Systemic therapy for biliary cancers
Introduction
Because biliary cancers are often asymptomatic until late in the course of the disease, they frequently manifest at an advanced and unresectable stage. Gallbladder cancer (GBC) frequently manifests as an incidental finding during laparoscopic surgery for ostensibly benign disease. Intrahepatic cholangiocarcinoma (IHCC) may arise in close approximation to the portal vein and in turn cause portal vein occlusion as it enlarges. In addition to local invasion in GBC, other negative prognostic factors in GBC include liver and lymph node involvement (1,2). In patients with IHCC negative prognostic signs include vascular invasion, multiple tumors, disease-positive tumor margins, large size, and lymph node metastases (3).
Many of the issues that pertain to chemotherapy trials in biliary cancers relate to their rarity with sparse randomized phase III data available to guide chemotherapy. In addition, many studies of biliary tract cancer to date have enrolled patients with both GBC and intra/extrahepatic cholangiocarcinoma, but are underpowered to evaluate differential activity of therapy at different anatomical sites along the biliary tree with rare exceptions such as the ABC-02 trial for which subgroup analysis was possible for gallbladder and cholangiocarcinoma subgroups (4). Developments in molecular profiling have led to a more sophisticated understanding of the genetic alterations driving these clinically distinct malignancies and going forward segregation by anatomic or molecular subtype will be optimal.
Role of systemic therapy
In the mid 1990s it was shown that overall survival (OS) was improved with the use of chemotherapy (5-fluorouracil and etoposide) when combined with best supportive care (BSC) as compared to BSC alone in advanced biliary cancer (5). In this trial over a 4-year period 90 patients were enrolled with thirty seven biliary cancer patients with the remainder pancreatic tumors. In the chemotherapy group median OS was seen 6 versus 2.5 months in the BSC group, (P<0.01). A benefit was seen in both pancreas and biliary cancers. Over subsequent years a variety of strategies have been assessed using single agent, doublet and triplet regimens with the goal of improving survival while maintaining quality of life in the palliative setting. In this review we report on the current data available for systemic therapy in biliary cancers in the metastatic and adjuvant setting as well as future strategies.
Systemic therapy for locally advanced or metastatic disease
There have been a number of non-randomized phase II trials and a paucity of phase III trials for locally advanced or metastatic disease in biliary cancer that have incorporated platinum based (Table 1) and non-platinum based chemotherapy (Table 2). The largest randomized phase III trial for advanced biliary cancer to date is the ABC-02 trial, which defined current standard of care therapy (4). In this multicenter study, 410 patients with locally advanced or metastatic cholangiocarcinoma, GBC or ampullary cancer were randomized to receive cisplatin (25 mg/m2) followed by gemcitabine (1,000 mg/m2) on day 1 and 8, every 21 days or gemcitabine alone (1,000 mg/m2) on 1, 8, and 15, every 28 days (4). Treatment was given for up to 6 months only in each arm. The rationale for this combination was based on promising data initially seen with cisplatin/gemcitabine combination in the ABC-01 trial (6); a randomized phase II study which evaluated gemcitabine and cisplatin versus gemcitabine alone with an improvement in both time to progression (TTP) and a 6-month progression free survival (PFS). In the ABC-02 trial the median PFS and response rates were higher in the cisplatin plus gemcitabine combination group. The primary median OS was 11.7 months with cisplatin and gemcitabine as compared to 8 months with gemcitabine alone [hazard ratio (HR) 0.64; 95% CI, 0.52–0.80; P<0.001]. The combination was safe with rates of neutropenia higher in the doublet arm (25.3% vs. 16.6%, P=0.03), however infection rates with associated neutropenia were comparable. The activity of gemcitabine plus cisplatin was also assessed in a Japanese study of 83 patients as compared to gemcitabine alone with survival times approximating those in the ABC-02 trial with a median OS of 11.2 months in the cisplatin plus gemcitabine combination group (7). Median PFS for cisplatin and gemcitabine combination was 8.0 months in the ABC-02 trial as compared to 5.8 months in the Japanese trial. This may be explained by the differing imaging schedules where radiological imaging was performed at week 12 and at week 24 in those who completed therapy followed by three monthly assessments thereafter for patients in the ABC-02 trial, as compared to six weekly in the Japanese trial. This schedule in the ABC-02 trial potentially limits the validity of PFS results in view of prolonged periods between imaging and discontinuation of therapy after 6 months. Presently, cisplatin plus gemcitabine has become standard of care therapy for patients with advanced biliary cancers, although in practice the regimen is frequently modified to use a lower dose of cisplatin and to continue treatment longer than 6 months in patients with stable or responding disease who are tolerating treatment well.
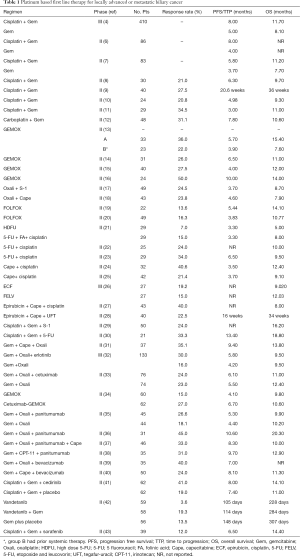
Full table
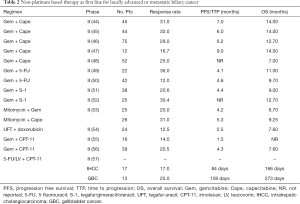
Full table
Oxaliplatin has been assessed in combination with gemcitabine in four previous non-randomized trials in advanced biliary cancer (13-16). A multicenter trial evaluated GEMOX utilizing gemcitabine (1,000 mg/m2) infused at 10 mg/m2 over 30 minutes on day 1 followed by oxaliplatin (100 mg/m2) on day two repeated every 14 days (13). Patients were divided into two groups, those with a good performance status (PS) of 0–2 and a bilirubin less than 2.5 times normal and those with a poor PS (>2) and/or had an elevated bilirubin or prior systemic therapy. As anticipated, median OS was twice that in the good PS patients as compared to the poor PS patients, 15.4 versus 7.6 months with therapy tolerable in both groups. A phase II trial of gemcitabine (1,000 mg/m2) on days 1, 8, 15 and oxaliplatin 100 mg/m2 on days 1 and 15 repeated every 28 days in 31 patients demonstrated a partial response rate of 26% and median OS of 11 months (14). A biweekly regimen of gemcitabine 1,000 mg/m2 followed by oxaliplatin 85 mg/m2 on days 1 and 15 of a 28 day cycle was found to be safe in 40 patients with advanced disease and had a response rate of 27.5% and a median OS of 12 months (15). An Italian study of oxaliplatin on day 1 and gemcitabine on day 1 and day 8 every 21 days in locally advanced (n=14) and metastatic (n=10) biliary cancer patients showed a response of 50% (complete response and partial response) and a median OS of 14 months in responders. In all studies of gemcitabine plus oxaliplatin the regimen was tolerable and safe with response rates between 22–50% and median OS 11–15.4 months observed which is comparable to gemcitabine and cisplatin. There has been no formal head to head comparison between gemcitabine plus cisplatin and gemcitabine plus oxaliplatin regimens. A potential limiting factor for an oxaliplatin based regimen is the possible concern about the dose density achievable with oxaliplatin given peripheral neuropathy may have a more noteworthy effect on patient quality of life than hematologic toxicity resulting in earlier discontinuation of oxaliplatin than that of cisplatin. Overall, selection of one first line regimen over another must take account of patient comorbidities and their potential toxicity profiles.
The combination of oxaliplatin with fluoropyrimidine therapy has also been evaluated in patients with advanced biliary cancers. One study used S-1, an oral fluoropyrimidine prodrug combined with oxaliplatin in a phase II Korean study in conjunction with an assessment of the potential impact of the CYP2A6 polymorphism (17). The second study used capecitabine as the fluoropyrimidine backbone (18). Both had similar response rates (24.5%, 23.8%) with a median OS less than that reported for cisplatin plus gemcitabine; 7.9 and 8.7 months respectively. FOLFOX was assessed in an Italian and Korean study with tolerability and similar survival results seen with gemcitabine and platinum therapy (19,20).
Cisplatin in combination with fluoropyrimidine therapy has been studied in both doublet and triplet regimens. A randomized phase II trial [European Organization for Research and Treatment of Cancer (EORTC) trial] assessed weekly high dose 5-FU (HDFU) with and without folinic acid and cisplatin in 58 treatment naive patients with advanced biliary carcinoma (21). One group (group A) received a 3 g/m2 infusion 5-FU weekly for 6 weeks followed by a 1 week of rest, repeated every 7 weeks. The second group (group B) received a continuous infusion of 2 g/m2 of 5-FU, leucovorin (LV) 500 mg/m2 weekly and cisplatin at a dose of 50 mg/m2 two weekly for 6 weeks, followed by 1 week of rest, every 7 weeks. Response rates were higher in the second group 15% vs. 7% with similar disease stabilization (group A: 46% and group B: 44%). However toxicity was higher in the second group with one death and therefore a phase III trial was not pursued. Cisplatin used in combination with infusional 5-FU or capecitabine has however shown to be safe with responses of 21.4–40.6% and median survival of 9.1–12.4 months (22-25).
Fluorouracil based chemotherapy has been used alone and in combination with other cytotoxic agents in advanced biliary cancer. The combination of gemcitabine plus capecitabine was originally evaluated in two studies with similar response rates and OS noted (44,45). Expansion of one of the studies for a larger cohort showed promising activity with a response rate of 29% and a median OS of 12.7 months (46). Thirty six percent of patients had GBC in this study. A smaller study of gemcitabine plus capecitabine was reported at this time in 12 patients (1 patient had GBC and 11 had cholangiocarcinoma) again showing a lower response but similar survival (47). The Southwest Oncology study of gemcitabine plus capecitabine showed a similar response rate (25%) but had an inferior survival (7 months) as compared to the three previously mentioned gemcitabine plus capecitabine trials (48). This may have been due to patient selection and characteristics; ampullary tumors were included in two of the studies (45,46). S-1, an oral prodrug of 5-FU has also been evaluated in combination with gemcitabine. Response rates were 20–30% and median OS ranged from 9–12.7 months. (51,52). Overall, the combination of gemcitabine and capecitabine is a reasonable alternative to gemcitabine platinum as first line therapy for ABC in patients for whom cisplatin or oxaliplatin is not recommended.
Triplet combinations using platinum with a fluoropyrimidine and an anthracycline have been evaluated. One was the only other phase III study in biliary cancer which assessed the addition of epirubicin an anthracycline with cisplatin and 5-FU (ECF regimen) and compared it to a non-platinum regimen of 5-FU, etoposide and leucovorin (FELV regimen) in 54 patients (26). Response rates were similar in both groups [ECF, 19.2% (95% CI, 6.55–39.3); FELV 15% (95% CI, 3.2–37.9), P=0.72]. There was no difference in survival between the groups while an increased rate of grade 3 or 4 neutropenia was seen with the FELV regimen compared to ECF (53.8% vs. 29.5%, P=0.020). The triplet regimen of epirubicin, cisplatin and capecitabine showed a high response rate (40%) but this did not translate to a superior survival with a median OS of 8 months seen (27). The addition of uracil/tegafur and LV to epirubicin and cisplatin was described in a Korean study of 40 patients for 11 had GBC (28). Tegafur-uracil (UFT) is an oral combination of two drugs; uracil, a competitive dihydropyrimidine dehydrogenase (DPD) inhibitor and tegafur, a prodrug which is converted by the liver to 5-FU. In this study the response rate was 22.5% with a median survival of 34 weeks similar to the capecitabine triplet combination. S-1 was assessed in a phase II trial with cisplatin and gemcitabine with a median survival of 16.1 months (29). This trial was one of the rare trials to show a median survival over 15 months and a phase III trial is underway comparing this regimen to cisplatin and gemcitabine. A phase II trial of gemcitabine, 5-FU, cisplatin (GFP) in 21 patients demonstrated a median OS of 18.8 months (30). In this regimen patients (8 patients with IHCC, 7 with GBC and 6 with extrahepatic cholangiocarcinoma) received either inpatient or outpatient GFP chemotherapy on a 4-week cycle for the first 2 months on a schedule of; gemcitabine at 1,000 mg/m2 on days 1, 8 and 15, and 5-FU (150 mg/m2) and cisplatin at 3 mg/m2 on days 1–5, 8–12 and 15–19. After the 2 months, an outpatient treatment regimen of gemcitabine (1,000 mg/m2) on days 1 and 15 along with 5-FU (500 mg/m2) and cisplatin (7 mg/m2) on days 1 and 15 was given. Seven patients (33.3%) had a partial response with grade 3/4 hematologic toxicity seen in six patients (28.6%). Using oxaliplatin as the platinum compound in triplet cytotoxic therapy has also been evaluated for advanced biliary cancer. A trial consisting of 37 patients assessed first line therapy utilizing oxaliplatin (100 mg/m2) three weekly combined with gemcitabine (1,000 mg/m2) on days 1 and 8 with oral capecitabine 1,500 mg/m2/day in a divided dose for 14 days out of a 21 day schedule (31). The response rate was 37.5% with a median OS of 13.8 months. The regimen was found to be both safe and tolerable. Overall, the use of platinum, fluoropyrimidine compounds in combination with gemcitabine were tolerable in the majority of trials.
The role of single agent therapy in biliary cancer is outlined in Table 3. Overall single agent chemotherapy with agents such as gemcitabine, 5-FU, mitomycin, docetaxel and oxaliplatin which were initially evaluated as therapy for advanced biliary cancers have in general provided underwhelming results but have provided the platform for investigation of combination strategies. Response rates for single agent gemcitabine range between 0–30% (19,49,58-63).
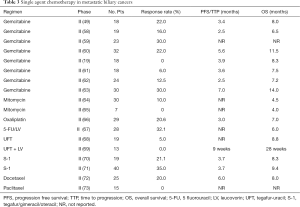
Full table
Second line and beyond therapy
There have been no randomized phase III studies of second line chemotherapy in advanced biliary cancer and thus no established standard second line therapy in this setting. Much of the second line data is from retrospective analysis and phase II trials. A retrospective analysis of 174 patients who received second line therapy following first line gemcitabine and cisplatin showed a 3.4% response rate and a PFS and OS of 3 and 6.6 months (74). Favorable prognostic factors for second line therapy included a good PS, low CA19-9 levels and absence of distant metastases by multivariate analysis. Also in this study a pooled analysis was performed combining this data along with five other series for a total of 499 patients which showed a higher response rate of 10.2% but a similar median OS of 6.3 months. In another French retrospective analysis a variety of second line therapy treatments were assessed on failure of cisplatin and gemcitabine in 603 patients. Among the 186 assessable patients a similar median OS of 6.7 months was noted and no regimen was deemed superior (75). Again potential putative markers of benefit for second line therapy included a good PS, CA19-9 levels ≤400 IU/mL and the duration of disease control on first line therapy. In addition, doublet fluoropyrimidine based chemotherapy was not superior to fluoropyrimidine monotherapy.
Additional data come from a review of 761 patients from 25 studies that included phase II trials, retrospective analyses and case reports that evaluated the role of second line therapy in advanced biliary cancers (76). Within this analysis the role of substituting chemotherapy type on progression i.e., gemcitabine based chemotherapy to 5-FU based therapy and vice-versa demonstrated no difference in benefit in either OS, response, disease control or PFS. Poor rates of outcome were also seen in a phase II trial assessing second line gemcitabine in 29 patients that had progressed on 5-FU therapy (77). A median TTP of 1.6 months (95% CI, 1.3–1.9 months) and median OS of 4.1 months (95% CI, 2.7–5.5 months) were observed. Putative predictive markers for outcome were commented on whereby those with a poor PS or low albumin levels (<3.5 g/dL) performing worse in this cohort. An albumin level >3.5 g/dL was also seen to predict for a longer benefit for second line therapy in another assessment (78). S-1 was also assessed on progression of gemcitabine therapy in advanced biliary cancer with modest activity (79). However one phase II report showed a response rate of 22.7% and a median OS of 13.5 months (95% CI, 7.1–23.1 months) and a median TTP of 5.4 months for S-1 as second line therapy in patients post progression on gemcitabine (80). This is likely related to patient selection as 64% of patients had recurrent disease, supported by the observation that for patients with recurrent disease there was a lower tumor volume 3.9 vs. 18.2 cm which was seen in de novo unresectable cases; P<0.01.
Overall for second line therapy improvements in therapeutic strategies are indisputably warranted for patients. Currently clinicians should select patients appropriately based on clinical PS with possibly albumin levels contributing to decisions. Outcomes seem to be comparable for doublet therapy as compared to single agent therapy with no standout therapy currently.
Other novel agents assessed in advanced biliary cancer
In the first line setting the addition of other agents to chemotherapy has been assessed. Erlotinib a tyrosine kinase inhibitor added to gemcitabine and oxaliplatin was evaluated in a phase III compared to gemcitabine and oxaliplatin alone (32). In this study the median PFS was 4.2 months (95% CI, 2.7–5.7) for the chemotherapy alone group as compared to the erlotinib plus chemotherapy group which had a PFS of 5.8 months (95% CI, 4.6–7.0) (HR, 0.80; 95% CI, 0.61–1.03; P=0.087). There was a statistically significant higher response in the erlotinib plus chemotherapy group however it did not translate to an improvement in OS which was identical in both groups; 9.5 months (95% CI, 7.5–11.5) in the chemotherapy alone group and 9.5 months (95% CI, 7.6–11.4) in the chemotherapy plus erlotinib group (HR, 0.93, 0.69–1.25; P=0.611). In a subgroup analysis the addition of erlotinib improved PFS in cholangiocarcinoma patients (5.9 vs. 3 months; HR, 0.73; 95% CI, 0.53–1.00; P=0.049. However this trial is notable for the lack of statistical power, an imbalance between groups for primary tumor location and the control group employing gemcitabine and oxaliplatin having an inferior survival as compared to previous trials that utilized this therapy backbone.
The uses of anti-EGFR antibodies cetuximab and panitumumab have been assessed in phase II trials in biliary cancer. Cetuximab failed to add benefit to gemcitabine and oxaliplatin in patients with locally advanced or metastatic biliary cancer in a randomized phase II trial (33). There was no difference in survival; 11.0 (range, 9.1–13.7) and 12.4 (range, 8.6–16.0) months in the chemotherapy alone group. Stratification by KRAS mutation status did not infer any advantage to response or PFS in patients treated with either gemcitabine and oxaliplatin alone or in combination with cetuximab in which the 36% of patients had a KRAS mutation (34). There have been four phase II trials assessing panitumumab in advanced biliary cancers (35-38). All have shown to be tolerable but none have shown an improvement in survival in combination with chemotherapy or identified a subgroup for which panitumumab may be effective.
Targeting the angiogenesis and the VEGF pathway has not demonstrated significant activity in advanced biliary cancer. Bevacizumab was used in two previous trials; one which correlated reductions in PET-CT SUV to improved survival and although a 40% response rate was seen the study failed to meet its target of a 6 months PFS rate of 70% (39). The second study evaluated gemcitabine and capecitabine with bevacizumab in a phase II trial with an OS (11.3 months) similar to that seen with cisplatin and gemcitabine (40). Cediranib in combination was compared with cisplatin and gemcitabine plus placebo in a phase II trial which did not show an improvement in outcome (41).
Vandetanib an oral multikinase inhibitor was evaluated as monotherapy compared with its combination with vandetanib plus gemcitabine or gemcitabine plus placebo in patients with advanced biliary cancer as first line therapy (42). There was no additional benefit with the addition of vandetanib to gemcitabine or single agent vandetanib therapy compared to gemcitabine alone. Other attempts include a phase II study that used a strategy without chemotherapy as first line therapy for advanced biliary cancer and combined sorafenib and erlotinib but was terminated with disappointing results with a median PFS of 2 months (95% CI, 2–3), and median OS of 6 months (81). A prior study of single agent sorafenib in the first line setting had no responses but did show a median OS of 9 months (82). Sorafenib in addition to cisplatin and gemcitabine was evaluated in 39 patients in a first line phase II study (43). An initial schedule in 16 patients employed gemcitabine 1,000 mg m/2 and cisplatin 25 mg m/2 on a 2 weeks on/1 week off cycle with sorafenib 400 mg prescribed twice daily but this regimen was altered due to unacceptable hematological toxicity and grade 3/4 hand foot syndrome events. Subsequently, patients received gemcitabine 800 mg m/2, cisplatin 20 mg m/2 and sorafenib 400 mg once daily. Median PFS and OS rates were 6.5 (95% CI, 3.5–8.3) and 14.4 months (95% CI, 11.6–19.2 months) with associated increased toxicity with grade 3 fatigue (16%), elevated liver function tests and hematologic toxicities such as thromboemboli (14%), hyponatraemia (16%) and hypophosphatemia (11%) noted. Furthermore pretreated tissues were evaluated for phosphorylated ERK (pERK) but there was no association between pERK staining and outcomes.
The utility of molecular profiling has identified potential targets that have allowed rationale design of clinical targeted agents. An ongoing phase 2 trial is assessing BGJ398 an oral pan FGFR inhibitor at a dose of 125 mg once a day on a 3 week on/1 week off schedule in patients with cholangiocarcinoma who have progressed post cisplatin and gemcitabine therapy or intolerant to cisplatin and harbor an FGFR2 fusion or other FGFR alteration (NCT02150967). Initial reports in 22 patients evaluable showed a disease control rate of 82% with 3 patients having a partial response and 15 with stable disease (83). In addition, the identification of mutations in isocitrate dehydrogenase 1 (IDH1) and IDH2 genes detected in ~23% of IHCC has identified another target for potential therapeutic manipulation (84). Ongoing clinical trials are evaluating the safety and activity of IDH1 inhibitor therapy including a phase I trial including patients with solid tumors that harbor an IDH1 mutation (NCT02073994) and also with AG-881 a dual IDH1 and IDH2 inhibitor in solid tumors that harbor an IDH1 and/or IDH2 mutation. (NCT02481154).
Future directions
Given the limitations seen with cytotoxic chemotherapy in the metastatic setting improvements and other strategies are warranted. Developments in targeting both the immune system and exploiting advances in next generation sequencing can help distinguish tumors based on their molecular profile and help guide rational trial investigation instead of classifying all gallbladder and cholangiocarcinoma as biliary tumors as has been performed in the past for clinical trials. There are a number of clinical trials ongoing which are critical to the ongoing attempts to develop improved outcomes for patients with biliary cancer (Table 4). Immunotherapy has transformed the treatment paradigm for tumors such as melanoma, renal cell carcinoma and lung carcinoma and attempts in other solid tumors are ongoing to determine if a benefit can also be seen. Currently there have been a number of trials assessing immunotherapy, peptide-based vaccines and dendritic cell based vaccines with some hopeful results which warrants further study (85,86). The safety and antitumor activity of pembrolizumab a humanized monoclonal anti-PD-1 antibody was evaluated in patients with PD-L1 positive biliary tract cancer as part of the ongoing multicohort, phase 1b trial using pembrolizumab monotherapy for pts with PD-L1-positive advanced solid tumors (KEYNOTE-028) (87). Pembrolizumab at a dose of 10 mg/kg every 2 weeks for up to 24 months or until confirmed progression or unacceptable toxicity was prescribed. Overall, 89 patients with biliary cancer were screened for PD-L1 expression with 37 (41.6%) considered PD-L1-positive. Tumors with ≥1% membranous staining in the tumor or stroma assessed by a prototype immunohistochemistry assay using the 22C3 antibody were considered PD-L1 positive. Of the 37 patients identified, 24 were enrolled. All patients had received at least one prior systemic therapy with 38% receiving ≥3 regimens. The overall response rate observed was 17.4% (95% CI, 5.0–38.8). With regards to safety, 15 patients (63%) had at least one adverse event of any grade, most commonly pyrexia and nausea. Four patients (17%) had grade 3 adverse events; anemia (n=1), autoimmune hemolytic anemia (n=1), colitis (n=1) and dermatitis (n=1). The results suggest that immunotherapy could have a role for biliary tract cancer and GBC, at least for a subset of the patients. The final results on this study are awaited.
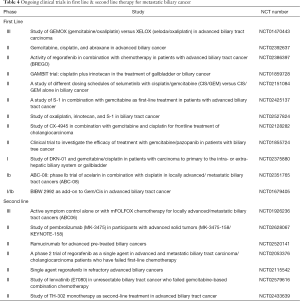
Full table
Conclusions
Biliary cancers are uncommon tumors associated with a poor outcome. There are currently no well-defined therapies in the adjuvant setting or second line setting with cisplatin and gemcitabine being standard of care therapy for advanced disease. There have been various phase II non-randomized trials assessing gemcitabine or fluoropyrimidine regimens either alone or in combination with platinum over the last decade. Overall, combination therapy is superior to single agent therapy in the first line setting and platinum agents such as oxaliplatin can be substituted for cisplatin if clinically contraindicated as both response and survival are similar albeit in non-randomized trials. For second line and adjuvant therapy continued enrollment on to clinical trials is paramount as no standard of care currently exists and no specific regimen has shown a significant better outcome. Targeting the EGFR pathway, VEGF pathway has currently not identified a subgroup of patients that may derive the greatest benefit. Limitations in chemotherapy have been exposed and future trials must have a logical design and incorporate biomarkers that can aid prognosis or predict benefit to therapy. Advances in genomic sequencing can allow identification of potential actionable targets that can be exploited therapeutically. This is already underway targeting FGFR2 fusions and IDH1/2 mutations in IHCC. Overall, this will require close collaboration among the oncology community and institutions so that desired and necessary improvements are met for this challenging disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- D'Hondt M, Lapointe R, Benamira Z, et al. Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol 2013;39:548-53. [Crossref] [PubMed]
- Goetze TO, Paolucci V. The prognostic impact of positive lymph nodes in stages T1 to T3 incidental gallbladder carcinoma: results of the German Registry. Surg Endosc 2012;26:1382-9. [Crossref] [PubMed]
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009;33:1247-54. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. [Crossref] [PubMed]
- Valle JW, Wasan H, Johnson P, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study - The UK ABC-01 Study. Br J Cancer 2009;101:621-7. [Crossref] [PubMed]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74. [Crossref] [PubMed]
- Meyerhardt JA, Zhu AX, Stuart K, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci 2008;53:564-70. [Crossref] [PubMed]
- Thongprasert S, Napapan S, Charoentum C, et al. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol 2005;16:279-81. [Crossref] [PubMed]
- Lee GW, Kang JH, Kim HG, et al. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol 2006;29:127-31. [Crossref] [PubMed]
- Kim ST, Park JO, Lee J, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 2006;106:1339-46. [Crossref] [PubMed]
- Williams KJ, Picus J, Trinkhaus K, et al. Gemcitabine with carboplatin for advanced biliary tract cancers: a phase II single institution study. HPB (Oxford) 2010;12:418-26. [Crossref] [PubMed]
- Andre T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [Crossref] [PubMed]
- Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer 2006;95:848-52. [Crossref] [PubMed]
- Halim A, Ebrahim MA, Saleh Y. A phase II study of outpatient biweekly gemcitabine-oxaliplatin in advanced biliary tract carcinomas. Jpn J Clin Oncol 2011;41:217-24. [Crossref] [PubMed]
- Gebbia N, Verderame F, Di Leo R, et al. A phase II study of oxaliplatin (O) and gemcitabine (G) first line chemotherapy in patients with advanced biliary tract cancers. J Clin Oncol 2005;23:4132. (Meeting Abstracts).
- Kim KP, Jang G, Hong YS, et al. Phase II study of S-1 combined with oxaliplatin as therapy for patients with metastatic biliary tract cancer: influence of the CYP2A6 polymorphism on pharmacokinetics and clinical activity. Br J Cancer 2011;104:605-12. [Crossref] [PubMed]
- Graham JS, Boyd K, Coxon FY, et al. A phase II study of capecitabine and oxaliplatin combination chemotherapy in patients with inoperable adenocarcinoma of the gall bladder or biliary tract. BMC Res notes 2016;9:161. [Crossref] [PubMed]
- Novarino AM, Satolli MA, Chiappino I, et al. FOLFOX-4 regimen or single-agent gemcitabine as first-line chemotherapy in advanced biliary tract cancer. Am J Clin Oncol 2013;36:466-71. [Crossref] [PubMed]
- Lee S, Kim KH, Kim HJ, et al. Oxaliplatin, 5-FU, and leucovorin (FOLFOX) in advanced biliary tract cancer. J Clin Oncol 2011;29:abstr 4106.
- Ducreux M, Van Cutsem E, Van Laethem JL, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer 2005;41:398-403. [Crossref] [PubMed]
- Ducreux M, Rougier P, Fandi A, et al. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol 1998;9:653-6. [Crossref] [PubMed]
- Taieb J, Mitry E, Boige V, et al. Optimization of 5-fluorouracil (5-FU)/cisplatin combination chemotherapy with a new schedule of leucovorin, 5-FU and cisplatin (LV5FU2-P regimen) in patients with biliary tract carcinoma. Ann Oncol 2002;13:1192-6. [Crossref] [PubMed]
- Hong YS, Lee J, Lee SC, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol 2007;60:321-8. [Crossref] [PubMed]
- Kim TW, Chang HM, Kang HJ, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol 2003;14:1115-20. [Crossref] [PubMed]
- Rao S, Cunningham D, Hawkins RE, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer 2005;92:1650-4. [Crossref] [PubMed]
- Park SH, Park YH, Lee JN, et al. Phase II study of epirubicin, cisplatin, and capecitabine for advanced biliary tract adenocarcinoma. Cancer 2006;106:361-5. [Crossref] [PubMed]
- Park KH, Choi IK, Kim SJ, et al. The efficacy of epirubicin, cisplatin, uracil/tegafur, and leucovorin in patients with advanced biliary tract carcinoma. Cancer 2005;103:2338-43. [Crossref] [PubMed]
- Kanai M, Hatano E, Kobayashi S, et al. A multi-institution phase II study of gemcitabine/cisplatin/S-1 (GCS) combination chemotherapy for patients with advanced biliary tract cancer (KHBO 1002). Cancer Chemother Pharmacol 2015;75:293-300. [Crossref] [PubMed]
- Yamashita Y, Taketomi A, Itoh S, et al. Phase II trial of gemcitabine combined with 5-fluorouracil and cisplatin (GFP) chemotherapy in patients with advanced biliary tree cancers. Jpn J Clin Oncol 2010;40:24-8. [Crossref] [PubMed]
- Petrioli R, Roviello G, Fiaschi AI, et al. Three-weekly oxaliplatin combined with gemcitabine and capecitabine in the first-line treatment of patients with advanced biliary tract cancer. Anticancer drugs 2015;26:682-6. [PubMed]
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Chen JS, Hsu C, Chiang NJ, et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol 2015;26:943-9. [Crossref] [PubMed]
- Leone F, Marino D, Cereda S, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2016;122:574-81. [Crossref] [PubMed]
- Hezel AF, Noel MS, Allen JN, et al. Phase II study of gemcitabine, oxaliplatin in combination with panitumumab in KRAS wild-type unresectable or metastatic biliary tract and gallbladder cancer. Br J Cancer 2014;111:430-6. [Crossref] [PubMed]
- Jensen LH, Lindebjerg J, Ploen J, et al. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann Oncol 2012;23:2341-6. [Crossref] [PubMed]
- Sohal DP, Mykulowycz K, Uehara T, et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann Oncol 2013;24:3061-5. [Crossref] [PubMed]
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48-54. [Crossref] [PubMed]
- Iyer RV, Groman A, Ma WW, et al. Gemcitabine (G), capecitabine (C) and bevacizumab (BV) in patients with advanced biliary cancers (ABC): final results of a multicenter phase II study. J Clin Oncol 2015;33:abstr 4078.
- Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol 2015;16:967-78. [Crossref] [PubMed]
- Santoro A, Gebbia V, Pressiani T, et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol 2015;26:542-7. [Crossref] [PubMed]
- Lee JK, Capanu M, O'Reilly EM, et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer 2013;109:915-9. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332-8. [Crossref] [PubMed]
- Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer 2005;104:2753-8. [Crossref] [PubMed]
- Riechelmann RP, Townsley CA, Chin SN, et al. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 2007;110:1307-12. [Crossref] [PubMed]
- Iyer RV, Gibbs J, Kuvshinoff B, et al. A phase II study of gemcitabine and capecitabine in advanced cholangiocarcinoma and carcinoma of the gallbladder: a single-institution prospective study. Ann Surg Oncol 2007;14:3202-9. [Crossref] [PubMed]
- Iqbal S, Rankin C, Lenz HJ, et al. A phase II trial of gemcitabine and capecitabine in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma: Southwest Oncology Group study S0202. Cancer Chemother Pharmacol 2011;68:1595-602. [Crossref] [PubMed]
- Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: results of a multicenter phase II study. J Clin Oncol 2001;19:4089-91. [PubMed]
- Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer 2005;103:111-8. [Crossref] [PubMed]
- Kim HS, Kim HY, Zang DY, et al. Phase II study of gemcitabine and S-1 combination chemotherapy in patients with metastatic biliary tract cancer. Cancer Chemother Pharmacol 2015;75:711-8. [Crossref] [PubMed]
- Kanai M, Yoshimura K, Tsumura T, et al. A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 2011;67:1429-34. [Crossref] [PubMed]
- Kornek GV, Schuell B, Laengle F, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol 2004;15:478-83. [Crossref] [PubMed]
- Furuse J, Okusaka T, Funakoshi A, et al. Early phase II study of uracil-tegafur plus doxorubicin in patients with unresectable advanced biliary tract cancer. Jpn J Clin Oncol 2006;36:552-6. [Crossref] [PubMed]
- Bhargava P, Jani CR, Savarese DM, et al. Gemcitabine and irinotecan in locally advanced or metastatic biliary cancer: preliminary report. Oncology (Williston Park) 2003;17:23-6. [PubMed]
- Chung MJ, Kim YJ, Park JY, et al. Prospective phase II trial of gemcitabine in combination with irinotecan as first-line chemotherapy in patients with advanced biliary tract cancer. Chemotherapy 2011;57:236-43. [Crossref] [PubMed]
- Feisthammel J, Schoppmeyer K, Mossner J, et al. Irinotecan with 5-FU/FA in advanced biliary tract adenocarcinomas: a multicenter phase II trial. Am J Clin Oncol 2007;30:319-24. [Crossref] [PubMed]
- Raderer M, Hejna MH, Valencak JB, et al. Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabine in patients with advanced biliary cancer. Oncology 1999;56:177-80. [Crossref] [PubMed]
- Kubicka S, Rudolph KL, Tietze MK, et al. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology 2001;48:783-9. [PubMed]
- Penz M, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol 2001;12:183-6. [Crossref] [PubMed]
- von Delius S, Lersch C, Schulte-Frohlinde E, et al. Phase II trial of weekly 24-hour infusion of gemcitabine in patients with advanced gallbladder and biliary tract carcinoma. BMC Cancer 2005;5:61. [Crossref] [PubMed]
- Lin MH, Chen JS, Chen HH, Su WC. A phase II trial of gemcitabine in the treatment of advanced bile duct and periampullary carcinomas. Chemotherapy 2003;49:154-8. [Crossref] [PubMed]
- Tsavaris N, Kosmas C, Gouveris P, et al. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest New Drugs 2004;22:193-8. [Crossref] [PubMed]
- Taal BG, Audisio RA, Bleiberg H, et al. Phase II trial of mitomycin C (MMC) in advanced gallbladder and biliary tree carcinoma. An EORTC Gastrointestinal Tract Cancer Cooperative Group Study. Ann Oncol 1993;4:607-9. [PubMed]
- von Eyben F, Hellekant C, Mattsson W, et al. Mitomycin C in advanced gallbladder carcinoma. Acta Radiol Oncol 1980;19:81-4. [Crossref] [PubMed]
- Androulakis N, Aravantinos G, Syrigos K, et al. Oxaliplatin as first-line treatment in inoperable biliary tract carcinoma: a multicenter phase II study. Oncology 2006;70:280-4. [Crossref] [PubMed]
- Choi CW, Choi IK, Seo JH, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol 2000;23:425-8. [Crossref] [PubMed]
- Ikeda M, Okusaka T, Ueno H, et al. A phase II trial of Uracil-tegafur (UFT) in patients with advanced biliary tract carcinoma. Jpn J Clin Oncol 2005;35:439-43. [Crossref] [PubMed]
- Mani S, Sciortino D, Samuels B, et al. Phase II trial of uracil/tegafur (UFT) plus leucovorin in patients with advanced biliary carcinoma. Invest New Drugs 1999;17:97-101. [Crossref] [PubMed]
- Ueno H, Okusaka T, Ikeda M, et al. Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 2004;91:1769-74. [Crossref] [PubMed]
- Furuse J, Okusaka T, Boku N, et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 2008;62:849-55. [Crossref] [PubMed]
- Papakostas P, Kouroussis C, Androulakis N, et al. First-line chemotherapy with docetaxel for unresectable or metastatic carcinoma of the biliary tract. A multicentre phase II study. Eur J Cancer 2001;37:1833-8. [Crossref] [PubMed]
- Jones DV Jr, Lozano R, Hoque A, et al. Phase II study of paclitaxel therapy for unresectable biliary tree carcinomas. J Clin Oncol 1996;14:2306-10. [PubMed]
- Fornaro L, Vivaldi C, Cereda S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res 2015;34:156. [Crossref] [PubMed]
- Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer 2015;121:3290-7. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref] [PubMed]
- Oh SY, Jeong CY, Hong SC, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 2011;29:1066-72. [Crossref] [PubMed]
- Kang EJ, Choi YJ, Kim JS, et al. Prognostic Factors for the Selection of Patients Eligible for Second-Line Chemotherapy in Advanced Biliary Tract Cancer. Chemotherapy 2014;60:91-8. [Crossref] [PubMed]
- Suzuki E, Ikeda M, Okusaka T, et al. A multicenter phase II study of S-1 for gemcitabine-refractory biliary tract cancer. Cancer Chemother Pharmacol 2013;71:1141-6. [Crossref] [PubMed]
- Sasaki T, Isayama H, Nakai Y, et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 2012;30:708-13. [Crossref] [PubMed]
- El-Khoueiry AB, Rankin C, Siegel AB, et al. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer 2014;110:882-7. [Crossref] [PubMed]
- El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs 2012;30:1646-51. [Crossref] [PubMed]
- Javle MM, Shroff RT, Zhu A, et al. A phase 2 study of BGJ398 in patients (pts) with advanced or metastatic FGFR-altered cholangiocarcinoma (CCA) who failed or are intolerant to platinum-based chemotherapy. J Clin Oncol 2016;34:abstr 335.
- Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012;17:72-9. [Crossref] [PubMed]
- Takahashi R, Yoshitomi M, Yutani S, et al. Current status of immunotherapy for the treatment of biliary tract cancer. Hum Vaccin Immunother 2013;9:1069-72. [Crossref] [PubMed]
- Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: Current status and emerging strategies. World J Gastrointest Oncol 2015;7:338-46. [Crossref] [PubMed]
- Bang YJ, Doi T, De Braud F, et al. 525 Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: Interim results of KEYNOTE-028. Eur J Cancer 2015;51:S112. [Crossref]


