
Intralesional curettage and surgical adjuvants in the treatment of giant cell tumor of bone: meta-analysis and systematic review
Highlight box
Key findings
• Intraoperative application of high-speed burring (HSB) or polymethyl methacrylate (PMMA) can decrease the recurrence rate of giant cell tumor of bone, while the use of phenol or H2O2 fails to make any significant difference.
What is known and what is new?
• Various surgical adjuvants have been used in conjunction with intralesional curettage to treat giant cell tumor of bone. However, findings from relevant studies are inconsistent, and no consensus has been reached.
• Through a systematic review and meta-analysis, we concluded that intraoperative application of HSB or PMMA has an additional antitumor effect, with a pooled ratio risk of 0.33 [95% confidence interval (CI): 0.22–0.49, P<0.001] and 0.59 (95% CI: 0.50–0.69, P<0.001), respectively. On the contrary, the use of phenol or H2O2 fails to make any significant difference, with a pooled relative risk of 0.84 (95% CI: 0.63–1.10, P=0.89).
What is the implication, and what should change now?
• Intralesional curettage is primarily used to treat giant cell tumors of the appendix. Through a systematic review and meta-analysis, we strongly recommend intraoperative high-speed deburring of the tumor cavity and reconstruction of the bone defect with PMMA. Chemical agents such as phenol or H2O2 can be used in conjunction with above adjuvants, but is not recommended to be used alone.
Introduction
Giant cell tumor of bone (GCTB) represents 4–5% of primary bone tumors (1). Despite its generally benign nature, GCTB can present with local aggressiveness and distant metastasis (1). GCTB predominantly occurs after skeletal maturity, exhibiting a slight female predilection, and has its peak incidence in the third and fourth decade of life (2). Clinically, GCTB has a predilection for the meta-epiphyseal region of long bones and is predominantly located in the distal femur and the proximal tibia (3).
Surgical options for GCTB include intralesional curettage and wide resection. En-bloc resection with a wide or marginal margin entails a lower risk of recurrence but necessitates major articular reconstruction with significant functional impairment (4-6). On the contrary, intralesional curettage through a broad cortical window provides favorable functional outcome and remains the treatment of choice for most patients. Research on curettage alone noted an elevated recurrence rate, ranging from 27% to 82% (7). Therefore, various surgical adjuvants have been introduced to eliminate tumor remnants and to improve local control rate. Adjuvants frequently used include high-speed burring (HSB), thermal procedures (argon beam coagulation, electrocautery, cryosurgery), and chemical agents (phenol, ethanol, hydrogen peroxide, zinc chloride, etc.) (8,9). After curettage, filling the cavity with polymethyl methacrylate (PMMA) and/or allograft/autograft is subsequently performed to provide structural support (8). Up to now, findings from studies evaluating the efficacy of various local adjuvants in the same cohort of patients are inconsistent, and a widely accepted consensus is still lacking (10-13).
Therefore, we conducted a systematic review and meta-analysis of studies revolving around intralesional curettage with local adjuvants to determine the effect of various surgical adjuvants in terms of local control. We present this article in accordance with the PRISMA reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-138/rc).
Methods
Systematic literature search
A literature search was performed to identify comparative studies that assessed the effects of local adjuvants on the recurrence of GCTB following intralesional curettage. The literature search was conducted via PubMed and Embase electronic databases by two independent authors. Terms used included “(giant cell tumor of bone (Title/Abstract) OR giant cell tumor of extremity (Title/Abstract) OR appendicular giant cell tumor (Title/Abstract)) AND (curettage (Title/Abstract) OR intralesional (Title/Abstract))”, and the results were limited to studies published from January 1995 till June 2022 in the English language. An additional search was manually performed through the reference lists of review articles and relevant studies. Two authors of this review (A.L. and Q.W.) independently screened the titles and abstracts of the identified papers and assessed the quality of the studies.
Inclusion and exclusion criteria
Studies were included if they performed intralesional curettage for pathologically confirmed GCTB, provided details on the application of various local adjuvants, followed for at least 18 months after surgery, and reported local recurrence as the primary outcome. The screeners excluded articles that incorporate pre-operative administration of bisphosphonate or denosumab. We also excluded research that only compares the recurrence of en-bloc resection versus intralesional curettage. Case reports, reviews, opinion articles, or technique notes were excluded based on the contents of the abstracts. Studies with a small number of subjects, with either cohort involving less than five patients, were also excluded. Once meeting our inclusion-exclusion criteria, a thorough full-paper assessment was performed for final inclusion. When critical data were missing, we either contacted the authors or removed the study.
Data extraction
Two investigators (A.L. and H.G.) independently examined each article and extracted the total number of patients and the total number of events for different treatment groups. When the total number of events for each arm was not explicitly reported, the study was excluded from the analysis. Since this research focuses on the addictive antitumor effect of various surgical adjuvants after intralesional, studies that only performed bloc resections were not considered.
Quality and publication bias assessment
The quality of eligible studies was assessed using the Newcastle Ottawa Quality assessment scale (NOS, http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm). NOS allocates a maximum of nine points for the quality of selection, comparability, exposure, and outcome of study participants. A study could earn a maximum of 2 points in the comparability category and a maximum of 1 point in the other categories, for a total of 9 points. A score of 7 or higher indicated high quality, a score between 6 and 4 indicated moderate quality, and a score of 3 or lower indicated low quality. The quality assessment was performed independently by two authors (H.G. and J.L.) and any disagreements were resolved through discussion with author A.L. Publication bias among the included studies was assessed through visual inspection of funnel plot asymmetry.
Outcome measurement
The primary endpoint for analysis was local recurrence rate, defined as radiological and pathological evidence of local disease recurrence necessitating further surgical intervention.
Statistical analysis
Stata/MP (Version17.0, StataCorp LLC, TX, USA) and Review Manager (RevMan, Version 5.4.1, The Cochrane Collaboration, 2020) were used for data analysis. Risk ratio (RR) and 95% confidence interval (CI) were reported. Heterogeneity among studies was assessed using the Cochrane Q test with the P value set at 0.1 for significance. The I-squared statistic is the percentage of total variation across studies due to heterogeneity. The random effect model was used for heterogeneous data, while the fixed effect model was advocated for homogenous data. A meta-analysis of pooled RR was performed, with P values less than 0.05 considered statistically significant.
Results
Literature search
Literature search through PubMed and Embase yielded 260 titles. Another 44 titles were manually identified through the reference lists of review articles and relevant studies. The abstracts were screened based on the inclusion and exclusion criteria, yielding 51 eligible papers included for full-text assessment. Twenty-seven studies were excluded due to: no comparison group (n=9), insufficient data (n=8), only compare the recurrence of en-bloc resection versus intralesional curettage (n=7) and case series involving less than five patients in the cohort (n=3). Finally, 24 studies were found to meet our criteria and were included in this meta-analysis (Figure 1).
Characteristics of included studies
The characteristics of included studies are listed in Table 1 and Table S1. Twenty-four studies involving 2,579 patients were included in this analysis (5,6,8-11,13-30). The sample size varies considerably across the studies from 18 to 330. Sixteen studies exclusively include appendicular giant cell tumors, while eight studies also include lesions in the axial skeleton. Seven studies only involve patients with primary GCTB, while two studies only include recurrent GCTB and fifteen studies include both primary and recurrent lesions. The median follow-up time ranges from 38 months to 134 months among the included studies. The risk of bias was evaluated with NOS, and the results suggested good quality of included studies, with all studies scoring ≥6 (Table S2). Publication bias was assessed with the funnel plot method, and symmetric plots were achieved, indicating no publication bias (Figure S1).
Table 1
| Study | Group | n | Treatment | Recurrence | |
|---|---|---|---|---|---|
| n | Rate, % | ||||
| Niu 2012 (14) | a | 59 | HSB + PMMA | 6 | 10.2 |
| b | 27 | HSB + BG | 3 | 11.1 | |
| c | 30 | HSB + PMMA + BG | 1 | 3.3 | |
| d | 9 | PMMA | 5 | 55.6 | |
| e | 32 | BG | 18 | 56.3 | |
| Errani 2010 (15) | a | 64 | HSB + phenol + PMMA | 8 | 12.5 |
| b | 136 | HSB + phenol + BG | 24 | 17.6 | |
| Klenke 2011 (16) | a | 22 | HSB + BG | 7 | 32.0 |
| b | 32 | HSB + phenol + BG | 11 | 34.0 | |
| c | 40 | HSB + phenol + PMMA | 6 | 15.0 | |
| Zou 2019 (17) | a | 12 | HSB + BG | 3 | 25.0 |
| b | 9 | HSB + PMMA | 2 | 22.2 | |
| Balke 2008 (8) | a | 46 | None | 30 | 65.2 |
| b | 9 | HSB | 2 | 22.2 | |
| c | 45 | PMMA | 16 | 35.6 | |
| d | 21 | HSB + PMMA | 5 | 23.8 | |
| e | 25 | HSB + H2O2 + PMMA | 4 | 16.0 | |
| f | 7 | PMMA + BG | 3 | 42.9 | |
| g | 18 | HSB + PMMA + BG | 2 | 11.1 | |
| h | 17 | HSB + H2O2 + PMMA + BG | 1 | 5.9 | |
| Becker 2008 (11) | a | 103 | None | 50 | 49.0 |
| b | 102 | PMMA | 22 | 22.0 | |
| c | 74 | Phenol + PMMA | 20 | 27.0 | |
| d | 27 | Phenol/ethanol/cyclophosphamide/cauterization | 4 | 15.0 | |
| Kivioja 2008 (10) | a | 147 | PMMA | 32 | 22.0 |
| b | 47 | BG | 24 | 52.0 | |
| Tang 2019 (18) | a | 94 | PMMA | 40 | 42.6 |
| b | 42 | BG | 18 | 42.9 | |
| Jones 2006 (9) | a | 6 | HSB + PMMA | 1 | 16.7 |
| b | 11 | HSB + BG | 0 | 0.0 | |
| c | 13 | BG | 5 | 38.5 | |
| Gaston 2011 (19) | a | 84 | HSB + PMMA | 12 | 14.3 |
| b | 246 | HSB + BG | 73 | 29.7 | |
| Pietschmann 2010 (20) | a | 34 | HSB + H2O2 + BG | 11 | 32.4 |
| b | 13 | HSB + BG | 7 | 53.9 | |
| Trieb 2001 (13) | a | 14 | BG | 3 | 21.0 |
| b | 12 | Phenol + BG | 3 | 25.0 | |
| Klenke 2011 (6) | a | 14 | HSB + phenol + PMMA | 2 | 14.3 |
| b | 14 | HSB + phenol + BG | 7 | 50.0 | |
| Gao 2014 (21) | a | 34 | HSB + BG | 12 | 35.3 |
| b | 31 | HSB + PMMA | 4 | 12.9 | |
| Benevenia 2017 (22) | a | 4 | HSB + H2O2 + BG | 1 | 25 |
| b | 17 | HSB + H2O2 + PMMA + BG | 3 | 17.6 | |
| c | 22 | HSB + H2O2 + PMMA | 6 | 27.3 | |
| O’Donnell 1994 (23) | a | 24 | HSB + PMMA | 4 | 16.7 |
| b | 11 | Phenol + PMMA | 2 | 18.2 | |
| c | 6 | HSB + phenol + PMMA | 1 | 16.7 | |
| d | 19 | PMMA | 8 | 42.1 | |
| Dürr 1999 (24) | a | 11 | HSB + phenol + BG | 1 | 9.1 |
| b | 7 | HSB + BG | 3 | 42.9 | |
| Ward 2002 (25) | a | 7 | HSB + phenol + PMMA | 1 | 14.3 |
| b | 6 | HSB + phenol + PMMA + BG | 0 | 0.0 | |
| c | 9 | HSB + phenol | 1 | 11.1 | |
| van der Heijden 2012 (26) | a | 96 | Phenol + PMMA | 29 | 30.2 |
| b | 27 | PMMA | 10 | 37.0 | |
| Boons 2002 (27) | a | 2 | HSB + BG | 0 | 0.0 |
| b | 4 | HSB + PMMA | 1 | 25.0 | |
| c | 12 | HSB + cryosurgery + BG | 5 | 41.7 | |
| d | 5 | HSB + cryosurgery + PMMA | 1 | 20.0 | |
| Balke 2009 (28) | a | 9 | None | 6 | 66.7 |
| b | 3 | H2O2 + PMMA | 0 | 0.0 | |
| c | 11 | PMMA | 5 | 45.5 | |
| d | 10 | HSB + H2O2 + PMMA | 3 | 30.0 | |
| e | 13 | HSB + PMMA | 2 | 15.4 | |
| van der Heijden 2014 (29) | a | 40 | Phenol + PMMA | 10 | 25.0 |
| b | 42 | Phenol + PMMA + BG | 13 | 31.0 | |
| c | 26 | HSB + PMMA | 8 | 31.0 | |
| d | 24 | HSB + BG | 9 | 38.0 | |
| Li 2016 (5) | a | 35 | HSB + BG | 8 | 22.9 |
| b | 49 | HSB + PMMA | 8 | 16.3 | |
| c | 16 | None | 6 | 37.5 | |
| d | 27 | H2O2 | 12 | 44.4 | |
| Kremen 2012 (30) | a | 108 | Phenol + H2O2 + PMMA | 11 | 10.2 |
| b | 55 | Phenol + H2O2 + BG | 9 | 16.4 | |
HSB, high-speed burring; PMMA, polymethyl methacrylate; BG, bone graft.
Recurrence
Five studies with nine subgroups evaluated the efficacy of HSB after intralesional curettage. The overall recurrence rates for patients treated with or without HSB are 11.9% (26/218) and 47.7% (92/193), respectively. The pooled RR for tumor recurrence is 0.33 (95% CI: 0.22 to 0.49, P<0.001), in favor of applying HSB after curettage for better local control (Figure 2A).
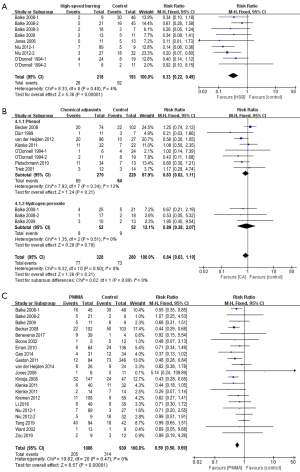
Nine studies involving eleven subgroups focused on the benefits of chemical adjuvants after intralesional curettage, with seven studies focusing on phenol and two studies focusing on hydrogen peroxide. The overall recurrence rates for patients treated with or without chemical adjuvants are 23.5% (77/328) and 26.1% (73/280), respectively. Although patients receiving local chemical adjuvants (either phenol or hydrogen peroxide) exhibit a lower recurrence rate, the discrepancy doesn’t reach statistical significance, with a pooled RR of 0.84 (95% CI: 0.63 to 1.10, P=0.89) (Figure 2B).
Nineteen studies reported the effect of using PMMA as void filler after curettage. The overall recurrence rates for patients treated with or without PMMA are 20.4% (205/1,006) and 33.4% (314/939), respectively. The pooled RR for tumor recurrence is 0.59 (95% CI: 0.50 to 0.69, P<0.001), suggesting that PMMA can significantly decrease the risk of local recurrence (Figure 2C).
Discussion
GCTB is one of the most controversial and discussed bone tumors. Treatment recommendations are mostly based on results from retrospective analyses of non-randomized series from single or multiple institutions. In the currently available literature, no consensus has been reached for preferential treatment in GCTB. In our systematic review and meta-analysis, we showed that intraoperative application of HSB and PMMA exhibited an additional antitumor effect, while the use of phenol or H2O2 failed to make any significant difference.
High speed burring
Several studies emphasized the role of HSB as a key factor for local control (8,9,14). It is assumed that burring of the tumor cavity can improve the thoroughness of tumor removal, thereby decreasing the risk of local recurrence. In a study conducted by Balke et al., HSB turned out to be the most relevant factor for reducing local recurrence (8). The likelihood of recurrence after curettage was 8 times higher than after the same procedure with additional burring (8). In another study by Niu et al., the recurrence rate for patients treated with chemical adjuvants and PMMA decreased from 55.6% to 7.9% with the addition of HSB (14). A similar antitumor effect was also observed in other studies (23,28), with no recurrence being reported by Jones et al. in patients treated with HSB and ethanol (9). In our analysis, the overall recurrence rates for patients treated with or without HSB are 11.9% (26/218) and 47.7% (92/193), respectively. The pooled RR for tumor recurrence is 0.33 (95% CI: 0.22 to 0.49, P<0.001), in favor of applying HSB after curettage for better local control (Figure 2A).
Despite the favoring oncological findings, the use of HSB present specific limitations. One such limitation is its potential impact on surrounding healthy bone tissue. The heat generated during burring might compromise the bone’s viability, potentially causing necrosis or weakening of the bone surrounding the tumor site. In addition, the aggressive nature of HSB might inadvertently cause damage to adjacent structures, especially in areas housing delicate structures or critical anatomical features, posing a risk of nerve or blood vessel injury (31).
Chemical adjuvants
Phenol is the most studied chemical agent used as a local adjuvant. The cytotoxic effect of phenol has been studied in vitro (32). It is believed that phenol induces tumor necrosis and coagulation of proteins at the surface of the curetted cavity (27), and the infiltration depth of phenol has been estimated at 0.2 mm (32). Up to date, the largest comparative study of phenolization was performed by Becker et al. (11). Cementation alone resulted in 22 relapses out of 102 patients (21.6%), while cementation enhanced by phenol resulted in 20 relapses out of 74 patients (27.0%), indicating no additional benefit after the application of phenol (8). On the other hand, van der Heijden et al. observed a reduction in recurrence rate from 53.9% after extended curettage to 32.4% after phenol enhancement (26). As included in our analysis, three studies demonstrated superior outcomes with the addition of phenol (20,24,26), while other studies observed no significant difference (11,13,16,23). In our analysis, patients with the additional treatment of phenol exhibit a lower pooled recurrence rate (69/276 vs. 64/228), but this discrepancy doesn’t reach statistical significance (P=0.21). Additionally, in spite that some data confirm low systemic toxicity from the use of phenol (33), it is a caustic substance and must be handled carefully to adjacent tissues and operating personnel (9).
Compared to phenol, H2O2 has no major side effects so it can be used as an alternative (8). Nicholson et al. examined the effect of H2O2 on giant cell tumor cells and osteoblasts grown in culture and observed cell lysis and death when exposed to the minimal concentration of H2O2 as it is commonly used in clinical practice (34). With curettage and cementation performed as the standard basic treatment, the likelihood of recurrence can be reduced by the factor of 7.9 with additional burring and H2O2 lavage (8). Meanwhile, the combination of all adjuncts (PMMA, burring, H2O2, n=42) reduces the likelihood of recurrence by the factor of 28.2 compared to curettage alone (8).
Other chemical agents such as ethanol and zinc chloride have also been tested as reasonable alternatives to phenol (35-37). Oh et al. reported that among patients who received anhydrous alcohol treatment, four (9.5%) developed local recurrence. In contrast, among the 31 patients who did not receive any adjuvant treatment, 15 (48.4%) experienced recurrence (34). Additionally, in a prospective comparative study on the inactivation effect of phenol and anhydrous ethanol, the recurrence rates of the two groups were 12% and 11%, respectively, after an average follow-up period of 58 months (35). Zhen et al. treated 92 patients with intralesional curettage, 50% zinc chloride, and bone grafting, and reported a recurrence rate of 13% (37). However, high-quality comparative studies revolving around alternative chemical agents in the treatment of GCTB are still lacking.
Cementation
PMMA is a thermal adjuvant that was first introduced in the treatment of GCTB in 1969 (11). Balke et al. analyzed the sole effect of PMMA without other adjuvants after curettage and showed that PMMA reduced the local recurrence rate by the factor of 8.2 (P=0.004) compared to curettage alone (8). Similar risk reductive effect was also observed in other studies (5,10,19,21-23). In contrast, some authors reported that the type of filling material didn’t correlate with local recurrence (4,5,9,14,15,17,18,22,25,27-30). In our analysis, the RR for tumor recurrence with or without PMMA is 0.59 (95% CI: 0.50 to 0.69, P<0.001), indicating that PMMA can significantly decrease the risk of developing local recurrence. This can be attributed to the toxicity of the acrylic monomer and thermal necrosis produced during cement polymerization (38). Besides, PMMA can extend the surgical margin by 1.5 to 2 mm in cancellous bone and 0.5 mm in cortical bone (38), therefore ensuring the complete removal of residual tumors in the subchondral bone or the joint cartilage where the use of HSB is limited by the potential complications (39). In addition, PMMA can provide instant mechanical support, thereby allowing for more aggressive tumor removal and early postoperative rehabilitation (16). In the meanwhile, reconstruction with PMMA can facilitate early radiographic detection of recurrence at the bone-cement interface (10).
The disadvantage of using bone cement in the subchondral region is that it may damage the adjacent articular cartilage and accelerate joint degeneration (19,40). A layer of bone graft between the cement and articular cartilage is assumed to be an attractive solution but has yet to gain popularity or show definite superiority (22,25,35). In cases of recurrence after cementation, cement generally needs to be removed before further curettage, and this may cause destruction to adjacent bone or cartilage (28).
HSB + chemical adjuvant + PMMA
Among the studies involved in our analysis, 11 studies incorporated 346 patients who were treated with the combination of HSB, chemical adjuvant and PMMA. Reported recurrence rates varied in the narrowest range (0–41.94%) among all used curettage variants with the mean and median recurrence rate being 14.74% and 14.29%, respectively (6,8,9,14-16,21,23,25,28,40). While the reported recurrence rates of resection distributed in the range of 0–27.03%, with the mean and median value being 9.67% and 6.60%, respectively (4-6,10-12,14,15,17,18,20,24,25,27,28,41-44). Even in recurrent cases, burring of the cavity and cementation significantly reduced the likelihood of re-recurrence by the factor of 5.508 (28).
Despite the introduction of various inactivation methods and filling materials, en-bloc resection remains the most effective method in decreasing recurrence rates, particularly when curettage is not feasible. However, wide resection often requires significant reconstruction and carries a higher risk of surgical complications and functional loss (10,12,43). The treatment algorithm should prioritize both local control and functional restoration. Taking into consideration the relatively benign nature of GCTB, we recommend wide resection be reserved for (I) tumors with extensive bone destruction and massive soft tissue compromise where joint preservation is impossible, (II) pathological fractures with joint invasion or unstable fractures, (III) multiple recurrences, or (IV) when expendable sites (head of the fibula or distal ulna) are affected (6,14-16,18).
There are several limitations in our study that need to be addressed. In this meta-analysis, we mainly focused on the surgery-related procedures in the treatment of GCTB and excluded studies incorporating denosumab in their treatment. The effect of denosumab in reducing local recurrence remains debatable, and is assumed to be influenced by the surgical techniques (45). Research indicates that administering denosumab before en-bloc resection might fortify the tumor, minimizing spillage, and subsequently lowering the local recurrence rate. Conversely, pre-curettage denosumab administration could induce osteosclerosis, potentially complicating intraoperative tumor identification and leading to an increased local recurrence rate (46). It was suggested that a clear surgical margin is more difficult to achieve after denosumab, and recurrence rates between 43% and 60% have been reported (7). Despite the ongoing debates regarding its impact on local recurrence, neoadjuvant denosumab demonstrates benefits in surgical downstaging. It is recommended for treating locally advanced tumors to facilitate a less invasive surgical resection (47). In addition, the paucity of high-quality comparative studies on the management of GCTB limited our ability to conduct comprehensive analyses for all intraoperative surgical adjuvants. The rarity of the tumor and the variety of local adjuvants make it difficult to outline the most adequate curettage technique. Last but not least, selection bias may exist since patients with grade III GCTB are more likely to be treated with aggressive procedures. The choice of local adjuvants was largely at the discretion of the treating surgeons.
Conclusions
Intralesional curettage is primarily used to treat giant cell tumors of the appendix. Through this systematic review and meta-analysis, we strongly recommend intraoperative high-speed deburring of the tumor cavity and reconstruction of the bone defect with PMMA. Chemical agents such as phenol or H2O2 can be used in conjunction with above adjuvants, but is not recommended to be used alone.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-138/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-138/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-138/coif). Q.W. received funding from the Natural Science Foundation of Liaoning Province (No. 2022-YGJC-09). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Flanagan AM, Larousserie F, O’Donnell PG, et al. A. Giant cell tumour of bone. 2020. In: WHO Classification of Tumours, 5th ed Soft Tissue and Bone Tumours (Internet). Lyon, France: International Arctic Research Center; (440-6).
- Aoude A, Nikomarov D, Perera JR, et al. Giant cell tumour of bone. Bone Joint J 2023;105-B:559-67. [Crossref] [PubMed]
- Rekhi B, Dave V. Giant cell tumor of bone: An update, including spectrum of pathological features, pathogenesis, molecular profile and the differential diagnoses. Histol Histopathol 2023;38:139-53. [PubMed]
- Pitsilos C, Givissis P, Papadopoulos P, et al. Treatment of Recurrent Giant Cell Tumor of Bones: A Systematic Review. Cancers (Basel) 2023;15:3287. [Crossref] [PubMed]
- Li D, Zhang J, Li Y, et al. Surgery methods and soft tissue extension are the potential risk factors of local recurrence in giant cell tumor of bone. World J Surg Oncol 2016;14:114. [Crossref] [PubMed]
- Klenke FM, Wenger DE, Inwards CY, et al. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res 2011;469:1181-7. [Crossref] [PubMed]
- Machak GN, Snetkov AI. The impact of curettage technique on local control in giant cell tumour of bone. Int Orthop 2021;45:779-89. [Crossref] [PubMed]
- Balke M, Schremper L, Gebert C, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol 2008;134:969-78. [Crossref] [PubMed]
- Jones KB, DeYoung BR, Morcuende JA, et al. Ethanol as a local adjuvant for giant cell tumor of bone. Iowa Orthop J 2006;26:69-76. [PubMed]
- Kivioja AH, Blomqvist C, Hietaniemi K, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop 2008;79:86-93. [Crossref] [PubMed]
- Arbeitsgemeinschaft Knochentumoren. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am 2008;90:1060-7. [Crossref] [PubMed]
- Turcotte RE, Wunder JS, Isler MH, et al. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res 2002;248-58. [Crossref] [PubMed]
- Trieb K, Bitzan P, Lang S, et al. Recurrence of curetted and bone-grafted giant-cell tumours with and without adjuvant phenol therapy. Eur J Surg Oncol 2001;27:200-2. [Crossref] [PubMed]
- Niu X, Zhang Q, Hao L, et al. Giant cell tumor of the extremity: retrospective analysis of 621 Chinese patients from one institution. J Bone Joint Surg Am 2012;94:461-7. [Crossref] [PubMed]
- Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1-7. [Crossref] [PubMed]
- Klenke FM, Wenger DE, Inwards CY, et al. Giant cell tumor of bone: risk factors for recurrence. Clin Orthop Relat Res 2011;469:591-9. [Crossref] [PubMed]
- Zou C, Lin T, Wang B, et al. Managements of giant cell tumor within the distal radius: A retrospective study of 58 cases from a single center. J Bone Oncol 2018;14:100211. [Crossref] [PubMed]
- Tang H, Moro A, Feng W, et al. Giant cell tumors combined with secondary aneurysmal bone cysts are more likely to develop postoperative recurrence: A retrospective study of 256 cases. J Surg Oncol 2019;120:359-65. [Crossref] [PubMed]
- Gaston CL, Bhumbra R, Watanuki M, et al. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J Bone Joint Surg Br 2011;93:1665-9. [Crossref] [PubMed]
- Pietschmann MF, Dietz RA, Utzschneider S, et al. The influence of adjuvants on local recurrence rate in giant cell tumour of the bone. Acta Chir Belg 2010;110:584-9. [Crossref] [PubMed]
- Gao ZH, Yin JQ, Xie XB, et al. Local control of giant cell tumors of the long bone after aggressive curettage with and without bone cement. BMC Musculoskelet Disord 2014;15:330. [Crossref] [PubMed]
- Benevenia J, Rivero SM, Moore J, et al. Supplemental Bone Grafting in Giant Cell Tumor of the Extremity Reduces Nononcologic Complications. Clin Orthop Relat Res 2017;475:776-83. [Crossref] [PubMed]
- O'Donnell RJ, Springfield DS, Motwani HK, et al. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am 1994;76:1827-33. [Crossref] [PubMed]
- Dürr HR, Maier M, Jansson V, et al. Phenol as an adjuvant for local control in the treatment of giant cell tumour of the bone. Eur J Surg Oncol 1999;25:610-8. [Crossref] [PubMed]
- Ward WG Sr, Li G 3rd. Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop Relat Res 2002;259-70. [Crossref] [PubMed]
- van der Heijden L, van de Sande MA, Dijkstra PD. Soft tissue extension increases the risk of local recurrence after curettage with adjuvants for giant-cell tumor of the long bones. Acta Orthop 2012;83:401-5. [Crossref] [PubMed]
- Boons HW, Keijser LC, Schreuder HW, et al. Oncologic and functional results after treatment of giant cell tumors of bone. Arch Orthop Trauma Surg 2002;122:17-23. [Crossref] [PubMed]
- Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol 2009;135:149-58. [Crossref] [PubMed]
- van der Heijden L, van der Geest IC, Schreuder HW, van de Sande MA, Dijkstra PD. Liquid nitrogen or phenolization for giant cell tumor of bone?: a comparative cohort study of various standard treatments at two tertiary referral centers. J Bone Joint Surg Am 2014;96:e35. [Crossref] [PubMed]
- Kremen TJ Jr, Bernthal NM, Eckardt MA, et al. Giant cell tumor of bone: are we stratifying results appropriately? Clin Orthop Relat Res 2012;470:677-83. [Crossref] [PubMed]
- Hu P, Zhao L, Zhang H, et al. Recurrence Rates and Risk Factors for Primary Giant Cell Tumors around the Knee: A Multicentre Retrospective Study in China. Sci Rep 2016;6:36332. [Crossref] [PubMed]
- Mittag F, Leichtle C, Kieckbusch I, et al. Cytotoxic effect and tissue penetration of phenol for adjuvant treatment of giant cell tumours. Oncol Lett 2013;5:1595-8. [Crossref] [PubMed]
- Quint U, Müller RT, Müller G. Characteristics of phenol. Instillation in intralesional tumor excision of chondroblastoma, osteoclastoma and enchondroma. Arch Orthop Trauma Surg 1998;117:43-6. [Crossref] [PubMed]
- Nicholson NC, Ramp WK, Kneisl JS, et al. Hydrogen peroxide inhibits giant cell tumor and osteoblast metabolism in vitro. Clin Orthop Relat Res 1998;250-60. [Crossref] [PubMed]
- Oh JH, Yoon PW, Lee SH, et al. Surgical treatment of giant cell tumour of long bone with anhydrous alcohol adjuvant. Int Orthop 2006;30:490-4. [Crossref] [PubMed]
- Lin WH, Lan TY, Chen CY, et al. Similar local control between phenol- and ethanol-treated giant cell tumors of bone. Clin Orthop Relat Res 2011;469:3200-8. [Crossref] [PubMed]
- Zhen W, Yaotian H, Songjian L, et al. Giant-cell tumour of bone. The long-term results of treatment by curettage and bone graft. J Bone Joint Surg Br 2004;86:212-6. [Crossref] [PubMed]
- Chao B, Jiao J, Yang L, et al. Comprehensive evaluation and advanced modification of polymethylmethacrylate cement in bone tumor treatment. J Mater Chem B 2023;11:9369-85. [Crossref] [PubMed]
- Duan DK, Zhang GC, Sun BJ, et al. Effect evaluation of denosumab combined with curettage and bone cement reconstruction in the treatment of recurrent giant cell tumor of bone around the knee joint. Eur Rev Med Pharmacol Sci 2023;27:5039-52. [PubMed]
- Vaishya R, Pokhrel A, Agarwal AK, et al. Current status of bone cementing and bone grafting for giant cell tumour of bone: a systemic review. Ann R Coll Surg Engl 2019;101:79-85. [Crossref] [PubMed]
- Harness NG, Mankin HJ. Giant-cell tumor of the distal forearm. J Hand Surg Am 2004;29:188-93. [Crossref] [PubMed]
- Rastogi S, Prashanth I, Khan SA, et al. Giant cell tumor of bone: Is curettage the answer? Indian J Orthop 2007;41:109-14. [Crossref] [PubMed]
- Kang L, Manoso MW, Boland PJ, et al. Features of grade 3 giant cell tumors of the distal radius associated with successful intralesional treatment. J Hand Surg Am 2010;35:1850-7. [Crossref] [PubMed]
- Panchwagh Y, Puri A, Agarwal M, et al. Giant cell tumor - distal end radius: Do we know the answer? Indian J Orthop 2007;41:139-45. [Crossref] [PubMed]
- Sun Z, Wu Z, Zhang L, et al. Association between preoperative denosumab and the risk of local recurrence in patients with giant cell tumor of bone: A meta-analysis and systematic review. J Cancer Res Ther 2023;19:25-33. [PubMed]
- Tsukamoto S, Mavrogenis AF, Kido A, et al. Current Concepts in the Treatment of Giant Cell Tumors of Bone. Cancers (Basel) 2021;13:3647. [Crossref] [PubMed]
- Tsukamoto S, Hindiskere S, Honoki K, et al. Outcome of re-operation for local recurrence following pre-operative denosumab administration and curettage for giant cell tumour of bone with difficult joint preservation. Int Orthop 2023;47:265-73. [Crossref] [PubMed]



