to accurately identify patients with a poor prognosis who should receive neoadjuvant chemotherapy as the initial treatment approach. From this perspective, Japan and regions other than Japan have adopted different paths.
In Japan
According to the nationwide registry of patients with gastric cancer (GC) in 1991 by the Japanese Research Society for Gastric Cancer, 3,871 patients (48.8%) were diagnosed with pathological T1 disease among 7,935 patients who underwent gastrectomy. The 5-year survival rate of this population is 90.4%, and most patients are curable with surgery alone (4). Focusing the indication for neoadjuvant chemotherapy on patients with a severe prognosis alone is a reasonable and acceptable concept in Japan. Therefore, neoadjuvant chemotherapy was initially investigated for patients with scirrhous GC or GC with extensive lymph node metastasis who had a dismal prognosis after upfront surgery (39-45). The 3-year OS was set as the primary endpoint because of its poor prognosis.
Borrmann type 4 and large type 3
Scirrhous GC or linitis plastica (LP) is a well-known hard-to-treat subset of GC that exhibits a diffusively invasive nature and poor survival outcome after curative resection. Scirrhous GC mainly focusing on the histologically wide spread of tumors with rigid fibrous proliferation, typically exhibiting wall thickness and luminal narrowing. Scirrhous GC referred not only limited or IIc-like advanced gastric cancer (AGC), but also to lesions with extensive infiltration like Borrman type IV. LP indicate the wall thickness with sclerosis of the gastric body and LP is currently used quite similar as the scirrhous GC (46). However, a global consensus on the definition of LP has not yet been reached (47). In terms of the pharmacological treatment, it would be acceptable not to precisely distinguish between these two categories. Due to its infiltrative and invasive nature, scirrhous GC or LP has a high likelihood of not achieving negative surgical margins or presenting peritoneal metastasis during laparotomy. Consequently, some clinicians argue against gastrectomy, considering this type of GC as an inoperable disease (48-51). In some patients, gastrectomy for scirrhous GC is recognized as a cytoreductive surgery, or a perioperative chemotherapy alone is inadequate for disease control of advanced signet ring cell GC (9). In Europe, the FLOT-9 trial is ongoing to evaluate the add-on effects of preventive hyperthermic intraperitoneal chemotherapy on perioperative FLOT (52). However, in several prospective or retrospective Japanese studies, almost 20% of patients with scirrhous GC achieved long-term survival of over 5 years after gastrectomy, even in patients with cytology-positive (CY1) or localized peritoneal metastasis (P1a) (53-55). The survival outcomes of patients with resected scirrhous GC were indeed dismal, but much better than those of patients with unresectable GC in the early 2000s (5-year OS: 2%) (56). Scirrhous GC with or without CY1 and/or P1a is recognized as a borderline resectable disease, and neoadjuvant chemotherapy has been investigated in this population in Japan (57).
The Borrmann classification is widely used in clinical practice and is readily available before surgery. The Borrmann classification classifies advanced GC into four types depending on gross appearance on endoscopy: type 1 (polypoid), type 2 (localized ulcer), type 3 (infiltrated ulcer), and type 4 (diffusely infiltrative) (58). Scirrhous GC mostly overlaps with Borrmann type 4 GC (46). In the early 2000s, the survival outcome of type 4 was dismal, even in Japan, with a 5-year OS rate of 21.4% (59). Considering the poor survival outcome of large-sized (≥8 cm) type 3 GC in a retrospective study such as that of type 4 GC, a single-arm phase II trial (Japan Clinical Oncology Group: JCOG0210) was conducted to assess the feasibility of S-1 plus cisplatin (SP) as neoadjuvant chemotherapy for type 4 and large type 3 GC (41). Consequently, 73.5% of the patients completed two courses of SP followed by gastrectomy, and the promising efficacy of neoadjuvant SP (3-year OS: 24.5%) was demonstrated. A subsequent randomized phase III trial (JCOG0501) was conducted to evaluate the safety and efficacy of neoadjuvant SP compared with upfront surgery (60). However, no survival improvement with additional SP was demonstrated (3-year OS: 60.9% in neoadjuvant chemotherapy vs. 62.4%) (43). Thus, D2 gastrectomy followed by adjuvant chemotherapy remains the standard of care for type 4 and large type 3, the same as for GC other than type 4. Although the JCOG0501 failed to show a survival benefit, the 3-year OS of this population was clearly improved compared with that of the early 2000s, remaining unsatisfactory and inferior to that of patients with pStage III GC other than type 4 (3-year OS: 77.7%) (61). Furthermore, 3-year progression-free survival (PFS) was 47.7% in both arms of JCOG0501, and approximately 80% of patients with recurrence had peritoneal metastasis (43). Peritoneum recurrence can cause cancer-associated symptoms, which considerably deteriorate quality of life.
A triplet regimen of docetaxel is anticipated to improve the survival outcome of patients with type 4 or large type 3 GC. The Osaka Gastrointestinal cancer chemotherapy Study Group (OGSG) 1902 is a single-arm phase II trial evaluating the efficacy of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) for type 4 and large type 3 GC (62). The 3-year PFS was set as the primary endpoint of this study. Patient accrual was completed, and the results are awaited. In contrast, the JCOG stomach group conducted a subsequent phase II study after JCOG0501. The JCOG2204 trial is a randomized phase II study with selective design to evaluate the efficacy, safety, and feasibility of neoadjuvant FLOT versus DOS for type 4 and large type 3 GC (57). The pathological response rate is the primary endpoint, and this study began in July 2023.
The PHOENIX GC-02 is also an ongoing randomized, phase III study comparing adjuvant S-1 plus docetaxel and S-1 plus paclitaxel with intraperitoneal paclitaxel for type 4 GC without non-curative factors and perioperative S-1 plus oxaliplatin (SOX) and perioperative SOX with intraperitoneal paclitaxel for type 4 GC with CY1 (63).
Extensive lymph node metastasis
Bulky N2 lymph node was defined as a lymph node that was either ≥3 cm in size or comprised at least two swollen lymph nodes (each ≥1.5 cm) located around the celiac artery (No. 7), common hepatic artery (No. 9), or splenic artery (No. 11) (39,42,59). Extensive lymph node metastasis is characterized by bulky N2 or paraaortic lymph node (PALN) (No. 16a2/b1) with a size ≥1 cm, indicating a poor survival outcome (less than 5%) (39). Neoadjuvant chemotherapy is expected to improve the survival outcomes of patients with extensive lymph node metastases. JCOG0405 was a single-arm phase II study aimed at elucidating the efficacy of neoadjuvant SP therapy for GC with extensive lymph node metastasis (39). Based on the favorable results of JCOG0405 (3-year OS: 59%), neoadjuvant SP followed by D2 plus para-aortic lymph node dissection is considered a tentative standard treatment for GC with extensive lymph node metastasis. To further improve OS, JCOG1704 was used to assess the safety and efficacy of neoadjuvant DOS (64). Recently, the major pathological response rate (≥ grade 2) was reported to be 56.5% (95% CI: 41.1–71.1%), numerically surpassing the expected and threshold values (by 40% and 25%, respectively). Survival data are awaited. Because of the rarity of such disease, JCOG1704 was terminated before reaching the planned enrollment number. Further large-scale phase III trials are required to confirm this hypothesis.
In Western countries, the FLOT-3 trial was conducted to evaluate neoadjuvant chemotherapy followed by gastrectomy in patients with limited metastatic G/GEJ cancer (65). Patients with retroperitoneal lymph node metastasis, defined as lymph nodes measuring ≥1 cm in the short-axis diameter or a single lymph node measuring ≥2 cm in the short axis, were enrolled in arm C of this trial. Even considering the difference in the definition of extensive lymph nodes, the median survival time of 22.9 months in arm C was poor compared with that of the JCOG0405. Therapeutic development for G/GEJ cancer with PALN metastasis has been conducted with other oligometastases in Western countries, and the aim of the treatment strategy with surgery and chemotherapy is not patient cure in this setting. The RENESSANCE (FLOT-5) trial is ongoing to evaluate patient selection for surgery after four-cycle FLOT as induction chemotherapy in the treatment of patients with limited metastatic G/GEJ cancer, mainly including retroperitoneal lymph node metastasis (66).
General type GC
The international wave of perioperative chemotherapy for locally advanced GC has also affected the attitude of Japanese clinicians towards the treatment of resectable stage GC other than type 4 and large type 3 (general type GC). The JCOG1509 trial is an ongoing randomized phase III trial to evaluate the superiority of neoadjuvant SOX for locally advanced general type GC compared with upfront surgery (jRCTs: 031180350). Before starting this trial, the accuracy of preoperative diagnosis was prospectively assessed in the JCOG1302A study (67). This observational study suggests that using cT3–4 and N+ as criteria could be the most reasonable approach for future neoadjuvant chemotherapy trials. This approach would reduce the inclusion of pStage I GC to 6.5% while maintaining the inclusion of pStage III patients at 64.5%.
The East other than Japan
Although upfront surgery followed by adjuvant chemotherapy is commonly adopted in clinical practice in Eastern countries, several clinical trials of perioperative chemotherapy have been conducted in Eastern countries. Patients with more advanced T stage (cT3 or 4) G/GEJ cancer were enrolled in Eastern clinical trials compared with Western countries (cT2 and more). The PRODIGY trial was a randomized phase III trial conducted mainly in Korea to evaluate the efficacy and safety of neoadjuvant DOS compared with that of upfront surgery followed by adjuvant chemotherapy with S-1 (18). Eligible patients in the PRODIGY trial had histologically proven G/GEJ adenocarcinoma with cT2–3N+ or T4anyN. PFS was set as its primary endpoint, demonstrating the superiority of the neoadjuvant arm over the upfront surgery arm (adjusted HR =0.70; 95% CI: 0.52–0.95; P=0.023). Although the primary endpoint was met, because of incomplete resection as a progressive disease in this study, this result was not accepted as clinically positive (68). OS did not improve with neoadjuvant DOS in the primary analysis. However, the final assessment recently demonstrated a statistically significant difference in OS between the neoadjuvant and upfront surgery arms (adjusted HR =0.72, 95% CI: 0.54–0.96) (69). Notably, in the cT4 subgroup, the HRs for disease progression and death were 0.68 (95% CI: 0.50–0.92) and 0.69 (95% CI: 0.51–0.95), respectively. The RESOLVE trial was conducted in China. This was a randomized phase III trial with a three-arm design to assess the efficacy and safety of perioperative or postoperative adjuvant SOX compared with adjuvant capecitabine plus oxaliplatin (CAPOX) (19). Patients with histologically confirmed cT4aN+ or cT4b G/GEJ adenocarcinoma underwent perioperative chemotherapy. This study met its primary endpoint, and the 3-year disease-free survival (DFS) was 51.1% (95% CI: 45.5–56.3%) in the adjuvant CAPOX group, 56.5% (95% CI: 51.0–61.7%) in the adjuvant SOX group, and 59.4% (95% CI: 53.8–64.6%) in the perioperative SOX group. The efficacies of perioperative SOX and adjuvant SOX were compared with the superiority and non-inferiority designs of adjuvant CAPOX. The HR for recurrence was 0.77 (95% CI: 0.61–0.97, P=0.028) for perioperative SOX versus adjuvant CAPOX, not exceeding the non-inferiority margin of 1.33. An updated survival analysis revealed a significant improvement in 5-year OS of perioperative SOX (60.0%) compared with that of adjuvant CAPOX (52.1%) (HR =0.79; 95% CI 0.62–1.00; P=0.049) (70), while non-inferiority of adjuvant SOX (61.0%) was not statistically demonstrated (HR =0.77; 95% CI: 0.61–0.98; P=0.033). It should be noted that although OS was the secondary endpoint, the addition of neoadjuvant chemotherapy showed an improvement of survival in Asian populations compared with surgery followed by adjuvant chemotherapy (69,70). A further two randomized phase III trials of neoadjuvant SOX (RESONANCE and RESONANCE II) were conducted in China (71,72). In addition, a randomized phase III was conducted to compare perioperative FLOT versus surgery followed by adjuvant SOX at a Chinese single center (NCT05264896).
The PRODIGY trial discussed the inadvertent inclusion of patients with cT1 or stage I disease. In this trial, patients with pT1 or pStage I G/GEJ cancer accounted for 7% and 9.7–11.9% of the adjuvant chemotherapy group, respectively (73). Nevertheless, refining the criteria to include only patients with cT4 tumors could reduce the proportion of pT1 patients to 5% while maintaining an acceptable sensitivity level of 80.1%. Therefore, the preferential use of neoadjuvant chemotherapy for patients with cT4 G/GEJ cancer is recommended in the clinical guidelines of both Korean and Chinese medical practice (20,74).
Global collaboration with East and West
Recently, international multi-center prospective clinical trials were planned to elucidate the efficacy of perioperative chemotherapy with immune checkpoint blockade for locally advanced G/GEJ cancer. The KEYNOTE-585 trial was the first randomized phase III trial conducted globally to evaluate the add-on effect of pembrolizumab, an anti-PD1 monoclonal antibody, on perioperative chemotherapy (75). Patients with G/GEJ cancer diagnosed as cT ≥3 or N+ were eligible for this trial. Although the incidence of pathological complete response (pCR) of pembrolizumab with chemotherapy (12.9%) was significantly better than that of chemotherapy alone (2.0%), a statistically significant improvement in event-free survival (EFS), which was its primary endpoint, was not observed (median EFS: 44.4 vs. 25.3 months; HR =0.81; 0.67–0.99; P=0.0198) (76).
The MATTERHORN trial was also a global phase III trial with the same concept using durvalumab, an anti-PD-L1 monoclonal antibody. The indication of this trial was same as that of the KEYNOTE-585 (77). A statistically significant improvement in the incidence of pCR was demonstrated in the durvalumab with FLOT (19%) arm compared with that in the FLOT alone arm (7%) (P<0.00001) (78). Patients from Asian countries were included in 19% of this study population. The upcoming result of the primary endpoint, EFS, is awaited.
A randomized, phase III trial to evaluate the efficacy of HLX10, an anti-PD1 monoclonal antibody, in combination with SOX was conducted for PD-L1-positive G/GEJ cancer with cT3–4N+ in Chinese patients alone (NCT04139135). Notably, a randomized phase II/III trial (DRAGON IV/CAP 05) was conducted to investigate the efficacy of neoadjuvant immunotherapy for patients with T3–4aN+M0 G/GEJ cancer randomly assigned (1:1:1) to three arms (control arm, SOX; experimental arms, SOX plus rivoceranib with or without camrelizumab anti-PD1 monoclonal antibody) in China alone (79). An increase in the incidence of pCR of SOX with camrelizumab and rivoceranib was reported compared with that of SOX alone (18.3% vs. 5.0%) (80). Furthermore, a randomized phase II/III trial evaluating the addition of SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGF-β, to SOX was conducted in the neoadjuvant setting at multi-centers in China (NCT05149807).
Biomarker-driven personalized therapy
HER2-positive G/GEJ cancer
In the ToGA trial, the addition of trastuzumab, a monoclonal antibody targeting HER2, to standard chemotherapy improved survival and enhanced antitumor activities in HER2-positive metastatic G/GEJ cancer (81). Thus, several clinical trials using trastuzumab to target HER2-positive G/GEJ cancer were conducted in the perioperative setting (82). Two phase II trials (NEOHX and HerFLOT) demonstrated that the incidences of pCR were 9.6% and 21%, respectively, which indicated promising antitumor activity (83).
The EORTC initiated a randomized phase III trial of perioperative chemotherapy using trastuzumab with or without pertuzumab for HER2-positive locally advanced G/GEJ cancer (84). However, this trial was terminated because of slow patient accrual. The Arbeitsgemeinschaft Internistische Onkologie (AIO) group also planned a randomized clinical trial (PETRARCA) designed as phase II/III, which was conducted to evaluate dual HER2 blockade with trastuzumab and pertuzumab in combination with FLOT for locally advanced HER2-positive G/GEJ cancer with cT2–4Nany or TanyN+M0. Although a significantly higher incidence of pCR was observed in the experimental arm (35%) than in the control arm (12%), it did not proceed to phase III because of the negative result of the JACOB trial in metastatic G/GEJ cancer (85).
A single-arm phase II study assessing the feasibility of neoadjuvant CRT with trastuzumab and pertuzumab demonstrated promising antitumor activity with manageable toxicity (86). In Japan, a randomized phase II trial (JCOG1301C) using trastuzumab with SP was conducted for HER2-positive locally advanced G/GEJ cancer with extensive lymph node metastasis (87). This trial showed enhancement of both radiological (50% to 84%) and histological response (≥ grade Ib) (24% to 50%) with the addition of trastuzumab. However, this failed to complete patient enrollment. Although the survival benefit remains unknown, the HER2-direct targeted agent recapitulated the attractive antitumor activity. Global collaboration is necessary to accomplish randomized phase III trial using targeted therapy, focusing on a limited number of patients with GC.
Microsatellite-instability-high (MSI-H) or deficient-mismatch-repair (dMMR) G/GEJ cancer
The remarkable efficacy of anti-PD-1 therapy has been reported in locally advanced MSI-H rectal cancers (88). In this study, 12 patients received single-agent dostarlimab, an anti-PD-1 inhibitor, for 6 months and demonstrated a complete clinical response. Notably, no case of disease progression was observed without additional radiotherapy or surgery. MSI-H is a tumor antigen-predictive marker of immune checkpoint inhibitors (89). Therapeutic development of operable-stage MSI-H G/GEJ cancer is an attractive field. Perioperative combination therapy with FLOT with or without atezolizumab, an anti-PD-L1 monoclonal antibody, was assessed in a randomized phase II (DANTE) trial for locally advanced G/GEJ cancer. The interim analysis of the trial showed a much higher incidence of pCR of FLOT with atezolizumab (63%) as compared with that of FLOT alone (27%) in MSI-H G/GEJ cancer (90). Furthermore, the NEONIPIGA trial, a phase II study of neoadjuvant nivolumab plus ipilimumab, an anti-cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) antibody for cT2–4NanyM0 MSI-H/dMMR G/GEJ cancer, demonstrated that the incidence of pCR was 58.6% with manageable toxicities, meeting the primary endpoint (91).
More recently, a single-arm phase II (INFINITY) study of another neoadjuvant dual immune check point blockade with anti-PD1 and CTLA-4 (durvalumab and tremelimumab) was conducted for patients with cT2NanyM0 MSI-H G/GEJ adenocarcinoma (92). According to the interim analysis of cohort 1 of the INFINITY trial, only 3-month immunotherapy achieved 60% in the incidence of pCR. Non-operative management (NOM) for clinical complete response after durvalumab and tremelimumab treatment will be assessed in cohort 2. Because of a more favorable survival outcome and significantly higher sensitivity to immune therapy, MSI-H/dMMR is recognized as having a distinct set of indications and therapeutic strategies.
Future perspectives
The indications for perioperative chemotherapy have been fixed since the 2000s in Eastern countries. Before the 2010s, the treatment strategy for locally advanced G/GEJ cancer varied between the West and the East. After various efforts to improve postoperative treatments, there has been a gradual development of neoadjuvant chemotherapy in East Asia. According to the current guidelines in Korea and China, perioperative or neoadjuvant chemotherapy is the standard therapeutic option for locally advanced G/GEJ cancer, especially cT4. Particularly in China, several randomized phase III trials including new agents have been performed (93). While in Japan, surgery followed by adjuvant chemotherapy remains the standard of care for locally advanced GC, except for extensive lymph node metastasis, unless JCOG1509 meets the primary endpoint. Furthermore, NEO-JPEG (JCOG2203) is ongoing randomized phase II/III trial to elucidate the safety and efficacy of neoadjuvant chemotherapy comparing to up-front surgery for resectable locally advanced GEJ cancer (jRCTs: 031230182) (94). Eastern countries are no longer a monolith regarding perioperative treatment for locally advanced G/GEJ cancer. Furthermore, international collaborative trials have been realized sharing the same indication (cT3–4NanyM0 or N+M0).
With the progress in the global consensus on perioperative chemotherapy, the aim of chemotherapy is to move forward in the treatment of resectable G/GEJ cancer. NOM is a new horizon in this field. The advancement of ctDNA analysis with high sensitivity will enable the detection of minimal residual disease after neoadjuvant chemotherapy (95). Based on the success of immune oncology, MSI-H G/GEJ cancer is currently a distinct subset of microsatellite-stable G/GEJ cancer. Immunotherapy for MSI-H G/GEJ cancer is expected to be the closest subset of NOM in the near future.
After the emergence of trastuzumab for HER2-positive G/GEJ cancer in the metastatic setting, biomarker-driven personalized therapy has become a trend in the development of pharmacological therapy (96). Recently, the successful results of the SPOTLIGHT and GLOW trials showed that claudin18.2 highly expressed G/GEJ cancer as an independent therapeutic subset in HER2-negative unresectable advanced or recurrent G/GEJ cancer (97,98). Furthermore, bemarituzumab, a novel targeting agent for fibroblast growth factor receptor 2 isoform IIb (FGFR2b) demonstrated the promising results in the randomized phase II (FIGHT) trial for patients with FGFR2b-selected unresectable advanced or recurrent AGC (99). However, to date, no randomized phase III trial for HER2-positive G/GEJ cancer has been successful in locally advanced stage. Patient accrual for a limited number of HER2-positive patients is a common barrier to completing clinical trials (84,87). Competitive agents targeting HER2 are another crucial issue in initiating clinical trials. In the field of perioperative or neoadjuvant chemotherapy for locally advanced G/GEJ cancer, biomarker-driven personalized therapy has yet to emerge (18).
In addition to MSI status, the improved efficacy of immunochemotherapy was consistently observed in patients with G/GEJ cancer with high PD-L1 expression (76,90). Although a subgroup analysis of PD-L1 status [combined positive score (CPS) ≥10] was not prespecified, the pembrolizumab group demonstrated a better trend towards improvement in EFS among patients with CPS ≥10 (HR =0.70; 95% CI: 0.46–1.04) (76). In the future immune oncology era, molecular biomarkers might be more optimal in patient selection for perioperative or neoadjuvant chemotherapy than the conventional TNM staging system.
Trimodal approach using chemotherapy, radiation therapy and surgery is another anticipating therapeutic strategy to improve the prognosis of patients with resectable AGC. The TOPGEAR trial is the international randomized phase III trial of perioperative ECF or FLOT with or without perioperative chemoradiation for resectable AGC (100). The interim analysis of TOPGEAR demonstrated the feasibility and safety of adding chemoradiation to perioperative chemotherapy (101). Further investigation on survival benefit is awaited.
Conclusions
Evidence gaps between Western and Eastern countries still exist regarding the indications for perioperative or neoadjuvant chemotherapy. However, emerging evidence closes this gap, and the concern of upfront surgery for patients with cT4 G/GEJ cancer is shared worldwide. Recent advances in targeted therapy and immune oncology in metastatic settings may offer various pharmacological therapies for locally advanced G/GEJ cancer. Thus, a global collaboration utilizing these therapeutic options is necessary for further improvement of locally advanced G/GEJ cancer treatments. Although the first global attempt of the KEYNOTE-585 failed to establish an international standard for treatment, this study provided a common platform for experts to engage in discussion. Thus, the door to the next era has already opened. An indication with precise molecular markers and global collaboration with immunochemotherapy would make further advancements in perioperative or neoadjuvant therapy for patients with locally advanced G/GEJ cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Yasuhide Yamada) for the series “Progress and Future Direction to Treat Advanced Gastric Cancer” published in Chinese Clinical Oncology. The article has undergone external peer review.
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-129/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-129/coif). The series “Progress and Future Direction to Treat Advanced Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Eom SS, Choi W, Eom BW, et al. A Comprehensive and Comparative Review of Global Gastric Cancer Treatment Guidelines. J Gastric Cancer 2022;22:3-23. [Crossref] [PubMed]
- Japanese Gastric Cancer Association Registration Committee. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer 2006;9:51-66. [Crossref] [PubMed]
- Kim JP, Lee JH, Kim SJ, et al. Clinicopathologic characteristics and prognostic factors in 10 783 patients with gastric cancer. Gastric Cancer 1998;1:125-33. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1005-20. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999;49:33-64, 1.
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Yoshikawa T, Terashima M, Mizusawa J, et al. Four courses versus eight courses of adjuvant S-1 for patients with stage II gastric cancer (JCOG1104 [OPAS-1]): an open-label, phase 3, non-inferiority, randomised trial. Lancet Gastroenterol Hepatol 2019;4:208-16. Erratum in: Lancet Gastroenterol Hepatol 2019;4:e3. [Crossref] [PubMed]
- Yoshida K, Kodera Y, Kochi M, et al. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol 2019;37:1296-304. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Park SH, Lim DH, Sohn TS, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial☆. Ann Oncol 2021;32:368-74. [Crossref] [PubMed]
- Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 2019;30:19-33. [Crossref] [PubMed]
- Kang YK, Yook JH, Park YK, et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol 2021;39:2903-13. [Crossref] [PubMed]
- Zhang X, Liang H, Li Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol 2021;22:1081-92. [Crossref] [PubMed]
- Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) 2021;41:747-95. [Crossref] [PubMed]
- Sano T, Sasako M, Mizusawa J, et al. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg 2017;265:277-83. [Crossref] [PubMed]
- Katai H, Sasako M, Fukuda H, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 2010;13:238-44. [Crossref] [PubMed]
- Katai H, Mizusawa J, Katayama H, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:142-51. [Crossref] [PubMed]
- Kurokawa Y, Doki Y, Mizusawa J, et al. Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): a phase 3, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2018;3:460-8. [Crossref] [PubMed]
- Katai H, Mizusawa J, Katayama H, et al. Single-arm confirmatory trial of laparoscopy-assisted total or proximal gastrectomy with nodal dissection for clinical stage I gastric cancer: Japan Clinical Oncology Group study JCOG1401. Gastric Cancer 2019;22:999-1008. [Crossref] [PubMed]
- Huang C, Liu H, Hu Y, et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: Five-Year Outcomes From the CLASS-01 Randomized Clinical Trial. JAMA Surg 2022;157:9-17. [Crossref] [PubMed]
- Son SY, Hur H, Hyung WJ, et al. Laparoscopic vs Open Distal Gastrectomy for Locally Advanced Gastric Cancer: 5-Year Outcomes of the KLASS-02 Randomized Clinical Trial. JAMA Surg 2022;157:879-86. [Crossref] [PubMed]
- Etoh T, Ohyama T, Sakuramoto S, et al. Five-Year Survival Outcomes of Laparoscopy-Assisted vs Open Distal Gastrectomy for Advanced Gastric Cancer: The JLSSG0901 Randomized Clinical Trial. JAMA Surg 2023;158:445-54. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Tsuji T, Matsuda S, Takeuchi M, et al. Updates of perioperative multidisciplinary treatment for surgically resectable esophageal cancer. Jpn J Clin Oncol 2023;53:645-52. [Crossref] [PubMed]
- Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228:449-61. [Crossref] [PubMed]
- Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077-9.
- Marano L, D’Ignazio A, Cammillini F, et al. Comparison between 7th and 8th edition of AJCC TNM staging system for gastric cancer: old problems and new perspectives. Transl Gastroenterol Hepatol 2019;4:22.
- Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
-
National Comprehensive Cancer Network - Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol 2017;18:1249-60. [Crossref] [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653-60. [Crossref] [PubMed]
- Kinoshita T, Sasako M, Sano T, et al. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG 0002). Gastric Cancer 2009;12:37-42. [Crossref] [PubMed]
- Iwasaki Y, Sasako M, Yamamoto S, et al. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 2013;107:741-5. [Crossref] [PubMed]
- Yoshikawa T, Sasako M, Yamamoto S, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 2009;96:1015-22. [Crossref] [PubMed]
- Iwasaki Y, Terashima M, Mizusawa J, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer 2021;24:492-502. [Crossref] [PubMed]
- Takahari D, Ito S, Mizusawa J, et al. Long-term outcomes of preoperative docetaxel with cisplatin plus S-1 therapy for gastric cancer with extensive nodal metastasis (JCOG1002). Gastric Cancer 2020;23:293-9. [Crossref] [PubMed]
- Sato Y, Kurokawa Y, Doki Y, et al. A Phase II study of preoperative chemotherapy with docetaxel, oxaliplatin and S-1 in gastric cancer with extensive lymph node metastasis (JCOG1704). Future Oncol 2020;16:31-8. [Crossref] [PubMed]
- Jung K, Park MI, Kim SE, et al. Borrmann Type 4 Advanced Gastric Cancer: Focus on the Development of Scirrhous Gastric Cancer. Clin Endosc 2016;49:336-45. [Crossref] [PubMed]
- Vivier-Chicoteau J, Lambert J, Coriat R, et al. Development and internal validation of a diagnostic score for gastric linitis plastica. Gastric Cancer 2020;23:639-47. [Crossref] [PubMed]
- Jafferbhoy S, Shiwani H, Rustum Q. Managing Gastric Linitis Plastica: Keep the scalpel sheathed. Sultan Qaboos Univ Med J 2013;13:451-3. [Crossref] [PubMed]
- Endo K, Sakurai M, Kusumoto E, et al. Biological significance of localized Type IV scirrhous gastric cancer. Oncol Lett 2012;3:94-9. [Crossref] [PubMed]
- Aranha GV, Georgen R. Gastric linitis plastica is not a surgical disease. Surgery 1989;106:758-62; discussion 762-3. [PubMed]
- Yang B, Wu G, Wang X, et al. Discussion of modifying stage IV gastric cancer based on Borrmann classification. Tumour Biol 2013;34:1485-91. [Crossref] [PubMed]
- Götze TO, Piso P, Lorenzen S, et al. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma – the phase III “PREVENT”- (FLOT9) trial of the AIO /CAOGI /ACO. BMC Cancer 2021;21:1158. [Crossref] [PubMed]
- Kodera Y, Ito S, Mochizuki Y, et al. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer 2012;15:335-7. [Crossref] [PubMed]
- Nakayama I, Chin K, Matsushima T, et al. Retrospective comparison of S-1 plus cisplatin versus S-1 monotherapy for the treatment of advanced gastric cancer patients with positive peritoneal cytology but without gross peritoneal metastasis. Int J Clin Oncol 2017;22:1060-8. [Crossref] [PubMed]
- Yamaguchi T, Takashima A, Nagashima K, et al. Efficacy of Postoperative Chemotherapy After Resection that Leaves No Macroscopically Visible Disease of Gastric Cancer with Positive Peritoneal Lavage Cytology (CY1) or Localized Peritoneum Metastasis (P1a): A Multicenter Retrospective Study. Ann Surg Oncol 2020;27:284-92. [Crossref] [PubMed]
- Yoshida M, Ohtsu A, Boku N, et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol 2004;34:654-9. [Crossref] [PubMed]
- Hashimoto T, Nakayama I, Ohashi M, et al. Randomized phase II study comparing neoadjuvant 5-fluorouracil/oxaliplatin/docetaxel versus docetaxel/oxaliplatin/S-1 for patients with type 4 or large type 3 gastric cancer. Future Oncol 2023;19:2147-55. [Crossref] [PubMed]
- Díaz Del Arco C, Ortega Medina L, Estrada Muñoz L, et al. Are Borrmann’s Types of Advanced Gastric Cancer Distinct Clinicopathological and Molecular Entities? A Western Study. Cancers (Basel) 2021;13:3081. [Crossref] [PubMed]
- Katai H, Ishikawa T, Akazawa K, et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 2018;21:144-54. [Crossref] [PubMed]
- Terashima M, Iwasaki Y, Mizusawa J, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer 2019;22:1044-52. [Crossref] [PubMed]
- Kakeji Y, Yoshida K, Kodera Y, et al. Three-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 plus docetaxel versus S-1 alone in stage III gastric cancer: JACCRO GC-07. Gastric Cancer 2022;25:188-96. [Crossref] [PubMed]
- Endo S, Terazawa T, Goto M, et al. Neoadjuvant docetaxel, oxaliplatin and S-1 therapy for the patients with large type 3 or type 4 gastric cancer (OGSG1902): protocol of a multi-center, phase II study. BMC Cancer 2022;22:811. [Crossref] [PubMed]
- Ishigami H, Tsuji Y, Shinohara H, et al. Intraperitoneal Chemotherapy as Adjuvant or Perioperative Chemotherapy for Patients with Type 4 Scirrhous Gastric Cancer: PHOENIX-GC2 Trial. J Clin Med 2021;10:5666. [Crossref] [PubMed]
- Yoshikawa T, Kurokawa Y, Kitabayashi R, et al. A phase II study of preoperative chemotherapy with docetaxel, oxaliplatin, and S-1 followed by gastrectomy with D2 plus para-aortic nodal dissection for gastric cancer with extensive lymph node metastasis: JCOG1704. J Clin Oncol 2023;41:354. [Crossref]
- Al-Batran SE, Homann N, Pauligk C, et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol 2017;3:1237-44. [Crossref] [PubMed]
- Al-Batran SE, Goetze TO, Mueller DW, et al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction – a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer 2017;17:893. [Crossref] [PubMed]
- Fukagawa T, Katai H, Mizusawa J, et al. A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer 2018;21:68-73. [Crossref] [PubMed]
- Mo DC, Qin L, Ye LJ. Neoadjuvant Docetaxel, Oxaliplatin, and S-1 in Resectable Advanced Gastric Cancer. J Clin Oncol 2021;39:3883-4. [Crossref] [PubMed]
- Kang YK, Kim HD, Yook JH, et al. Neoadjuvant docetaxel, oxaliplatin, and s-1 plus surgery and adjuvant s-1 for resectable advanced gastric cancer: Final survival outcomes of the randomized phase 3 PRODIGY trial. J Clin Oncol 2023;41:4067. [Crossref]
- Zhang X, Li Z, Liang H, et al. Overall survival of perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy: An updated analysis of RESOLVE trial. ESMO Congress 2023. Madrid, Spain, 2023.
- Wang X, Li S, Xie T, et al. Early results of the randomized, multicenter, controlled evaluation of S-1 and oxaliplatin as neoadjuvant chemotherapy for Chinese advanced gastric cancer patients (RESONANCE Trial). J Clin Oncol 2020;38:280. [Crossref]
- Wang X, Li S, Sun Y, et al. The protocol of a prospective, multicenter, randomized, controlled phase III study evaluating different cycles of oxaliplatin combined with S-1 (SOX) as neoadjuvant chemotherapy for patients with locally advanced gastric cancer: RESONANCE-II trial. BMC Cancer 2021;21:20. [Crossref] [PubMed]
- Kim HD, Lee JS, Yook JH, et al. Radiological criteria for selecting candidates for neoadjuvant chemotherapy for gastric cancer: an exploratory analysis from the PRODIGY study. Gastric Cancer 2022;25:170-9. [Crossref] [PubMed]
- Kim TH, Kim IH, Kang SJ, et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J Gastric Cancer 2023;23:3-106. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Fuchs CS, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol 2019;15:943-52. [Crossref] [PubMed]
- Shitara K, Rha SY, Wyrwicz LS, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol 2024;25:212-24. [Crossref] [PubMed]
- Janjigian YY, Van Cutsem E, Muro K, et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol 2022;18:2465-73. [Crossref] [PubMed]
- Janjigian YY, Al-Batran SE, Wainberg ZA, et al. Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): Interim results of the global, phase III MATTERHORN study. ESMO Congress 2023. Madrid, Spain, 2023.
- Zheng Y, Wang Z, Yan C, et al. Protocol for a randomized controlled trial of perioperative S-1 plus oxaliplatin combined with apatinib and camrelizumab in patients with resectable, locally advanced gastric or gastroesophageal junction adenocarcinoma. Ann Transl Med 2020;8:1684. [Crossref] [PubMed]
- Li C, Zheng Y, Shi Z, et al. Perioperative camrelizumab I combined with rivoceranib I and chemotherapy (chemo) versus chemo for locally advanced resectable gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The first interim analysis of a randomized, phase III trial (DRAGON IV). ESMO Congress 2023. Madrid, Spain, 2023.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Lavacchi D, Fancelli S, Buttitta E, et al. Perioperative Tailored Treatments for Gastric Cancer: Times Are Changing. Int J Mol Sci 2023;24:4877. [Crossref] [PubMed]
- Hofheinz RD, Hegewisch-Becker S, Kunzmann V, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer 2021;149:1322-31. [Crossref] [PubMed]
- Wagner AD, Grabsch HI, Mauer M, et al. EORTC-1203-GITCG – the “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer 2019;19:494. [Crossref] [PubMed]
- Tabernero J, Hoff PM, Shen L, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 2018;19:1372-84. [Crossref] [PubMed]
- Stroes CI, Schokker S, Creemers A, et al. Phase II Feasibility and Biomarker Study of Neoadjuvant Trastuzumab and Pertuzumab With Chemoradiotherapy for Resectable Human Epidermal Growth Factor Receptor 2-Positive Esophageal Adenocarcinoma: TRAP Study. J Clin Oncol 2020;38:462-71. [Crossref] [PubMed]
- Tokunaga M, Machida N, Mizusawa J, et al. A randomized phase II trial of preoperative chemotherapy of S-1/CDDP with or without trastuzumab followed by surgery in HER2 positive advanced gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1301C (Trigger Study). J Clin Oncol 2022;40:285. [Crossref]
- Cercek A, Lumish M, Sinopoli J, et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med 2022;386:2363-76. [Crossref] [PubMed]
- Shimozaki K, Nakayama I, Hirota T, et al. Current Strategy to Treat Immunogenic Gastrointestinal Cancers: Perspectives for a New Era. Cells 2023;12:1049. [Crossref] [PubMed]
- Al-Batran SE, Lorenzen S, Thuss-Patience PC, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase Iib trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol 2022;40:4003. [Crossref]
- André T, Tougeron D, Piessen G, et al. Neoadjuvant Nivolumab Plus Ipilimumab and Adjuvant Nivolumab in Localized Deficient Mismatch Repair/Microsatellite Instability-High Gastric or Esophagogastric Junction Adenocarcinoma: The GERCOR NEONIPIGA Phase II Study. J Clin Oncol 2023;41:255-65. [Crossref] [PubMed]
- Pietrantonio F, Raimondi A, Lonardi S, et al. INFINITY: A multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol 2023;41:358. [Crossref]
- Tong X, Zhi P, Lin S. Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer. J Gastric Cancer 2023;23:182-93. [Crossref] [PubMed]
- Kita R, Yanagimoto Y, Imazeki H, et al. Protocol digest of a randomized controlled adaptive Phase II/III trial of neoadjuvant chemotherapy for Japanese patients with oesophagogastric junction adenocarcinoma: Japan Clinical Oncology Group Study JCOG2203 (NEO-JPEG). Jpn J Clin Oncol 2024;54:206-11. [PubMed]
- Parsons HA, Blewett T, Chu X, et al. Circulating tumor DNA association with residual cancer burden after neoadjuvant chemotherapy in triple-negative breast cancer in TBCRC 030. Ann Oncol 2023;34:899-906. [Crossref] [PubMed]
- Nakamura Y, Kawazoe A, Lordick F, et al. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol 2021;18:473-87. [Crossref] [PubMed]
- Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2023;401:1655-68. Erratum in: Lancet 2023;402:290; Lancet 2024;403:30. [Crossref] [PubMed]
- Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med 2023;29:2133-41. [Crossref] [PubMed]
- Wainberg ZA, Enzinger PC, Kang YK, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 2022;23:1430-40. [Crossref] [PubMed]
- Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015;15:532. [Crossref] [PubMed]
- Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 2017;24:2252-8. [Crossref] [PubMed]


