
TPD52 is a prognostic biomarker and potential therapeutic target for ER+/PR+/HER2+ breast cancer
Highlight box
Key findings
• Elevated tumor protein D52 (TPD52) expression in breast cancer (BRCA) strongly associates with adverse prognosis and significantly impacts survival outcomes. It demonstrates correlations with clinical variables, molecular pathways, and immune cell infiltration. TPD52 serves as an independent prognostic biomarker for BRCA and shows promise as a potential therapeutic target, especially in refractory estrogen receptor-positive (ER+)/progesterone receptor-positive (PR+)/human epidermal growth factor receptor 2-positive (HER2+) cases unresponsive to endocrine and targeted treatments.
What is known and what is new?
• Known: TPD52 expression is increased in many tumors and existing therapies have improved breast cancer outcomes, yet resistance remains a challenge.
• New contribution: TPD52 emerges as an independent prognostic biomarker and a potential therapeutic target in treatment-resistant cases.
What is the implication, and what should change now?
• Implication: TPD52’s role in prognosis offers insights into personalized treatment approaches.
• Action needed: prioritize research on TPD52-targeted therapies for refractory breast cancer.
Introduction
Breast cancer (BRCA) is the most common malignancy in women. In 2025, it is predicted that there will be approximately 2.47 million new cases of BRCA diagnosed worldwide, leading to an estimated 768,646 deaths (1). Approximately 70% of all invasive BRCAs are hormone receptor-positive (HR+) (2), and even the women with early-stage HR BRCA who receive standard endocrine therapy for 5 years remain at risk of distant recurrence for at least 15–20 years after treatment discontinuation (3,4). Even with combination endocrine therapy, approximately 25–35% of these tumors are resistant to hormonal therapy (5). The late recurrence (>5 years after diagnosis) of HR+ BRCA is a major clinical challenge. Human epidermal growth factor receptor 2-positive (HER2+) BRCA accounts for 15–20% of breast malignancies and is characterized by aggressiveness and a high recurrence rate (6). Although its prognosis has improved with the emergence of several HER2-targeted drugs, many patients fail to respond to treatment, resulting in cancer progression. Identifying novel biomarkers associated with tumor type and prognosis is important for evaluating and treating refractory BRCAs.
Tumor protein D52 (TPD52) is an oncogene isolated from an amplified region of human chromosome 8q21. TPD52 family reportedly plays an important role in the proliferation and metastasis of various cancer cells (7-9). It participates cancer progression through multiple mechanisms, including regulating the proliferation and apoptosis of cancer cells (7). Studies have shown that overexpression of TPD52 is associated with poor survival in BRCA (10), prostate (11), colorectal (12), and lung squamous cell carcinomas (13). These findings suggest that TPD52 may hold promise as a potential therapeutic target for the treatment of various human cancers. Although the clinical significance of TPD52 has been demonstrated in many cancers, its expression and prognosis in BRCA subtypes and its related mechanisms remain unknown.
This study comprehensively evaluated the prognostic value of TPD52 expression in BRCA using The Cancer Genome Atlas (TCGA, https://cancergenome.nih.gov/) and Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) databases and further investigated the biological mechanism of action of TPD52 in BRCA pathogenesis using gene set enrichment analysis (GSEA) function and pathway analyses. Single-sample GSEA (ssGSEA) and Tumor Immune Estimation Resource (TIMER, http://timer.comp-genomics.org/) were used to explore the relative proportions of infiltration of different types of immune cells in the tumor microenvironment. Additionally, we verified the protein expression of TPD52 in clinical samples of BRCA patients from the Department of Pathology of Shanghai First Maternity and Infant Hospital using immunohistochemistry (IHC) and assessed its prognostic value. We present this article in accordance with the REMARK reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-23-156/rc).
Methods
Patient information
RiboNucleic acid RNA sequencing data and corresponding clinical information of patients with BRCA were collected from the TCGA data repository and included 1,109 BRCA and 113 healthy control samples (a total of 113 healthy breast tissue samples were acquired, representing a subset of the adjacent non-tumor tissues corresponding to the 1,109 cases of BRCA. Upon further review, it was discovered that one healthy tissue sample did not have a corresponding cancerous tissue counterpart. To maintain the integrity of paired comparisons between cancerous and non-cancerous tissues, this sample was excluded from the analysis. Consequently, our final dataset for comparative purposes included 112 matched pairs of healthy and cancerous breast tissue samples. The ‘low’ and ‘high’ TPD52 expression groups were determined based on transcriptome data derived. Expression levels were quantified as log2-transformed transcripts per million [log2(TPM+1)]. The median TPD52 expression value served as the threshold; samples with expression above the median were categorized as ‘high’ expression, whereas those below were designated as ‘low’ expression. The pan-cancer analysis of TPD52 expression using the “Gene_DE” module of TIMER2 (TIMER version 2) was conducted. To verify the expression of TPD52 in BRCA tissues, the gene expression profiles of GSE42568 and GSE18672 were downloaded from the GEO database. The selection criteria for the dataset were as follows: (I) excluding patients with a history of neoadjuvant treatment; (II) excluding patients with other neoplasm cancer status; and (III) excluding patients with less than 30 days from the last follow-up.
GSEA of TPD52
Expression datasets [high-throughput sequencing (HTSeq-Counts)] were compared between low- and high-TPD52 expression groups to identify the differentially expressed genes using the DESeq2 R package (version 1.34.0). Then, GSEA was performed using the clusterProfiler R package (version 4.2.2) to identify significant functions and pathways between the low- and high-TPD52 expression groups. The TPD52 expression level served as a phenotypic label.
Construction of PPI network
We established a TPD52-related protein-protein interactions (PPI) network using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING, https://string-db.org/) database with a minimum required interaction score of >0.4. The interactions were visualized in Cytoscape 3.7.1 after hiding disconnected nodes for better clarity.
Analysis of immune infiltration
ssGSEA from the gene set variation analysis (GSVA) package (version 1.42.0) was used to examine the relative proportions of different types of immune cell infiltration in tumor microenvironments to study the relationship between TPD52 and immune infiltration. The correlation between TPD52 and immune cell infiltration level was determined using Spearman correlation. Finally, TIMER software was used to validate the correlation between the different TPD52 expression levels and the infiltration of immune cells in BRCA samples from the TCGA database.
Immunohistochemistry
Between January 2010 and December 2015, 238 BRCA and 19 non-malignant tissues were obtained from the Department of Pathology of Shanghai First Maternity and Infant Hospital, preserved in paraffin, and sectioned. The selection criteria for the patients were as follows: (I) excluding patients with a history of neoadjuvant treatment; (II) excluding patients with other neoplasm cancer status; and (III) excluding patients who did not sign the informed consent form. None of the patients received chemotherapy or radiotherapy before surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and has been approved by the Ethics Committee of the Shanghai First Maternity and Infant Hospital [Institutional Review Board (IRB) number KS1412]. Informed consent was obtained from all individual participants included in this study. The expression of TPD52 was assessed according to the immunoreactive score (IRS). For IHC staining, all the steps were performed according to a standard LSAB protocol (Dako, Carpinteria, CA, USA). The primary antibody was rabbit antibody against human TPD52 (Abcam, Cambridge, UK; ab182578). The secondary antibodies were biotinylated swine anti-rabbit antibody (Dako). The protein level of TPD52 was detected mainly in the plasma membrane and cyto-plasma. The omission of the primary antibody served as the negative control. To facilitate statistical evaluation, TPD52 protein expression levels were reclassified according to a semi-quantitative scheme using the IRS. Low levels of expression were defined as IRS <6, and high levels of expression were defined as IRS ≥6. The statistical methods used for correlation, survival, and regression analyses were the same as those used for TCGA data.
Statistical analysis
All statistical analyses were performed using R (version 4.1.2, 2021-11-01, R Foundation, Vienna, Austria), and the expression of TPD52 was compared between BRCA and control groups using the Wilcoxon rank-sum test, and between tumor tissues and adjacent healthy tissues using the Wilcoxon signed-rank test. The relationship between TPD52 expression and clinicopathologic characteristics was assessed using the Wilcoxon signed-rank test or Kruskal-Wallis test and logistic regression. The association between the expression of TPD52 and survival outcome, along with other clinicopathologic characteristics, was determined using Cox regression and Kaplan-Meier analyses. In the Cox regression analysis, P<0.05 was considered statistically significant. The median expression value of TPD52 was considered the cut-off value.
Results
Pan-cancer TPD52 expression analysis
We assessed TPD52 expression using pan-cancer data from TCGA and Genotype-Tissue Expression (GTEx, https://www.gtexportal.org/home/). The analysis revealed that TPD52 expression was higher in 23 tumors, namely ACC, BRCA, CESC, CHOL, COAD, DLBC, ESCA, LAML, LIHC, LUAD, LUSC, OV, PAAD, PCPG, PRAD, READ, SKCM, STAD, TGCT, THCA, THYM, UCEC, and UCS. In contrast, its expression was low in KIRC and KIRP (Figure 1).

Clinicopathologic features of patients with BRCA
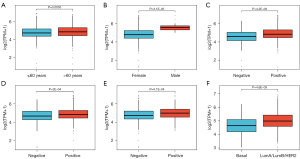
The clinicopathologic characteristics of the patients were obtained from the TCGA repository and are shown in Table 1. Upon scrutiny of the data, we found that the cohort with elevated TPD52 expression exhibited a significantly increased prevalence of ER (P<0.001), PR (P=0.004), and HER2 (P=0.005) positivity compared to the cohort with diminished expression. Moreover, relative to the basal subtype (‘Basal’ refers to a subtype with gene expression patterns similar to basal epithelial cells), the luminal subtypes Luminal A (LumA)/Luminal B (LumB)/HER2 subtype exhibited increased TPD52 expression.
Table 1
| Characteristic | High expression (n=631) | Low expression (n=503) | P value |
|---|---|---|---|
| Age, n (%) | 0.002** | ||
| ≤60 years | 321 (51.0) | 303 (60.2) | |
| >60 years | 309 (49.0) | 200 (39.8) | |
| Gender, n (%) | 0.005** | ||
| Female | 618 (98.1) | 503 (100.0) | |
| Male | 12 (1.9) | 0 | |
| Pathologic stage, n (%) | 0.77 | ||
| Stage I/II | 459 (74.8) | 377 (75.7) | |
| Stage III/IV | 155 (25.2) | 121 (24.3) | |
| ER, n (%) | <0.001*** | ||
| Negative | 104 (17.3) | 141 (29.3) | |
| Positive | 497 (82.7) | 341 (70.7) | |
| PR, n (%) | 0.004** | ||
| Negative | 174 (29.0) | 180 (37.4) | |
| Positive | 425 (71.0) | 301 (62.6) | |
| HER2, n (%) | 0.005** | ||
| Negative | 304 (74.1) | 282 (82.9) | |
| Positive | 106 (25.9) | 58 (17.1) | |
| PAM50, n (%) | <0.001*** | ||
| Basal | 75 (13.7) | 80 (25.7) | |
| LumA/LumB/HER2 | 472 (86.3) | 231 (74.3) |
**, P<0.01, *** P<0.001. The total number of patients with high TPD52 expression is 631, and the total number with low TPD52 expression is 503. Some clinical characteristics sums differ due to missing data for certain patients. For example, one patient’s age is missing in the high expression group, accounting for the discrepancy by one. TCGA, The Cancer Genome Atlas; TPD52, tumor protein D52; PR, progesterone receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Overexpression of TPD52 in BRCA
TPD52 expression was significantly augmented in the 1,109 BRCA cases compared to that in the 112 healthy tissue samples (P=9.6E−43) as per the results of the Wilcoxon rank-sum test (Figure 2A). Furthermore, compared to 112 adjacent healthy tissue samples, TPD52 expression was markedly elevated in BRCA specimens (P=2.2E−17), as evidenced by Wilcoxon signed-rank tests (Figure 2B). Receiver operating characteristic (ROC) analysis revealed that the area under the curve (AUC) for TPD52 mRNA expression in BRCA was 0.891 [95% confidence interval (CI): 0.866–0.915] (Figure 2C), with an optimal cut-off value of 4.467. To validate these findings across additional datasets, we evaluated TPD52 expression using the GSE42568 and GSE18672 datasets from the GEO database. The ensuing analysis corroborated the increased TPD52 expression in BRCA relative to that in healthy tissue samples (Figure 2D,2E).

Effects of TPD52 overexpression on clinicopathologic characteristics
As shown in Figure 3, increased TPD52 expression significantly correlated with gender (male vs. female, P=3.1E−05), age (>60 vs. ≤60 years, P=0.0055), ER status (positive vs. negative, P=4.2E−09), PR status (positive vs. negative, P=2E−04), HER2 status (positive vs. negative, P=9.1E−04), and PAM50 subtypes (basal vs. LumA/LumB/HER2, P=4.6E−09), where ‘PAM50’ represents the prediction analysis of microarray 50 gene signature used to classify BRCAs into molecular subtypes.
The ROC curve showed a strong diagnostic value for TPD52, with an AUC of 0.891 (95% CI: 0.866–0.915) (Figure 2C). In contrast, the survival curve for overall survival (OS), obtained from the Kaplan-Meier plotter, confirmed that a low level of TPD52 represents a good prognosis (Figure 4).

Logistic regression was used to examine the association between TPD52 expression and clinicopathologic attributes (Table 2). We found that upregulated TPD52 significantly correlated with age [odds ratio (OR) =1.458 for >60 vs. ≤60 years, P=0.002), ER status (OR =1.976 for positive vs. negative, P<0.001), PR status (OR =1.461 for positive vs. negative, P=0.004), HER2 status (OR =1.695 for positive vs. negative, P=0.004), and PAM50 subtypes (OR =2.180 for LumA/LumB/HER2 vs. basal, P<0.001). In summary, elevated TPD52 expression was intimately linked to more adverse clinicopathologic features and a predisposition towards a poorer prognosis.
Table 2
| Characteristics | Total | Univariate analysis | |
|---|---|---|---|
| Odds ratio (95% CI) | P value | ||
| Age (>60 vs. ≤60 years) | 1,133 | 1.458 (1.221–1.696) | 0.002** |
| ER status (positive vs. negative) | 1,083 | 1.976 (1.688–2.264) | <0.001*** |
| PR status (positive vs. negative) | 1,080 | 1.461 (1.205–1.716) | 0.004** |
| HER2 status (positive vs. negative) | 750 | 1.695 (1.337–2.054) | 0.004** |
| PAM50 (LumA/LumB/HER2 vs. basal) | 858 | 2.180 (1.827–2.532) | <0.001*** |
**, P<0.01; ***, P<0.001. CI, confidence interval; TPD52, tumor protein D52; PR, progesterone receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
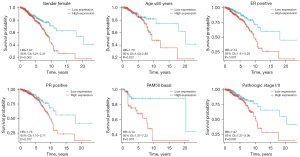
Univariate analysis for OS with Cox regression model showed that poor OS prominently correlated with TPD52 expression [high vs. low; P=0.002, hazard ratio (HR) =1.661 (95% CI: 1.199–2.301)], age (>60 vs. ≤60 years; P<0.001, HR =2.133 (95% CI: 1.556–2.923)], pathologic stage [stage III/IV vs. stage I/II; P<0.001, HR =2.448 (95% CI: 1.763–3.398)], PR [positive vs. negative; P=0.006, HR =0.637 (95% CI: 0.460–0.881)], and HER2 [positive vs. negative; P=0.039, HR =1.672 (95% CI: 1.027–2.723)] (Table 3). However, multivariate Cox regression analysis revealed that TPD52 expression [high vs. low; P=0.008, HR =1.597 (95% CI: 1.128–2.261)], age [>60 vs. ≤60 years; P<0.001, HR =2.637 (95% CI: 1.862–3.735)], pathologic stage [stage III/IV vs. stage I/II; P<0.001, HR =2.948 (95% CI: 2.098–4.143)], and PR status (positive vs. negative; P<0.001, HR =0.450 (95% CI: 0.319–0.634)] could independently predict adverse OS. Thus, patients with elevated TPD52 expression had a 1.597 times higher risk of adverse OS than patients with low TPD52 expression. Figure 5 is a subgroup OS analysis of TPD52 expression in BRCA patients. With the prolongation of survival time, the OS rate of patients with low TPD52 expression is higher than that of patients with high TPD52 expression. Among them, the OS rate of patients with low TPD52 expression is significantly higher among BRCA patients who are female, ≤60 years old, ER/PR positive, stage I/II and PAM50 Basal. Higher than patients with high TPD52 expression.
Table 3
| Characteristics | Total | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| TPD52 | 1,132 | |||||
| Low | 503 | Reference | Reference | |||
| High | 629 | 1.661 (1.199–2.301) | 0.002** | 1.597 (1.128–2.261) | 0.008** | |
| MKI67 | 1132 | |||||
| Low | 511 | Reference | Reference | |||
| High | 621 | 1.218 (0.886–1.675) | 0.22 | 1.073 (0.752–1.532) | 0.70 | |
| Age, years | 1,132 | |||||
| ≤60 | 624 | Reference | Reference | |||
| >60 | 508 | 2.133 (1.556–2.923) | <0.001*** | 2.637 (1.862–3.735) | <0.001*** | |
| Gender | 1,132 | |||||
| Female | 1,120 | Reference | ||||
| Male | 12 | 0.805 (0.112–5.761) | 0.83 | |||
| Pathologic stage | 1,111 | |||||
| Stage I/II | 835 | Reference | Reference | |||
| Stage III/IV | 276 | 2.448 (1.763–3.398) | <0.001*** | 2.948 (2.098–4.143) | <0.001*** | |
| Tumor position | 1,132 | |||||
| Left | 591 | Reference | ||||
| Right | 541 | 0.739 (0.539–1.014) | 0.061 | |||
| PR | 1,079 | |||||
| Negative | 354 | Reference | Reference | |||
| Positive | 725 | 0.637 (0.460–0.881) | 0.006** | 0.450 (0.319–0.634) | <0.001*** | |
| ER | 1,082 | |||||
| Negative | 245 | Reference | ||||
| Positive | 837 | 0.776 (0.540–1.114) | 0.17 | |||
| HER2 | 750 | |||||
| Negative | 586 | Reference | ||||
| Positive | 164 | 1.672 (1.027–2.723) | 0.039* | |||
| PAM50 | 858 | |||||
| Basal | 155 | Reference | ||||
| LumA/LumB/HER2 | 703 | 0.980 (0.639–1.503) | 0.93 | |||
*, P<0.05; **, P<0.01; ***, P<0.001. BRCA, breast cancer; OS, overall survival; CI, confidence interval; TPD52, tumor protein D52; PR, progesterone receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Univariate analysis for disease-specific survival (DSS) using a Cox regression model showed that poor DSS prominently correlated with TPD52 expression (high vs. low; P=0.049, HR =1.533 (95% CI: 1.001–2.349)], age (>60 vs. ≤60 years; P=0.023, HR =1.615 (95% CI: 1.068–2.441)], pathologic stage (stage III/IV vs. stage I/II; P<0.001, HR =3.318 (95% CI: 2.176–5.059)], PR status (positive vs. negative; P<0.001, HR =0.417 (95% CI: 0.272–0.639)], and ER status (positive vs. negative; P=0.038, HR =0.616 (95% CI: 0.390–0.973)] (Table 4). However, multivariate Cox regression analysis revealed that TPD52 expression [high vs. low; P=0.051, HR =1.580 (95% CI: 0.997–2.505)], age [>60 vs. ≤60 years; P=0.008, HR =1.855 (95% CI: 1.177–2.923)], pathologic stage [stage III/IV vs. stage I/II; P<0.001, HR =3.954 (95% CI: 2.538–6.160)], and PR status [positive vs. negative; P<0.001, HR =0.279 (95% CI: 0.160–0.487)] could independently predict adverse DSS (Table 4). Thus, patients with elevated TPD52 had a 1.58 times higher risk of adverse DSS than patients with low TPD52 expression.
Table 4
| Characteristics | Total | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| TPD52 | 1,113 | |||||
| Low | 497 | Reference | ||||
| High | 616 | 1.533 (1.001–2.349) | 0.049* | 1.580 (0.997–2.505) | 0.051 | |
| Age, years | 1,113 | |||||
| ≤60 | 614 | Reference | ||||
| >60 | 499 | 1.615 (1.068–2.441) | 0.023* | 1.855 (1.177–2.923) | 0.008** | |
| Gender | 1113 | |||||
| Female | 1101 | Reference | ||||
| Male | 12 | 1.263 (0.175–9.087) | 0.82 | |||
| Pathologic stage | 1,093 | |||||
| Stage I/II | 826 | Reference | ||||
| Stage III/IV | 267 | 3.318 (2.176–5.059) | <0.001*** | 3.954 (2.538–6.160) | <0.001*** | |
| Tumor position | 1,113 | |||||
| Left | 579 | Reference | ||||
| Right | 534 | 0.723 (0.475–1.099) | 0.13 | |||
| PR | 1,061 | |||||
| Negative | 347 | Reference | ||||
| Positive | 714 | 0.417 (0.272–0.639) | <0.001*** | 0.279 (0.160–0.487) | <0.001*** | |
| ER | 1,064 | |||||
| Negative | 238 | Reference | ||||
| Positive | 826 | 0.616 (0.390–0.973) | 0.038* | 1.186 (0.656–2.144) | 0.57 | |
| HER2 | 740 | |||||
| Negative | 579 | Reference | ||||
| Positive | 161 | 1.488 (0.743–2.979) | 0.26 | |||
| PAM50 | 841 | |||||
| Basal | 151 | Reference | ||||
| LumA/LumB/HER2 | 690 | 0.666 (0.398–1.116) | 0.12 | |||
*, P<0.05; **, P<0.01; ***, P<0.001. BRCA, breast cancer; DSS, disease-specific survival; CI, confidence interval; TPD52, tumor protein D52; PR, progesterone receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
TPD52-related signaling pathway based on GSEA
In our analysis, two signaling pathways that were positively enriched in the high TPD52 expression phenotype group were filtered out. These included the response to estradiol and the response to progesterone signaling pathways (Figure 6A). However, the signaling pathways of fatty acid, cholesterol, glucose, and alcohol metabolism, as well as AMP-activated protein kinase (AMPK) signaling pathways, were negatively enriched in the high TPD52 expression group.
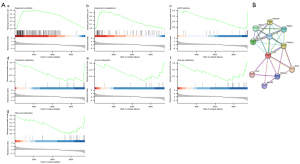
TPD52-associated PPI network
A TPD52-associated PPI network was established with 11 points, 30 edges, and an average point degree of 5.45 (Figure 6B). The PPI network revealed that several genes were closely related to TPD52, including DNAJC6, HSPA8, VAMP8, TBC1D8B, RAB5C, TPD52L1, CALU, TPD52L2, MRPS2, and MAL2.
Correlation analysis between TPD52 expression levels and degree of immune cell infiltration in BRCA
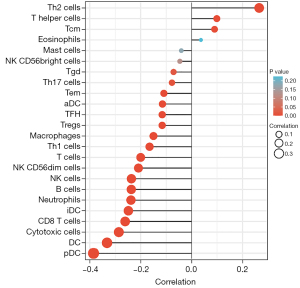
We further investigated the association between TPD52 and immune cell infiltration using ssGSEA. T helper type 2 (Th2) cells, T helper cells, and central memory T (Tcm) cells were positively correlated with TPD52, whereas plasmacytoid dendritic cells (pDCs), dendritic cells (DCs), cytotoxic cells, cytotoxic T (CD8 T) cells, immature dendritic cells (iDCs), neutrophils, B cells, natural killer (NK) cells, NK CD56dim cells, T cells, T helper type 1 (Th1) cells, macrophages, regulatory T cells (Tregs), T follicular helper (TFH) cells, activated dendritic cells (aDCs), effector memory T (Tem) cells, T helper 17 (Th17) cells and gamma delta T (Tgd) cells were negatively correlated with TPD52 in BRCA (Figure 7). The three cell types with the largest absolute values of positive and negative correlation coefficients (Th2 cells, T helper cells, and Tcm cells for positive correlation and pDCs, DCs, and cytotoxic cells for negative correlation) were subsequently validated using the TIMER and an integrated repository portal for tumor-immune system interactions (TISIDB) databases, and the results were consistent.
Protein expression of TPD52 and clinicopathologic characteristics of patients with BRCA
We performed additional immunohistochemical staining to further clarify the protein expression levels of TPD52 in BRCA tissues, comparing 238 BRCA samples to 19 healthy breast tissue specimens obtained from Shanghai First Maternity and Infant Hospital, with follow-up data available for the corresponding patient cohort.
The present study investigated the correlation between TPD52 overexpression and clinicopathologic characteristics in patients with BRCA from the Shanghai First Maternity and Infant Hospital. Immunohistochemical analysis revealed that the expression of TPD52 in BRCA tissues was also higher than in healthy tissues (Figures 8,9). Our findings indicate a statistically significant association between increased TPD52 expression and age, ER status, PR status, HER2 status, and PAM50 status (Table 5 and Figure 10), consistent with TCGA data. Similarly, logistic regression analysis (Table 6) revealed that TPD52 overexpression was significantly associated. Logistic regression analysis demonstrated a significant correlation between TPD52 overexpression and several clinicopathologic features, consistent with TCGA data, but with more pronounced differences.
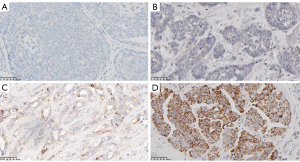
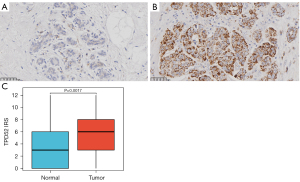
Table 5
| Characteristics | High expression (n=121) | Low expression (n=117) | P value |
|---|---|---|---|
| Age, n (%) | 0.005** | ||
| ≤60 years | 59 (48.8) | 78 (66.7) | |
| >60 years | 62 (51.2) | 39 (33.3) | |
| Pathologic stage, n (%) | 0.77 | ||
| Stage I/II | 96 (79.3) | 91 (77.8) | |
| Stage III/IV | 25 (20.7) | 26 (22.2) | |
| ER, n (%) | 0.003** | ||
| Negative | 13 (10.7) | 30 (25.6) | |
| Positive | 108 (89.3) | 87 (74.4) | |
| PR, n (%) | 0.003** | ||
| Negative | 26 (21.5) | 46 (39.3) | |
| Positive | 95 (78.5) | 71 (60.7) | |
| HER2, n (%) | <0.001*** | ||
| Negative | 76 (62.8) | 100 (85.5) | |
| Positive | 45 (37.2) | 17 (14.5) | |
| PAM50, n (%) | 0.005** | ||
| Basal | 11 (9.1) | 26 (22.2) | |
| LumA/LumB/HER2 | 110 (90.9) | 91 (77.8) |
**, P<0.01; ***, P<0.001. TPD52, tumor protein D52; PR, progesterone receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
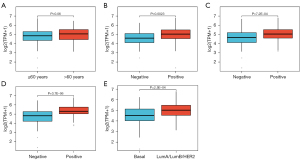
Table 6
| Characteristics | Total | Univariate analysis | |
|---|---|---|---|
| Odds ratio (95% CI) | P value | ||
| Age (>60 vs. ≤60 years) | 238 | 2.102 (1.577–2.626) | 0.005** |
| PR status (positive vs. negative) | 238 | 2.367 (1.796–2.938) | 0.003** |
| ER status (positive vs. negative) | 238 | 2.865 (2.155–3.574) | 0.004** |
| HER2 status (positive vs. negative) | 238 | 2.491 (1.846–3.137) | 0.006** |
| PAM50 (LumA/LumB/HER2 vs. basal) | 238 | 2.857 (2.099–3.615) | 0.007** |
**, P<0.01. CI, confidence interval; TPD52, tumor protein D52; PR, progesterone receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
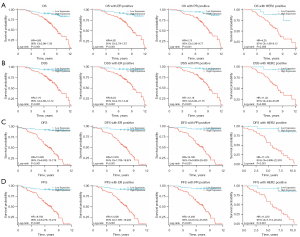
A detailed analysis of the survival curves [OS, DSS, disease-free survival (DFS) and progression-free survival (PFS)] for different patient subgroups (Figure 11A-11D) highlighted a significant impact of TPD52 expression on the survival of patients with different subtypes. In our hospital data, DFS and PFS demonstrated statistical significance, distinguishing our findings from TCGA data.
Discussion
Despite significant advancements in the therapeutic management of BRCA aimed at enhancing its prognosis, in recent years, challenges remain for patients with some types of BRCA, such as poor prognosis, insensitivity to conventional treatment, and early metastasis, which seriously threaten their life and health. The discovery of novel predictors and therapeutic targets is critical for such patients with refractory BRCA. In the current investigation, it has been observed that the mRNA and protein expression of TPD52 exhibited upregulation in BRCA tissues as compared to that in healthy tissues. Using TCGA data mining, we showed that high TPD52 expression may lead to a poor prognosis. High TPD52 expression correlated with poor OS and DSS in patients with BRCA. Elevated TPD52 expression was closely related to ER/PR/HER2-positive BRCA. Analysis of clinical samples from patients in our hospital showed that heightened TPD52 expression exhibited a significant association not only with OS and DSS but also with DFS and PFS among individuals afflicted with BRCA. The heightened expression of TPD52 is closely correlated with the positive status of ER, PR, and HER2. ER/PR-positive patients often experience long-term recurrence and metastasis, requiring a long follow-up period. However, many samples in TCGA or GEO databases have limited follow-up time; therefore, no statistical difference in DFS/PFS was found. Patients from our hospital were followed up for a long time, and more patients who failed ER+/PR+/HER+-related endocrine therapy and targeted therapy were included in the study.
Progesterone and estrogen are important drivers of BRCA cell proliferation. The implementation of functional enrichment analysis revealed a conspicuous inverse correlation between TPD52 expression and the response to estradiol. Our study found that patients with HR+ tumors demonstrated TPD52 expression, and individuals manifesting amplified TPD52 expression exhibited a more unfavorable prognosis. Therefore, it is speculated that the failure of anti-estrogen endocrine therapy in such patients, resulting in recurrence and metastasis, may be related to the poor response to estradiol in patients with high TPD52 expression (14,15). This provides new ideas for targeted therapy in patients with BRCA who have failed HR+ therapy.
In this study, we used PPI network analysis to explore the potential interaction between TPD52 and BRCA, aiming to reveal the cellular and molecular basis of TPD52 as a prognostic marker and potential therapeutic target for BRCA. In the network, proteins that directly interact with TPD52, such as DNAJC6, HSPA8, VAMP8, etc., have certain literature support, indicating that these proteins play roles in cellular stress response, molecular chaperone network, organelle transport and signal transduction. In particular, DNAJC6 and HSPA8, as molecular chaperones, are involved in the recognition and processing of misfolded proteins, which is an important mechanism for cells to cope with the pressure of malignant transformation and promote tumor development. Therefore, these findings highlight that TPD52 and its interacting partners may play a central regulatory role in malignant transformation of cells and adaptation to the tumor microenvironment. Further verification through statistical methods, we found that high expression of TPD52 is positively correlated with poor clinical prognosis of BRCA patients. This supports our hypothesis that TPD52 and its interaction network may play a promoter role in the development and progression of BRCA. This central role of TPD52 is related not only to the diversity of its direct interacting proteins but also to the complexity of its differential expression in different subtypes of BRCA. Combined with clinical data, we observed a statistically significant correlation between high TPD52 expression and DSS and DFS. This enhances the clinical significance of the PPI network analysis results and hints at the potential application value of TPD52 in the diagnosis and treatment of BRCA. In future work, verifying these interactions through functional experiments and exploring their role in pathological processes will further confirm the potential of TPD52 and its interaction network as targets in the treatment of BRCA.
With the burgeoning acknowledgment of the pivotal role played by the immune system in the genesis and progression of neoplasms, tumor immunotherapy has witnessed a precipitous and meteoric surge in its advancements in recent times (16-18). However, whether tumor immune infiltration in BRCA is associated with TPD52 expression remains unclear. To explore the underlying mechanism of action of TPD52 in BRCA tumorigenesis, we investigated the potential relationship between TPD52 and immune cell infiltration. Our findings revealed a substantial positive correlation be-tween TPD52 expression and Th2 cells while exhibiting a negative correlation with Th1 cells. A shift in the Th1/Th2 balance leads to cancer cells evading immune system surveillance (19,20), which may play a crucial role in the development and progression of BRCA. Furthermore, utilizing the ssGSEA metastasis algorithm, we observed significant negative correlations between TPD52 and various immune cell types in BRCA, including pDC, DC, cytotoxic cells, CD8+ T cells, iDCs, neutrophils, B cells, NK cells, NK CD56dim cells, and T cells. DCs, including pDCs, are essential contributors to immune defense against cancer (21,22). Activation of pDCs can prompt their differentiation into mature DCs, which possess a remarkable ability to acquire, process, and present antigens to T cells. Through this process, DCs effectively integrate environmental signals, thus establishing a vital connection between innate and adaptive immunity (23,24). NK cells, CD8 T cells, iDCs, neutrophils, B cells, NK CD56dim cells, and T cells play vital roles in antitumor immunity, and the downregulation of these immune cell types may facilitate the progression of BRCA. TPD52 expression indicates immune cell infiltration within tumor cells, thus serving as a valuable reference for BRCA immuno-therapy. Consequently, TPD52 likely plays a significant role in immune cell infiltration and holds promise as a prognostic biomarker in BRCA.
Furthermore, pathway enrichment analysis revealed that TPD52 was significantly negatively related to fatty acid, cholesterol, glucose, and alcohol metabolism pathways and negatively related to the AMPK signaling pathway, suggesting that TPD52 may affect the pathogenesis of proliferation and invasion in BRCA through the above pathways. AMPK is a central regulator of energy homeostasis, and its deregulation leads to cancer (25). Emerging evidence suggests that diminished AMPK activity promotes the development and growth of BRCA (26). TPD52 negatively correlates with AMPK, resulting in altered cellular metabolic processes that possibly drive tumor growth and progression in BRCA. Additionally, epidemiological studies have shown that patients with diabetes who receive metformin may have a low risk of BRCA owing to the potency of metformin to activate AMPK (27-29). The findings from this study provide compelling evidence supporting the role of TPD52 as a driver in cancer progression, implying that it is not only a prospective prognostic biomarker but also an enticing therapeutic target impacting crucial oncogenesis-related pathways in BRCA. Although our study reveals the prognostic relevance of TPD52 in BRCA, it also has certain limitations. We did not perform in vitro experiments to elucidate the functional role of TPD52, which limits our ability to infer mechanistic insights. In addition, the number of samples from our institution that participated in the study and could be followed over the long term was not large enough. Future expanded cohort studies and laboratory investigations are anticipated, particularly in patients with ER/PR/HER-positive refractory BRCA. Despite its limitations, this study provides clues regarding the function of TPD52 in BRCA and identifies potential therapeutic targets and prognostic markers for the management of BRCA. Taken together, we speculate that the prognosis of BRCA can be improved by controlling blood glucose and blood lipids and avoiding alcoholism in patients with high TPD52 expression.
Conclusions
Our study establishes a significant association between TPD52 overexpression and an unfavorable prognosis in patients diagnosed with BRCA. Our analysis suggests that TPD52 overexpression could serve as an independent predictive factor for reduced survival rates, specifically in patients presenting with ER/PR/HER-positive refractory BRCA. Thus, TPD52 may be a potential immunotherapy target in BRCA. Moreover, the AMPK pathway may be essential for TPD52 regulation in BRCA.
Acknowledgments
The authors acknowledge any support given to help this work.
Funding: This research was funded by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-23-156/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-156/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-23-156/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-23-156/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and has been approved by the Ethics Committee of the Shanghai First Maternity and Infant Hospital (Institutional Review Board number KS1412). Informed consent was obtained from all individual participants included in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO International Agency for Research on Cancer. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/tables?cancers=20&single_unit=100000&years=2025&types=1; 2023 [Accessed 6 June 2023].
- De Marchi T, Foekens JA, Umar A, et al. Endocrine therapy resistance in estrogen receptor (ER)-positive breast cancer. Drug Discov Today 2016;21:1181-8. [Crossref] [PubMed]
- Richman J, Dowsett M. Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer. Nat Rev Clin Oncol 2019;16:296-311. [Crossref] [PubMed]
- Pan H, Gray R, Braybrooke J, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 2017;377:1836-46. [Crossref] [PubMed]
- Kettner NM, Vijayaraghavan S, Durak MG, et al. Combined Inhibition of STAT3 and DNA Repair in Palbociclib-Resistant ER-Positive Breast Cancer. Clin Cancer Res 2019;25:3996-4013. [Crossref] [PubMed]
- Sareyeldin RM, Gupta I, Al-Hashimi I, et al. Gene Expression and miRNAs Profiling: Function and Regulation in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer. Cancers (Basel) 2019;11:646. [Crossref] [PubMed]
- Ummanni R, Teller S, Junker H, et al. Altered expression of tumor protein D52 regulates apoptosis and migration of prostate cancer cells. FEBS J 2008;275:5703-13. [Crossref] [PubMed]
- Sims AH, Finnon P, Miller CJ, et al. TPD52 and NFKB1 gene expression levels correlate with G2 chromosomal radiosensitivity in lymphocytes of women with and at risk of hereditary breast cancer. Int J Radiat Biol 2007;83:409-20. [Crossref] [PubMed]
- Abe Y, Mukudai Y, Kurihara M, et al. Tumor protein D52 is upregulated in oral squamous carcinoma cells under hypoxia in a hypoxia-inducible-factor-independent manner and is involved in cell death resistance. Cell Biosci 2021;11:122. [Crossref] [PubMed]
- Shehata M, Bièche I, Boutros R, et al. Nonredundant functions for tumor protein D52-like proteins support specific targeting of TPD52. Clin Cancer Res 2008;14:5050-60. [Crossref] [PubMed]
- Fan Y, Hou T, Gao Y, et al. Acetylation-dependent regulation of TPD52 isoform 1 modulates chaperone-mediated autophagy in prostate cancer. Autophagy 2021;17:4386-400. [Crossref] [PubMed]
- Petrova DT, Asif AR, Armstrong VW, et al. Expression of chloride intracellular channel protein 1 (CLIC1) and tumor protein D52 (TPD52) as potential biomarkers for colorectal cancer. Clin Biochem 2008;41:1224-36. [Crossref] [PubMed]
- Kumamoto T, Seki N, Mataki H, et al. Regulation of TPD52 by antitumor microRNA-218 suppresses cancer cell migration and invasion in lung squamous cell carcinoma. Int J Oncol 2016;49:1870-80. [Crossref] [PubMed]
- Daniel AR, Gaviglio AL, Knutson TP, et al. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene 2015;34:506-15. [Crossref] [PubMed]
- Santen RJ, Song RX, Zhang Z, et al. Adaptive hypersensitivity to estrogen: mechanism for sequential responses to hormonal therapy in breast cancer. Clin Cancer Res 2004;10:337S-45S. [Crossref] [PubMed]
- Del Paggio JC. Immunotherapy: Cancer immunotherapy and the value of cure. Nat Rev Clin Oncol 2018;15:268-70. [Crossref] [PubMed]
- Adams S, Gatti-Mays ME, Kalinsky K, et al. Current Landscape of Immunotherapy in Breast Cancer: A Review. JAMA Oncol 2019;5:1205-14. [Crossref] [PubMed]
- Emens LA, Adams S, Cimino-Mathews A, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of breast cancer. J Immunother Cancer 2021;9:e002597. [Crossref] [PubMed]
- Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol 1995;7:793-8. [Crossref] [PubMed]
- Ruterbusch M, Pruner KB, Shehata L, et al. In Vivo CD4(+) T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu Rev Immunol 2020;38:705-25. [Crossref] [PubMed]
- Kvedaraite E, Ginhoux F. Human dendritic cells in cancer. Sci Immunol 2022;7:eabm9409. [Crossref] [PubMed]
- Kießler M, Plesca I, Sommer U, et al. Tumor-infiltrating plasmacytoid dendritic cells are associated with survival in human colon cancer. J Immunother Cancer 2021;9:e001813. [Crossref] [PubMed]
- Anderson DA 3rd, Murphy KM, Briseño CG. Development, Diversity, and Function of Dendritic Cells in Mouse and Human. Cold Spring Harb Perspect Biol 2018;10:a028613. [Crossref] [PubMed]
- Fu C, Zhou L, Mi QS, et al. Plasmacytoid Dendritic Cells and Cancer Immunotherapy. Cells 2022;11:222. [Crossref] [PubMed]
- Patra KC, Weerasekara VK, Bardeesy N. AMPK-Mediated Lysosome Biogenesis in Lung Cancer Growth. Cell Metab 2019;29:238-40. [Crossref] [PubMed]
- Yi Y, Chen D, Ao J, et al. Transcriptional suppression of AMPKα1 promotes breast cancer metastasis upon oncogene activation. Proc Natl Acad Sci U S A 2020;117:8013-21. [Crossref] [PubMed]
- Cai H, Everett RS, Thakker DR. Efficacious dose of metformin for breast cancer therapy is determined by cation transporter expression in tumours. Br J Pharmacol 2019;176:2724-35. [Crossref] [PubMed]
- Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res 2010;16:1695-700. [Crossref] [PubMed]
- Faria J, Negalha G, Azevedo A, et al. Metformin and Breast Cancer: Molecular Targets. J Mammary Gland Biol Neoplasia 2019;24:111-23. [Crossref] [PubMed]


