Chinese physician perception on the treatment of chemotherapy-induced anemia: online cross-section survey study
Highlight box
Key findings
• The physician-reported dose reduction or delay (DDR) was 24% corresponding to the previous result 26.4% in a survey based on patient data. However, the study indicated that the initial hemoglobin (Hb) level for erythropoiesis-stimulating agent treatment is 80 g/L in clinical practice which is different from guidelines’ recommendation.
What is known and what is new?
• The prevalence, incidence and treatment patterns of anemia in cancer patients has been studied since that the anemia may decrease quality of life, affect anti-cancer treatment and shorten survival.
• We conducted the first survey to look into the physicians’ insights of DDR in chemotherapy induced anemia (CIA) and the considerations of CIA management in China.
What is the implication, and what should change now?
• The result of the survey showed the unmet medical need and treatment gaps in CIA. In the future, the Chinese physicians should pay more attention to cancer patients with Hb level80–100 g/L in clinical practice.
Introduction
Anemia, which is one of the most prevalent complications of cancer and cancer treatment, can be classified into two forms: (I) the occurrence of anemia during treatment as an adverse event of anticancer therapy [chemotherapy-induced anemia (CIA)]; or (II) anemia occurrence as a manifestation of the disease itself [cancer-related anemia (CRA)], resulting from systemic processes and immune system activation in cancer (1). CIA generally has a higher prevalence, with about 89.5% of patients with cancer developing anemia during chemotherapy. Anemia can result in a decreased quality of life (QoL), and the dose reduction or delay (DDR) of chemotherapy. In a post-hoc analysis which compared the anemia vs. non-anemia in cancer patients, they found the anemia show a negative impact in QoL with statistically significant and clinically relevant difference (anemia vs. non-anemia: 45.6 vs. 58, respectively; mean difference: −12.4, P<0.001) (2). A randomized controlled trial (RCT) which assessed the impact of the relative dose intensity (RDI) on patient outcome of non-Hodgkin’s lymphoma (NHL) patients treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), the result found that those treated with RDI >70% had a longer 5-year survival than those treated with RDI ≤70%. It is essential to prompt clinical management of CIA to avoid modifications of scheduled chemotherapy resulting in which the poor prognosis and the decreased overall survival of cancer patients (3).
RBC transfusions i.v.ironi.v.iron It is usually recommended by most guidelines that cancer patients with hemoglobin (Hb) level <8 g/dL receive the RBC transfusions, those with an Hb <10 g/dL may consider erythropoiesis-stimulating agents (ESAs) therapy and those with iron deficiency [ID, defined as transferrin saturation (TSAT) <20% or serum ferritin (SF) <100 ng/mL] should receive iron treatment (4).
Different form global guidelines, China Society of Clinical Oncology (CSCO) guideline recommended RBC transfusions with Hb <6 g/dL due to the blood shortage problems in China (5). RBC transfusions can rapidly increase Hb resulting in a fast recovery in QoL and anemia related symptoms. Risks associated with RBC transfusions include transfusion-related reactions (e.g., hemolytic, non-hemolytic, febrile, lung injury), transfusion associated circulatory overload (TACO), and bacterial contamination (6).
It is a controversial question that the proper use of ESAs in CIA. Numerous RCTs have proved ESAs can reduce RBC transfusions rate, sustained correction of anemia and improvement in QoL. ESAs may prompt tumor growth, decreased survival in cancer anemia patients along with high risks of thromboembolic events (7).
In CIA patients with absolute ID, IV iron monotherapy has demonstrated efficacy and safety with no clinically significant adverse events especially with third generation compounds. In CIA patients with functional ID, i.v.iron in combination with ESAs for CIA has the capacity to increase Hb level, improve QoL, reduce transfusion requirements and lower ESAs doses. The toxicity of i.v.iron included immunological effects, transfusion reactions, pains, allergic reaction, infection etc. (7). In terms of oral iron, European Society of Medical Oncology (ESMO) guideline indicated that oral iron did not result in better outcomes compared with control arm with no iron (8). National Comprehensive Cancer Network (NCCN) guideline pointed out that many CIA patients do not respond to oral iron (6). CSCO guideline recommended oral iron (Evidence 2A) and noted that oral iron is convenient but worse treatment outcome compared with i.v.iron (5).
Therefore, the management of CIA remains challenging: the potential risk and benefits in providing patient-centered care need to be balanced, the disease is multifactorial, and each of three above-mentioned recommended treatments has a unique set of strengths and limitations. The well-known European Cancer Anaemia Survey (ECAS) discussed the incidence, prevalence and principles of treatment of CIA as clinical practice based on patient data in 2004 (9). Song et al. published the survey conducted in 2012 provided the prevalence and treatment of CRA data in China (10). However, most survey based on the patient data could not reveal the process of evaluation and decision-making for CIA treatment from a clinician’s perspective. As the comparison of CSCO, NCCN and ESMO guidelines, the standard of CIA treatment in China will vary from United States and Europe like the initial Hb for RBC transfusions. In order to better understand the diagnosis, treatment, and unmet medical needs of patients with the clinical practice of CIA in China, the China Medical Education Association (CMEA), in conjunction with Cancer Hope Medium, initiated the first national survey of Chinese physicians regarding the diagnosis and treatment of CIA. We present this article in accordance with the SURGE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-9/rc).
Methods
Data sources and statistics
According to China Health Statistical Yearbook 2022 (11), there were 28.8 thousand doctors practicing in cancer hospitals in China, and based on the assumption of 95% confidence level and 5% margin of error, 265 samples were calculated using surveyplanet.com. And eventually 301 questionnaires were collected. The questionnaire was developed and standardized according to CSCO guidelines.
CMEA sent an online survey of 12-item questionnaire (Appendix 1) via wjx.cn from 1st Sep. to 22nd Oct. 2022 aiming to gather questionnaires in China nationwide. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all individual participants. The questionnaire evaluated the impact of anemia on chemotherapy interruption, the initial treatment, the target Hb of CIA in clinical practice and the current status of ESA prescription in clinical practice, as well as scored the reasons for not using ESA (including safety issues, drug access in practice or adherence) and the risk options of the current treatment, including ESA, RBC transfusions and i.v.iron.
Statistical analysis
Descriptive statistics were applied to describe the investigated hospitals, physicians, prescription behavior, DDR, and treatment concept. The collected data were entered into a database and are presented as the number and percentage or as the median and range, as appropriate.
The questionnaires were statistically analyzed, which included the description of the distribution of relevant answers and the frequency and incidence of relevant items, among other issues. If a question was not selected or vacant, the response of the questionnaire would be excluded from the final analysis. Stratified analysis was performed according to the hospital level, including tier IIIA, IIIB, IIA, and IIB. The scoring scheme (1–10 points) for responses was as follows: 0 point = not filled in, 1–3 points = “disagree”, 4–6 points = “generally agree”, 7–9 points = “highly agree”, and 10 points = “completely agree”.
Results
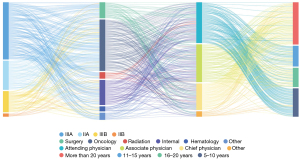
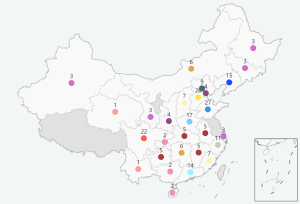
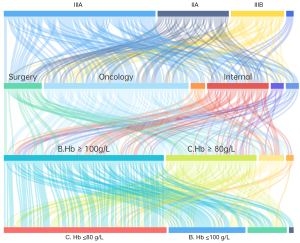
A total of 331 questionnaires among 5,000 web visits were gathered, covering 247 hospitals in 29 provinces across China (Figure 1) (provinces/autonomous regions/municipalities directly under the central government/special administrative regions), of which 130 (53%) were tier IIIA hospitals, 50 (20%) were tier IIIB hospitals, 59 (24%) were tier IIA hospitals, and 8 (3%) were tier IIB hospitals. The distribution of the hospital level of the respondents is shown in Figure 2.
Among the responses, 217 (63%) were from oncology specialists, including 128 (39%) from oncology departments of tier III hospitals and 89 (27%) from oncology departments of hospitals at other levels. Of the oncologists who participated in the survey, 85 (39%) were attending physicians, 60 (28%) were associate chief physicians, 52 (24%) were chief physicians, and 20 (9%) had others positions; moreover, 63 (29%) had worked for more than 20 years, 59 (27%) for 5 to 10 years, 38 (18%) for 11 to15 years, 36 (17%) for 16 to 20 years, and 21 (10%) for less than 5 years (Figure 2).
CIA diagnosis and treatment concepts
The questionnaire included items on the occurrence of anemia leading to chemotherapy dose reduction or chemotherapy delay, factors affecting the treatment of anemia, and the usage of ESAs, i.v.iron products, blood transfusion, and other anemia treatments.
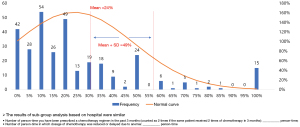
Of the 331 retrieval questionnaires, 315 indicated a prescription of chemotherapy within the past 3 months, and the average number of patients who had been prescribed chemotherapy within the past 3 months (calculated as 2 if the same patient received 2 doses of chemotherapy in 3 months) was 52 [standard deviation (SD) 82]; among the chemotherapy-prescribed patients, the mean number of those who developed anemia leading to DDR was 9 (SD 16), and the frequency of chemotherapy DDR due to anemia was 24% (SD 25%). The mean event number of chemotherapy DDR due to anemia was 8 (SD 15), and the proportion of DDR was 23% (SD 26%) in tier IIIA hospitals, while the mean event number of DDR was 10 (SD 16) and the proportion was 25% (SD 24%) in the other hospital levels (Figure 3).
In addition, in a full analysis of 328 valid questionnaires, 60% (n=195) of responding physicians rated Hb ≥100 g/Las favorable without limiting treatment availability and 28% rated (n=92) Hb ≥80 g/L as favorable without limiting treatment availability. The distribution of physicians investigated in the tier IIIA hospitals and other hospital levels on this issue was consistent with the overall distribution (Figure 4).
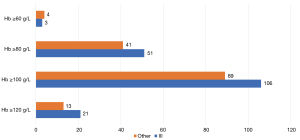
Evaluation, management, and treatment timing of anemia
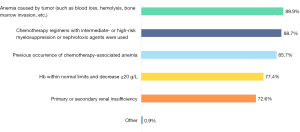
Among the 328 valid questionnaires, the top three factors deemed of special interest for anemia management were “anemia due to the tumor disease itself” (n=295, 89.9%), “chemotherapy regimens or nephrotoxic drugs for intermediate- and high-risk myelosuppression” (n=291), and “previous chemotherapy”. Other issues physicians were concerned about were “Hb within the standard range but decreasing by ≥20 g/L after chemotherapy” (n=254) and “primary or secondary renal insufficiency” (n=238) (Figure 5).
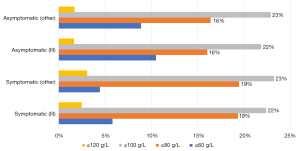
For chemotherapy patients with symptoms of anemia, the Hb range (≤60 g/L, ≤80 g/L, ≤100 g/L, ≤120 g/L) considered necessary for anemia correction that was most commonly selected by responding physicians was <80 g/L(n=149, 45%), which was followed by <100 g/L (n=127, 39%); for chemotherapy patients without symptoms of anemia, the most common Hb range that initiated anemia was also <80 g/L (n=146, 45%), which was followed by <100 g/L (n=106, 32%). However, when Hb ≤60 g/L, the majority of responding physicians prioritized treating symptomatic patients for anemia but not asymptomatic patients (20% vs. 10%). The distribution of views of physicians investigated in tier IIIA hospitals and other levels of hospitals were similar to the overall distribution (Figure 6).
ESA treatment
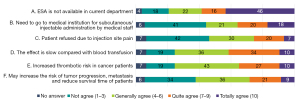
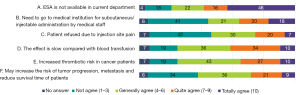
Of the 327 physicians with valid responses concerning ESAs, 32.4% (n=106) indicated that their hospitals never used ESAs for CIA treatment (Figure 7). The main reasons for not using ESA to correct anemia (the ranking of views in this survey was weighted according to the option score, total number of questionnaires, and total score) were as follows: “ESA is not currently available in our department”, “The effect is slow compared with blood transfusion”, and “There is increased thrombotic risk in cancer patients”. 32.4% of the respondents had never used ESAs (Figure 8). The proportion of respondents from tier IIIA hospitals who strongly agreed that “ESA is not currently available in our department” was lower than that of hospitals at other levels (38% vs. 50%), while the proportion who strongly agreed that “The effect is slow compared with blood transfusion” was higher than that of other hospital levels (34% vs. 29%).
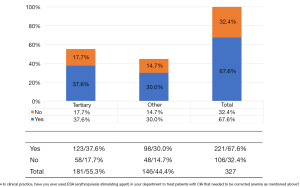
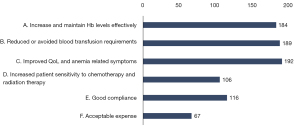
Of the 327 respondents, 67.6% (n=221) indicated that they had used ESAs for anemia correction (Figure 8). Of these respondents, 58% (n=128) believed that Hb ≤80 g/Lrequired the initiation of ESAs for anemia to continue chemotherapy. The most commonly cited advantages of ESA medication were “improving patients’ QoL and anemia-related symptoms” (n=192), “reducing or avoiding transfusion requirements” (n=189), and “effectively increasing and maintaining Hb levels” (n=184) (Figure 9).Respondents from both tier III hospitals and other hospitals also supported the need to initiate ESAs when Hb <80 g/Land held similar views on the advantages of ESA medication. Interestingly, most of the physicians chose Hb ≥10 g/L as the preferred benefited Hb for chemotherapy but initiated ESAs treatment mostly at Hb <80 g/L not 100 g/L (Figure 10).
The most commonly cited reasons for insufficient prescription of ESAs were the following: “The effect is slow compared with blood transfusion”, “There is uncreased thrombotic risk”, and “Some patients do not respond” (Figure 11).
I.v.iron supplementation
Among the 326 physicians with valid responses concerning i.v.iron, the main advantages of i.v.iron use for anemia correction were indicated to be “improved hematopoietic response and reduced blood transfusion” (35%), “rapid correction of i.v.iron deficiency” (30%), “improved response to ESA therapy” (21%), and “no gastrointestinal-related adverse effects” (14%). The distribution of views of the tier III hospitals and the hospitals at other levels was similar to that of the overall population.
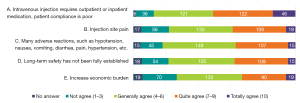
The top three reasons for insufficient clinical use of i.v.iron were “intravenous injection requiring outpatient or inpatient medication, with poor patient compliance”, “injection site pain”, and “numerous adverse reactions, such as hypotension, nausea, vomiting, diarrhea, pain, and hypertension”. Among the tier IIIA hospitals, the second most common reason for restrained use of i.v.iron was “Long-term safety has not been fully established”. The distribution of views at other hospital levels was consistent with that of the overall population (Figure 12).
RBC transfusions
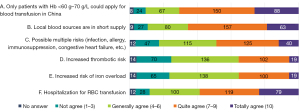
Among the 331 physicians with valid responses concerning blood transfusion, the main challenges encountered by blood transfusion in correcting anemia were “Only patients with Hb <60–70 g/L can apply for blood transfusion in China”, “Local blood sources are in short supply”, and “Hospitalization is required for RBC transfusions”. The views of tier IIIA hospitals and other levels on this issue were distributed similarly to that of the overall population (Figure 13).
Discussion
This is the first study to conduct a survey of physicians regarding CIA diagnosis and treatment in China and involved 31 provinces and 247 hospitals across the country. The surveyed population was representative, with 63% of the physician respondents being from oncology specialties, different hospital tiers, physician seniority, and occupations being covered. The questionnaire was primarily aimed at characterizing the current hotspots in CIA diagnosis and treatment, including the analysis of the Hb cutoff value for anemia initiation and treatment as well as the advantages and disadvantages of conventional treatment options such as ESAs, i.v.iron, and blood transfusion. Previous web-based surveys published in 2012 mainly focused on the perception of CIA in Italy and the understanding of possible deviations in clinical practice management of thromboembolism events, cancer progression, or decreased overall survival probably related to ESA use (12). Since then, after nearly a decade of development, cancer treatment options have proliferated, with emerging target therapies, immunotherapies and antibody-drug conjugates, being able to prolong the survival time of patients with cancer and drastically changing the treatment landscape. However, chemotherapy still remains the backbone of treatment in patients with cancer who are negative for actionable molecular biomarkers.
Chemotherapy effectuates temporary or permanent marrow suppression to cause anemia via treatment-related infections or off-target effects on RBC production damage (13).Anemia can worsen tumor hypoxia, precipitating disease progression and metastases and reducing tumor sensitivity to antitumor treatment (14). For instance, anemia can reduce the patients’ tolerance to chemotherapy, leading to its interruption. The incidence of anemia during chemotherapy may be as high as 89.5%. In our study, the survey indicated that the average frequency of chemotherapy DDR due to anemia was 24%, which is similar to a previously reported DDR of 26.4% in all chemotherapy cycles (3). A previous study reported that 60% of patients with chemotherapy DDR had concurrent anemia, which was a greater proportion than that of those with concurrent chemotherapy-induced neutropenia (CIN); however, the study did not examine whether anemia led to DDR (3,15). Our survey suggested that anemia leads to chemotherapy DDR in nearly a quarter of patients, indicating that physicians should not only pay attention to the management and prevention of neutropenia but also take CIA management into consideration. Moreover, 60% and 28% of percent physicians indicated that Hb ≥100 and Hb ≥80 g/L, respectively, were more conducive to completion of the scheduled chemotherapy. Interestingly, a 2012 survey revealed that 20% of Chinese cancer patients with Hb ≤80 g/L could not receive treatment directly, which is consistent with the DDR of 24% in our study (10). In addition, although Hb ≥80 g/L is the recommended lower limit for chemotherapy in the relevant guideline (5), two-thirds of physicians in our survey believed Hb ≥100 g/Lto be more conducive to chemotherapy.
Regarding the initiation Hb for anemia treatment, nearly half (45%) of the physicians considered Hb <80 g/Lto be suitable regardless of whether patients with CIA had symptoms of anemia, which is the lowest Hb value recommended by the CSCO Cancer-Associated Anemia Guideline (2022 edition), which suggests an Hblevel ≤110 g/L and for ESA treatment to be initiated when Hb ≤100 g/L (5).
In this survey, 32.4% of physician respondents indicated that they did not use ESAs to correct anemia due to the concern of the slow onset of action, increased risk of tumor progression, and inaccessibility of ESAs, with the latter response being stated in 30% of respondents in tier IIIA hospitals and 50% of those from hospitals of other levels. These reasons differ considerably from those provided by US physicians, who indicated a disinclination to using ESA due to tumor progression and considerations of black box warnings and risk evaluation and mitigation strategy (16).
The advantages of i.v.iron therapy are the rapid correction of ID, improvement in response to ESA therapy, hematopoietic response, and reduction in blood transfusion; meanwhile, the disadvantages are gastrointestinal irritation with oral iron, the need for outpatient or inpatient medication with i.v.iron supplementation, poor patient compliance, and a broad variety of adverse effects (such as hypotension, nausea, vomiting, diarrhea, pain, hypertension, dyspnea, pruritus, and headache). According to our survey, the advantages of i.v.iron therapy in both the tier IIIA hospitals and hospitals at other levels mainly included the improvement of hematopoietic reactions, reduction of blood transfusion, and rapid correction of ID. Meanwhile, reasons for restrained use from physicians of all hospital levels included “poor compliance of intravenous medication” and “more adverse reactions”; however, physicians from tier IIIA hospitals also indicated particular concern for the safety of long i.v.iron, as this can cause vascular extravasation leading to local swelling, pain, and additional injuries and thus needs to be administered as outpatient or inpatient care. A lack of nursing experience of i.v.iron in primary hospitals may be one of the reasons why the respondent physicians in the other levels of hospitals had a greater concerning for “injection site pain” (Appendix 1).
The advantage of blood transfusion is that it can rapidly increase Hb levels and can be used in patients who do not respond to ESA therapy. Meanwhile, the disadvantages include the need for indications for blood transfusion (guidelines mainly recommend patients with Hb <60 g/L); the need for inpatient blood transfusion; the short duration of Hb maintenance; the risk of iron overload, allergy, immunosuppression, congestive heart failure, thrombosis, and other issues (17); and limitations imposed by blood source tension, blood transfusion matching, etc. In this survey, both the overall and stratified analyses identified the main challenges of transfusion to be the “limitation of transfusion indications”, “shortage of local blood sources”, and “need for hospitalization for RBC transfusions”. The distribution of views on blood transfusion was equivalent between tier III hospitals and other levels of hospitals, suggesting that the challenges faced in blood transfusion are widespread in hospitals at all levels in China.
Conclusions
This study is the first to conduct a large-scale survey on the diagnosis and treatment of CIA in China, covering a wide geographical distribution with an expansive diversity and representativeness of hospitals and physicians. We found that in China, nearly one-quarter of patients undergoing chemotherapy with concurrent anemia may experience interruption of chemotherapy and that the initiation of anemia treatment is not adequately timed. In treating CIA, most physicians prioritize the completion of chemotherapy via Hb level over treating the symptoms of anemia. Compared with the severity and high risks of rapidly decreasing white blood cell count and/neutrophils and platelet ratios, those related to RBC proceed relatively slowly, with the symptoms of anemia being delayed or tolerable, all of which may explain why anemia may be relatively overlooked. However, it should be noted that anemia can cause the interruption of chemotherapy, which can increase the cost of treatment, reduce the QoL, and affect the overall survival of patients with cancer.
Although most physicians realize that a higher Hb level (e.g., ≥100 g/L) is more conducive to the completion of scheduled chemotherapy, the anemia treatment rate of patients with cancer in China remains low, indicating that the limitation of current anemia treatment may be a leading cause of insufficient treatment of anemia. In this survey, up to one-third of the responding physicians had not used ESA in their clinical practice, and in addition to poor accessibility, efficacy, safety, and treatment adherence of ESA, other reasons for physician reluctance to use ESA included its slow onset, increased risk of thrombosis, and lack of patient response. Other anemia treatment options also have challenges of insufficient clinical application; for example, i.v.iron may involve outpatient/inpatient treatment, injection site pain, and considerable adverse reactions, while blood transfusion is limited by “indications for blood transfusion”, “lack of donor blood resources”, and “the need for inpatient treatment” (Appendix 1). Therefore, in order to better benefit patients, it is necessary to further develop new drugs for the treatment of anemia and to further standardize CIA diagnosis and treatment among Chinese physicians.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (grant No. 82373359); CSCO Cancer Research Fund (grant Nos. Y-HR2020MS-0298 and Y-pierrefabre202102-0066); and Beijing Science and Technology Innovation Medical Development Foundation Key Project (grant No. KC2022-ZZ-0091-6); and China Medical Education Association.
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-9/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-9/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-9/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xu H, Xu L, Page JH, et al. Incidence of anemia in patients diagnosed with solid tumors receiving chemotherapy, 2010-2013. Clin Epidemiol 2016;8:61-71. [Crossref] [PubMed]
- Barca-Hernando M, Muñoz-Martin AJ, Rios-Herranz E, et al. Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis. Cancers (Basel) 2021;13:2517. [Crossref] [PubMed]
- Family L, Xu L, Xu H, et al. The effect of chemotherapy-induced anemia on dose reduction and dose delay. Support Care Cancer 2016;24:4263-71. [Crossref] [PubMed]
- Tartarone A, Lerose R, Tartarone M. Erithropoiesis stimulating agents in the treatment of chemotherapy induced anemia: what do guidelines say? AME Med J 2023;8:32. [Crossref]
- Ma J, Wang J. Guidelines of Chinese Society of Clinical Oncology (CSCO): Clinical practice in tumor-related anemia 2022. Beijing: People’s Medical Publishing House; 2022.
- Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines® Insights: Hematopoietic Growth Factors, Version 1.2022. J Natl Compr Canc Netw 2022;20:436-42. [Crossref] [PubMed]
- Abdel-Razeq H, Hashem H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol 2020;145:102837. [Crossref] [PubMed]
- Aapro M, Beguin Y, Bokemeyer C, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29:iv96-iv110. Erratum in: Ann Oncol 2018;29:iv271. [Crossref] [PubMed]
- Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293-306. [Crossref] [PubMed]
- Song Z, Lu S, Feng J, et al. Prevalence and Treatment of Cancer-related Anemia: A Nationwide Survey in China. China Cancer 2019;28:718-22.
- National Bureau of Statistics of China. China Health Statistical Yearbook 2022. Beijing: China Statistics Press; 2022.
- Cortinovis D, Beretta G, Piazza E, et al. Chemotherapy-induced anemia and oncologist perception on treatment: results of a web-based survey. Tumori 2013;99:45-50. [Crossref] [PubMed]
- Anand S, Burkenroad A, Glaspy J. Workup of anemia in cancer. Clin Adv Hematol Oncol 2020;18:640-6. [PubMed]
- Madeddu C, Gramignano G, Astara G, et al. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front Physiol 2018;9:1294. [Crossref] [PubMed]
- Dinan MA, Hirsch BR, Lyman GH. Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J Natl Compr Canc Netw 2015;13:e1-7. [Crossref] [PubMed]
- Hoque S, Chen BJ, Schoen MW, et al. End of an era of administering erythropoiesis stimulating agents among Veterans Administration cancer patients with chemotherapy-induced anemia. PLoS One 2020;15:e0234541. [Crossref] [PubMed]
- The society of chemotherapy, Chinese Anti-Cancer Association. A consensus on the clinical diagnosis, treatment, and prevention of cancer- and chemotherapy-related anemia in China (2019 edition). Chinese Journal of Clinical Oncology 2019;46:869-75.