A prospective, multicenter phase II trial of low-dose erlotinib as maintenance treatment after platinum doublet chemotherapy for advanced non-small cell lung cancer harboring EGFR mutation
Introduction
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) have been shown to improve progression-free survival (PFS) compared with chemotherapy when given as first-line treatment for patients with non-small cell lung cancer (NSCLC) with activated EGFR mutation (1,2). However, about 1 week is needed to confirm the existence of EGFR mutation in most hospitals in Japan. Therefore, some situations arise in which cytotoxic drugs are used as first-line therapy.
In Japan, three types of EGFR-TKI are currently available. Gefitinib was approved first, and the approved dose was 250 mg/day, approximately one-third of the maximum tolerated dose, because two randomized trials showed similar efficacy between 250 and 500 mg of gefitinib (3). Erlotinib was the second EGFR-TKI approved, with a dose the same as the maximum tolerated dose of 150 mg/day based on the OSI-774 phase I study (4). However, those data were determined based on EGFR mutation-negative patients.
On the other hand, erlotinib as a maintenance drug after first-line chemotherapy also demonstrated significantly prolonged PFS and overall survival (OS) in the overall population of a study of patients with advanced NSCLC (5,6). However, considering that serious treatment-related adverse events were reported in 12% of patients from the erlotinib group, 150 mg/day of erlotinib might be excessive for EGFR-mutated lung adenocarcinoma, especially in a maintenance setting (5). Some reports have shown favorable effects of low-dose (i.e., 25 or 50 mg/day) erlotinib (7-9). Based on such evidence, we initiated a prospective, multicenter phase II trial of low-dose erlotinib as maintenance treatment after platinum doublet chemotherapy in advanced NSCLC harboring EGFR mutation.
Methods
Study design and patients
This multicenter phase II trial was conducted to evaluate the efficacy and safety of low-dose erlotinib as maintenance treatment after platinum doublet chemotherapy in advanced NSCLC harboring EGFR mutations.
Patients with histologically or cytologically confirmed, stage IV or postoperative recurrent NSCLC harboring EGFR mutation, aged ≥20 and ≤85 years were enrolled in this study. EGFR mutation status was assessed by SRL Inc. (Tokyo, Japan) or BML Inc. (Tokyo, Japan) using the Cycleave PCR method or PCR invader method, respectively. Furthermore, other inclusion criteria were: no prior EGFR-TKI therapy; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–3; and absence of active interstitial lung disease. All patients had to have received at least one prior platinum-based chemotherapy regimen without disease progression. Patients had to have retained sufficient deglutition function to take oral medicine. Patients had to have adequate liver [serum bilirubin ≤ upper normal limit (UNL); aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2 UNL in the absence of demonstrable liver metastases; or 3 UNL in the presence of liver metastases], renal (serum creatinine ≤1.5 times UNL) and bone marrow (white blood cells ≥3×109 L−1 and platelets ≥100×109 L−1) function. Previous radiotherapy for the treatment of bone metastases or brain metastases was allowed, provided that the measurable lesions were outside the radiation fields.
The study protocol was approved by the ethics review board at each participating site and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent before undergoing any study procedure.
Patient evaluation
Baseline assessment comprised of a complete medical history, physical examination, evaluation of PS, complete blood cell count and blood chemistry, chest X-rays, computed tomography (CT) of the chest, abdomen, pelvis, magnetic resonance imaging (MRI) of the brain and a whole-body radionuclide bone scan. Tumor assessment was performed using CT, MRI, and bone scan at baseline, and at least every 2 months until disease progression was confirmed. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. All patients underwent follow-up assessments and monitoring every week within the first 2 weeks, and at least every month after 4 weeks.
Treatment
Patients received 25 mg of erlotinib once a day until the occurrence of progressive disease (PD) or unacceptable toxicity. Patients with PD continued at an increased dose of 150 mg/day.
Statistical analysis
The primary endpoint of this study was the objective response rate (ORR) at 25 mg/day of erlotinib as determined by RECIST version 1.1 (10). Secondary end-points were OS, PFS, disease control rate (DCR) and safety during the entire study period. PFS was defined as the period from enrolment until the date of confirmation of PD or the date of death from any cause, whichever was earlier. OS was defined as the period from enrolment until death due to any cause.
All patients who were eligible and received at least 1 dose of 25 mg of erlotinib were included in the safety and efficacy analysis. For ORR, we calculated the proportion of patients and 95% exact confidence intervals (CIs). Survival rates for PFS and OS were estimated by the Kaplan-Meier method. Forty patients were needed to reject a null ORR of 50% at a significance level of 5% and power of 80% with an expected ORR of 70%. This threshold was based on a prior study in which the ORR of erlotinib in patients with EGFR mutation was 70.6% (11).
This study is registered with the University Hospital Medical Information Network in Japan (No. UMIN000005468). UMIN000005468 as efficacy of low-dose erlotinib as maintenance therapy in patients of lung adenocarcinoma with EGFR mutation (http://www.umin.ac.jp/ctr/).
Results
Patient characteristics
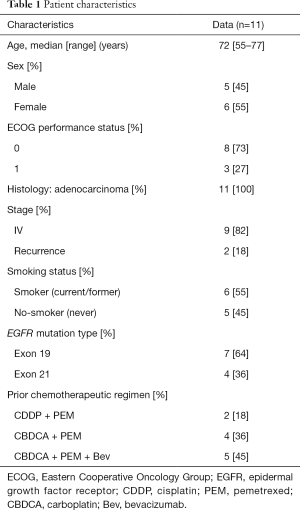
This study was stopped early, after completion of only 28% of planned enrollment, due to poor accrual. A total of 11 patients were enrolled into the study between April 2011 and April 2014 at two institutes in Japan, and efficacy and safety were evaluated among all of them. Patient characteristics are presented in Table 1.
Full table
Response to treatment
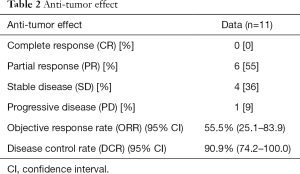
No patient achieved complete response (CR), while 6 (55.5%) achieved partial response (PR) and 4 (36.4%) showed stable disease (SD), with a DCR of 90.9% (Table 2).
Full table
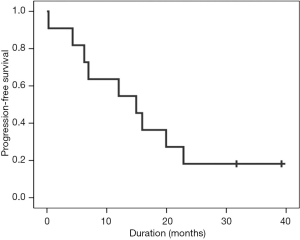
At the point of data cutoff (April 2016), 9 of the 11 patients had shown PD. Median PFS was 14.9 months (95% CI, 5.2–24.6 months) (Figure 1) and a median OS was not calculable. The recurrence included brain metastases (n=3), local (n=2), pulmonary metastasis (n=2), local plus brain metastasis (n=1) and bone metastasis (n=1) (Table 3).

Full table
Safety and toxicity
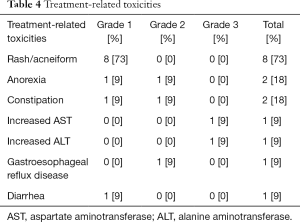
Toxicity was assessed in all patients (Table 4). One patient was forced to interrupt treatment because of adverse events (grade 3 increases in AST and ALT).
Full table
The most frequent toxicity was skin disorder, which was generally very mild. Eight patients developed grade 1 skin rash. Diarrhea was observed in one patient, but was grade 1. Other toxicities were also very mild and no interstitial lung disease was observed. No treatment-related deaths occurred.
Discussion
This represents the first prospective study to investigate the efficacy and safety of low-dose erlotinib (25 mg/day) as maintenance treatment after platinum doublet chemotherapy in NSCLC harboring EGFR mutation. The purpose of this study was to evaluate the efficacy of lower-dose erlotinib, because the full dose seems too toxic in some patients, especially in a maintenance setting. This study used ORR as the primary end point. If PD had been observed with 25 mg of erlotinib, therapy could have been continued at an increased dose of 150 mg/day until next PD was observed. Because of changes to the guidelines, including the increasing priority of EGFR-TKIs, accrual to this study was slow and the Data and Safety Monitoring Board recommended discontinuing this study after achieving only 28% of planned enrollments. The response rate for this treatment was 56%, quite similar to that with erlotinib at 50 mg/day for advanced EGFR-mutated NSCLC patients with a history of 1–3 prior chemotherapies (9). Our trial showed a PFS of 14.9 months, comparable to 44.6 weeks in EGFR-mutated patients on maintenance therapy with 150 mg/day of erlotinib in the SATURN trial (12). Good PFS seemed to result from a selection bias that patients in this study responded to the front-line treatment to some extent. However, low-dose erlotinib has the possibility of achieving efficacy in at least some EGFR-mutated NSCLC patients.
Regarding the effects of EGFR-TKIs, the cerebrospinal fluid concentration and penetration rate of erlotinib were significantly higher than those of gefitinib in the previous study (13). The frequency of recurrent brain metastasis was considered to be lower in patients treated with erlotinib than in those treated with gefitinib. Another study also found that patients with PD after gefitinib treatment could be successfully controlled by changing to standard-dose erlotinib (14). In contrast, the recurrence site most often seen after administration of low-dose erlotinib was the brain in our study. This was attributed to the decreased concentration of erlotinib, as with gefitinib therapy. In our trial, brain metastases were evaluated strictly using MRI and small metastases not demonstrable on CT could be detected. This might be another reason for increased rates of brain metastasis. For those who developed brain metastases, disease control was obtained with dose escalation of erlotinib in our study. Recently, third-generation EGFR-TKIs that can control brain metastases much better than other EGFR-TKIs have become available (15,16). This offers a reliable treatment option for patients who develop resistance against erlotinib with T790M. However, physicians should be careful in the treatment of patients with uncontrolled brain metastasis to avoid worsening the disease.
On the other hand, adverse events were generally very mild. In particular, skin rash was less than grade 2 in all patients. No patients discontinued this treatment because of erlotinib-induced toxicities, except for a short interruption due to grade 3 elevations in ALT and AST. Skin conditions of cancer patients, such as rash and paronychia, affect quality of life in daily life, particularly for female patients, so this treatment might be very attractive for them.
Maintenance therapy with erlotinib for patients harboring EGFR mutation usually continues more than 10 months, so the cost of treatment for patients could represent a serious problem (12). Enormous medical costs have been becoming an increasingly serious issue in Japan. From the perspective of the financial burdens of medical care, low-dose erlotinib treatment could decrease the expense while achieving almost the same efficacy with fewer adverse events in some patients.
Our study has several limitations. First, this study was stopped after achieving only 28% of planned enrollments without evaluation of the primary end point. Recent guidelines have recommended EGFR-TKIs as front-line therapy for patients with NSCLC harboring EGFR mutations. The opportunity to use cytotoxic agents in front-line therapy is thus decreasing. However, maintenance therapy with low-dose erlotinib might be feasibly adapted as a kind of maintenance continuation if first response to full-dose EGFR-TKI is obtained and disease symptoms disappear.
Second, presence of EGFR-mutation at the recurrence site was not confirmed in patients with postoperative recurrence. PFS of the two patients with recurrence was comparably short. The possibility must be considered that the EGFR mutation was no longer present in some cases.
In conclusion, this study was not able to demonstrate the primary end-point because of early study closure due to poor accrual, but maintenance therapy with low-dose erlotinib may offer an effective and useful option in selected NSCLC patients harboring EGFR mutation.
Acknowledgements
The authors gratefully acknowledge all persons, patients included.
Funding: This work was supported by a grant from the National Center for Global Health and Medicine (No. 22-119).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the ethics review board of National Center for Global Health and Medicine (NCGM-G-000917-01) and Funabashi Municipal Medical Center (FMMC-G-829) at each participating site and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent before undergoing any study procedure.
References
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J Clin Oncol 2003;21:2237-46. [corrected]. [Crossref] [PubMed]
- Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 2001;19:3267-79. [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [Crossref] [PubMed]
- Coudert B, Ciuleanu T, Park K, et al. Survival benefit with erlotinib maintenance therapy in patients with advanced non-small-cell lung cancer (NSCLC) according to response to first-line chemotherapy. Ann Oncol 2012;23:388-94. [Crossref] [PubMed]
- Szejniuk WM, McCulloch T, Røe OD. Effective ultra-low doses of erlotinib in patients with EGFR sensitising mutation. BMJ Case Rep 2014;2014. pii: bcr2014204809.
- Lind JS, Postmus PE, Heideman DA, et al. Dramatic response to low-dose erlotinib of epidermal growth factor receptor mutation-positive recurrent non-small cell lung cancer after severe cutaneous toxicity. J Thorac Oncol 2009;4:1585-6. [Crossref] [PubMed]
- Yamada K, Aono H, Hosomi Y, et al. A prospective, multicentre phase II trial of low-dose erlotinib in non-small cell lung cancer patients with EGFR mutations pretreated with chemotherapy: Thoracic Oncology Research Group 0911. Eur J Cancer 2015;51:1904-10. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4113-20. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415-9. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]