
Paraneoplastic leukocytosis secondary to carcinosarcoma: a report of two cases and literature review
Highlight box
Key findings
• This article describes the rare occurrence of paraneoplastic leukemoid reaction (PLR) in two cases of carcinosarcoma of different origin. It also explains its diagnostic method and management, as well as the poor prognosis involved.
What is known and what is new?
• PLR has been described in other solid tumors, but its occurrence in carcinosarcomas is rare.
• Our patients showed a progressive worsening in their renal function associated with hyperviscosity secondary to hyperleukocytosis. The determination of interleukin-17 (IL-17), vascular endothelial growth factor A (VEGF-A), interleukin-6 (IL-6), and erythrocyte sedimentation rate are an innovation in the diagnosis of this entity.
What is the implication, and what should change now?
• Try to rule out PLR early in patients with newly diagnosed carcinosarcoma and neutrophilia, in order to improve their prognosis.
• The determination of IL-17, VEGF-A, and IL-6 as biomarkers of this entity.
Introduction
Neutrophils are polymorphonuclear cells in the family of white blood cells. They share a common ancestor with monocytes, colony-forming unit-granulocyte-macrophage (CFU-GM) (1). They are part of the innate immune system and have a short life span in peripheral circulation of approximately 6–8 hours. They migrate to tissues in which they detect signs of inflammation induced by pathogenic microorganisms or tumor cells, among others, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-17 (IL-17). When they reach them, the endothelial cells of the target tissue display molecules called selectins (P-selectin, E-selectin) which capture the circulating neutrophils and attach them to the endothelium, to which they become adhered through integrins (integrin beta 2). Then, they cross the endothelium, reach the site of inflammation, and release cytokines such as IL-8 to recruit other cells in the immune system, including macrophages or mastocytes. Some of their actions include phagocytosis, followed by neutrophil degranulation, in which neutrophil extracellular traps (NETs) are released to create an antimicrobial medium if the infectious process is mediated by fungi and/or bacteria, or as part of the tumor microenvironment (TME) in neoplastic processes (1,2).
Neutrophilia is an increase in the number of neutrophils over 7.5×103/µL. The mechanism that causes it is an increase in the spinal production of neutrophils and their release into peripheral circulation. It is necessary to establish a difference between primary neutrophilia, which includes myeloproliferative syndromes such as chronic myeloid leukemia, juvenile myelomonocytic leukemia or chronic neutrophilic leukemia; and secondary neutrophilia, which is more common and includes bacterial infections, tobacco consumption and physical or emotional stress. Other less common causes of secondary neutrophilia include uremia, myocardial lesions, or neoplastic processes (2-5).
An increase in leukocytes over 50×103/µL is called a leukemoid reaction; and when it is associated with a solid tumor, it is considered a paraneoplastic syndrome called paraneoplastic leukemoid reaction (PLR) (6). Multiple cases have been reported of paraneoplastic leukocytosis associated with tumors in the lung, head and neck, gastric adenocarcinoma, melanoma, hepatocarcinoma and kidney cancer. However, it is rare in gynecological tumors (7).
We present two cases of a leukemoid reaction observed in the Medical Oncology Department of the University Hospital of Salamanca between May and September 2023. The main objectives of our article are to describe the unusual appearance of paraneoplastic leukocytosis at the diagnosis of carcinosarcoma, explain in a detailed way its diagnostic procedure and to show the poor prognosis to which it is associated. We present both cases in accordance with the CARE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-146/rc).
Case presentation
Case 1
The patient is a 60-year-old woman without relevant medical history. She does not receive regular medication and has no toxic habits.
She was referred by her primary physician to the Department of Internal Medicine in August 2023 with asthenia, lumbar pain, and weight loss of 12 kg of 3 months of evolution. The physical examination revealed a palpable hypogastric mass, and the blood analysis showed neutrophils 29×103/µL, C-reactive protein (CRP) 6.65 mg/dL and procalcitonin (PCT) 14.21 ng/mL with no signs of an active infection (no fever and no microorganisms found in the blood or the urine cultures). An abdominal, pelvic, and thoracic computed tomography (CT) scan revealed a heterogenous solid mass with necrotic areas originating in the uterus, with loss of the flat plane in the abdominal wall, bladder, cecal pole and adjacent loops of the ileum and jejunum, together with adenopathy in the root of the mesentery and the free intraperitoneal fluid. She underwent laparotomy with hysterectomy, double adnexectomy, omentectomy, partial cystectomy and resection of the cecal pole. The anatomopathological diagnosis was carcinosarcoma of the uterus that was vimentin+, PAX8+, CK7+, CK20+, WT1−, estrogen receptor (ER)−, progesterone receptor (PR)−, patchy p16, wild type p53, MSH-2+, MSH-6+, MLH-1−, PMS-2−, and with loss of expression of MLH-1 and PMS-2 [mismatch repair deficiency− (MMRd−)] (Figure 1). Ten days after the intervention, the patient showed a progressive worsening in her renal function associated with hyperviscosity secondary to hyperleukocytosis caused by 120.6×103/µL neutrophils (Table 1). An analysis was conducted and it revealed IL-6 68.4 pg/mL, CRP 24.2 mg/dL, erythrocyte sedimentation rate (ESR) 59 mm, IL-17 3.6 pg/mL, and vascular endothelial growth factor A (VEGF-A) 393.7 pg/mL. A molecular biology analysis was performed and it did not detect mutations in JAK2, CALR, or MPL, which ruled out the diagnosis of essential thrombocythemia and polycythemia vera; and new blood cultures, urine cultures, and polymerase chain reaction (PCR) tests for viruses were performed without isolation of pathogens.
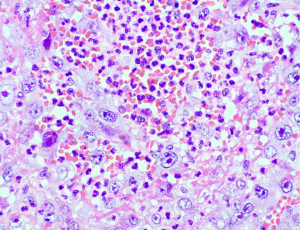
Table 1
| Values | Day 1 | Day 2 (PO) | Day 4 | Day 8 | Day 13 | Day 15 | Day 16 (ChT) | Day 17 |
|---|---|---|---|---|---|---|---|---|
| Leukocytes (×103/μL) | 32.03 | 28.01 | 41.37 | 67.5 | 125.1 | 182.2 | 170.5 | 124.7 |
| Neutrophils (×103/μL) | 29.45 | 24.38 | 38.91 | 63.81 | 120.6 | 177.8 | 165.6 | 121.8 |
| CRP (mg/dL) | 6.65 | 6.68 | 8.07 | 11.54 | 24.2 | 46.09 | 56.12 | 36.08 |
| PCT (ng/mL) | 14.21 | 3.22 | 0.49 | NV | 2.03 | 3.53 | NV | NV |
| LDH (U/L) | 254 | 232 | 350 | NV | 421 | 878 | 987 | 1,468 |
| Creatinine (mg/dL) | 0.34 | 0.39 | 0.69 | 0.55 | 0.48 | 0.64 | 1.14 | 1.87 |
| Glomerular filtration (CKD-EPI) (mL/min/1.73 m2) | 90 | 90 | 90 | 90 | 90 | 90 | 53 | 29 |
PO, postoperative; ChT, chemotherapy; CRP, C-reactive protein; PCT, procalcitonin; NV, not valuable; LDH, lactate dehydrogenase; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
The clinical diagnosis was paraneoplastic neutrophilia, and the patient started chemotherapy with a regimen of paclitaxel and carboplatin. The patient showed a poor evolution and died 96 hours later after presenting anuria, hypotension, and hypoxia, which led to multiple organ dysfunction syndrome.
Case 2
The patient is a 65-year-old man with a medical history of renal lithiasis and hepatitis B with indetectable viral load, without chronic medication.
He was admitted as an emergency in May 2023 with hypotension, decreased consciousness, and fever of 38.5 °C. An analysis showed creatinine 3.16 mg/dL, sodium 125 mmol/L, potassium 5.5 mmol/L, CRP 13.46 mg/dL, PCT 0.9 ng/mL, hemoglobin 11.5 g/dL, leukocytes 30.46×103/µL, neutrophils 28.26×103/µL. Blood cultures, urine cultures and CRP tests for viruses were performed and no pathogens were isolated. An abdominal ultrasound revealed a heterogeneous parenchyma on the right kidney that was larger than the left one (16 vs. 10 cm), with calyceal dilation and renal calyces occupied by isoechoic material. The initial diagnosis was xanthogranulomatous pyelonephritis. The patient started empiric antibiotic therapy with piperacillin/tazobactam and an emergency right nephrectomy with placement of a left double J stent was conducted (Figure 2). The anatomopathological sample showed that it was a poorly differentiated renal parenchyma carcinoma with sarcomatoid dedifferentiation (40% pleomorphic sarcomatoid carcinoma) epithelial membrane antigen (EMA)+, E-cadherin+, vimentin+, positive p53 stain 95%, with very high mitotic index (Figure 3). The patient was discharged after an improvement in the renal function and normal levels of leukocytes. He was afterwards readmitted to the Department of Medical Oncology, in which an abdominal, pelvic, and thoracic CT scan revealed involvement of the lungs, bones, liver, and retroperitoneum. At that time, the blood analysis showed 44.68×103/µL leukocytes, 42.39×103/µL neutrophils, and CRP 23.66 mg/dL. He was admitted and started intravenous antibiotic therapy after suspicion of sepsis. New blood cultures, urine cultures, and PCR tests for viruses were negative. During hospitalization, the patient showed an increase in leukocytosis in spite of the antibiotic treatment. Given the lack of isolated microorganisms in the microbiological tests, it was considered a case of paraneoplastic leukocytosis. Chemotherapy was administered with paclitaxel and carboplatin 1 week after the CT scan. When the patient started chemotherapy, he presented 55.08×103/µL leukocytes, 53.16×103/µL neutrophils, creatinine 1.83 mg/dL, and lactate dehydrogenase (LDH) 267 U/L. Eight days after receiving chemotherapy, the patient was admitted as an emergency with oligoanuria and decreased consciousness. He presented creatinine 6.25 mg/dL, phosphate 12.4 mg/dL, sodium 134 mmol/L, potassium 7.8 mmol/L, LDH 238 U/L, CRP 13.74 mg/dL, hemoglobin 9.9 g/dL, leukocytes 1.05×103/µL, and neutrophils 0.71×103/µL (Table 2). The clinical diagnosis was acute exacerbation of multifactorial mixed (renal and prerenal) chronic kidney disease associated with tumor lysis syndrome and grade 3 neutropenia. He started treatment with calcium gluconate, insulin, bicarbonate, and exchange resins. After 5 days, the patient presented an improvement in his clinical and analytical symptoms. However, given his poor clinical condition at that time, performance status (PS) 3, he was referred to the Unit of Palliative Care and died 2 months later.

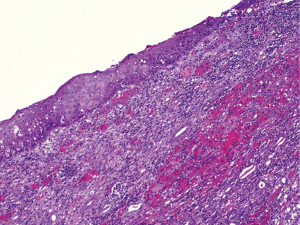
Table 2
| Values | April 2022 | May 2023 | Day +0 (PO) | Day +4 (PO) | Day +20 (CT) | Day +27 (ChT) | Day +35 |
|---|---|---|---|---|---|---|---|
| Leukocytes (×103/μL) | 6.31 | 30.46 | 18.24 | 9.42 | 44.68 | 55.08 | 1.05 |
| Neutrophils (×103/μL) | 3.5 | 28.26 | 16.34 | 8.4 | 42.39 | 53.16 | 0.71 |
| Hemoglobin (g/dL) | 4.6 | 11.5 | 9 | 9.5 | 8.3 | 10.5 | 9.9 |
| CRP (mg/dL) | 0.67 | 13.46 | NV | NV | 23.66 | 12.02 | 13.74 |
| PCT (ng/mL) | NV | 0.9 | NV | NV | 0.77 | NV | NV |
| LDH (U/L) | 175 | NV | NV | NV | NV | 267 | 238 |
| Creatinine (mg/dL) | 1.72 | 3.16 | 1.69 | 1.93 | 4.43 | 1.83 | 6.25 |
| Glomerular filtration (CKD-EPI) (mL/min/1.73 m2) | 42 | 20 | 43 | 36 | <15 | 39 | <15 |
PO, postoperative; CT, computed tomography; ChT, chemotherapy; CRP, C-reactive protein; NV, not valuable; PCT, procalcitonin; LDH, lactate dehydrogenase; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Ethical consideration
All procedures performed in this study were in accordance with the ethical standards of the University Hospital of Salamanca and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Uterine carcinosarcoma is a very aggressive type of cancer with fatal prognosis. In this carcinoma, there are heterologous elements, epithelial and stromal cells, and even areas with characteristics similar to those of osteoclasts and myoblasts. It had been previously classified as a uterine sarcoma and is currently categorized as a carcinoma that originates in a monoclonal cancerous cell with metaplastic sarcomatous activity (8). It is very rare and represents less than 5% of all uterine tumors. Its incidence is 1–4 cases per 100,000 women in the United States. A previous history of pelvic irradiation is associated with a higher risk for this type of tumor (8,9). Renal tumors with sarcomatoid differentiation represent 1–3% of all renal carcinomas. They appear more frequently in men and have immunohistochemical characteristics of epithelial and stromal cells. This type of tumor has been categorized by the Word Health Organization (WHO) as an unclassifiable renal carcinoma, and it must be differentiated from clear cell carcinoma. The term “carcinosarcoma” may also be used, with the same characteristics that have been reported in the case of the uterine tumor described (8,10).
The first case presented here showed PLR with extreme leukocytosis that led to hyperviscosity syndrome and multiple organ dysfunction after chemotherapy administration that ended with the patient’s life. The combination of paclitaxel and carboplatin was chosen according to the study by Powell et al. (11).
In the second case, although there is currently evidence of response to immunotherapy in patients with sarcomatoid variants of renal carcinoma (12), the high tumor burden, the poor condition of the patient and the objective of obtaining a rapid response to avoid hyperviscosity motivated the initial choice of chemotherapy. The first cycle showed, after 8 days, a sudden decrease in the number of neutrophils, from 53.16×103/µL to 0.71×103/µL, which led to kidney failure secondary to significant cell lysis.
Different authors have concluded that paraneoplastic leukocytosis is the secondary effect of a paracrine mechanism used by the tumor cells to stimulate their own growth (6,8). This mechanism includes the acquisition of colony-stimulating factor receptors, granulocyte colony-stimulating factor (G-CSF), by the tumor cells, as has been reported in several articles (6).
By this same logic, the tumor cells secrete proinflammatory cytokines IL-6, IL-17, or the proangiogenic cytokine VEGF, which contribute to the tumor growth through pathways regulated by STAT3 and promote proliferation of myeloid cells, which is yet another mechanism that contributes to the increased number of neutrophils (2,3,8). The role of IL-6 has been studied in autoimmune diseases, acute inflammatory processes and tumors, and its increase is considered a biomarker for a poor prognosis. The first case presented in this article showed a significant increase of IL-6 in peripheral blood, and a less significant increase of IL-17 and VEGF. Given the lack of other causes that explained the clinical symptoms, such as infection or an underlying myeloproliferative neoplasm, a diagnosis of paraneoplastic leukocytosis was reached. In the second case, the marked decrease in the number of neutrophils after the administration of chemotherapy supported our hypothesis that this was a leukemoid reaction with a paraneoplastic origin.
The management of this syndrome is based on the treatment of the underlying tumor. The complications derived from the leukemoid reaction are generally secondary to the increase in blood viscosity. In the case of our first patient, the sudden worsening of renal function, together with hypotension and hypoxia, led to suspicion that the death had been caused by multiple organ dysfunction triggered by renal and respiratory failure, and the latter may have been induced by massive pulmonary embolism.
Conclusions
Carcinosarcomas and sarcomatoid dedifferentiation carcinoma are tumors with a very poor prognosis in which each case must be evaluated individually. During evolution and even at diagnosis, complications leading to death are not infrequent. Paraneoplastic neutrophilia is a rare and serious entity that requires significant clinical suspicion for its diagnosis. The complications derived from the leukemoid reaction are generally secondary to the increase in blood viscosity. The management of this syndrome is based on the treatment of the underlying tumor. The use of some biomarkers such as IL-17, VEGF-A, and IL-6 must be taken into account in order to reach a diagnosis of certainty. Joint assessment by oncologists, hematologists, and internists of situations such as extreme neutrophilia in oncology patients could accelerate the implementation of treatment and improve survival in this type of patient. However, the evidence is not conclusive.
Acknowledgments
The authors would like to thank Teresa and Valentin for their bravery and affection.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-146/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-146/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-146/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the University Hospital of Salamanca and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang XW, Wald A, Salzmann M, et al. Cytokine alterations during paraneoplastic neutrophilia and leukemoid reaction in patients with advanced melanoma. Cancer Immunol Immunother 2023;72:509-13. [Crossref] [PubMed]
- Rozman C, Cardellach F. Medicina Interna de Farreras. v.2. 18 ed. Barcelona: Elsevier; 2016.
- Kasper D, Hauser S, Jameson L, et al. Harrison: principios de medicina interna. v.1. 19th ed. Barcelona: MacGrawHill; 2016.
- San Miguel JF, Sánchez-Guijo F Hematología. Manual básico razonado. 5th ed. Barcelona: Elsevier; 2020.
- Cruz Hernández JJ, Rodríguez Sánchez CA, Del Barco-Morillo E, et al. Oncología Clínica. 6th ed. Barcelona: Elsevier; 2017.
- Fredeau L, Bohelay G, Shourick J, et al. Paraneoplastic neutrophilic leukaemoid reaction in a patient with melanoma: association between tumour volume and leucocytosis. Br J Dermatol 2020;183:579-80. [Crossref] [PubMed]
- Chakraborty S, Keenportz B, Woodward S, et al. Paraneoplastic leukemoid reaction in solid tumors. Am J Clin Oncol 2015;38:326-30. [Crossref] [PubMed]
- Niederhuber JE, Armitage JO, Doroshow JH, et al. Abeloff's Clinical Oncology. 6th ed. Philadelphia: Elsevier; 2020.
- Pezzicoli G, Moscaritolo F, Silvestris E, et al. Uterine carcinosarcoma: An overview. Crit Rev Oncol Hematol 2021;163:103369. [Crossref] [PubMed]
- Chiu KC, Lin MC, Liang YC, et al. Renal carcinosarcoma: case report and review of literature. Ren Fail 2008;30:1034-9. [Crossref] [PubMed]
- Powell MA, Filiaci VL, Hensley ML, et al. Randomized Phase III Trial of Paclitaxel and Carboplatin Versus Paclitaxel and Ifosfamide in Patients With Carcinosarcoma of the Uterus or Ovary: An NRG Oncology Trial. J Clin Oncol 2022;40:968-77. [Crossref] [PubMed]
- Iacovelli R, Ciccarese C, Bria E, et al. Patients with sarcomatoid renal cell carcinoma - re-defining the first-line of treatment: A meta-analysis of randomised clinical trials with immune checkpoint inhibitors. Eur J Cancer 2020;136:195-203. [Crossref] [PubMed]


