
A case report of successful combined intra-arterial immunotherapy and cytokine genetic therapy treatment in a patient with recurrent glioblastoma
Highlight box
Key findings
• Concurrent administration of cytokine genetic therapy (CGT) that has a direct antitumor effect can be a promising option in the treatment of glioblastoma.
What is known and what is new?
• The disturbed proliferation of neuroepithelial cells is a possible cause of glial tumors, and CGT drugs may have a direct antiproliferative effect on them.
• CGT may also regulate dysfunctions in microglia and correct changes in cytokine milieu that usually accompany glioblastoma growth.
• Besides, CGT can decrease transendothelial electrical resistance in endothelial cells of brain microvessels, which can be connected with possible additional local effect. We fixed a complete tumor regression after the combined treatment with intra-arterial bevacizumab and following CGT with the prolonged survival period up to 25 months.
What is the implication, and what should change now?
• This case study expands the range of treatment options for glioblastoma that may lead to improved recurrence-free survival. CTG as an option can also be used to address the problem of intolerance to cytostatic drugs and their high toxicity.
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor with the prevalence rate of 3.5–5.26 cases/100,000 people annually (1). It has extremely poor prognosis due to short overall survival (OS) time and frequent recurrence.
Rationale and knowledge gap
According to various sources, the life span of patients with GBM is not longer than 9 to 17 months after the diagnosis (1,2); other researchers report median OS of 3 to 12 months (3,4). No more than 15% of patients live more than 3 years, and no more than 5% of patients live for at least 5 years.
Because of infiltrative growth pattern, the tumor often propagates to functionally significant brain areas. This, together with the working age of patients, makes the treatment of this neoplasm an immediate and urgent problem.
The standard therapy for patients with GBM involves surgical resection of the tumor mass and chemoradiation treatment.
Cytoreduction is expected after surgery. Its scope has almost no influence on the prognosis (5) even if radically performed because residual microscopic sites may remain in the brain and eventually lead to recurrence (6). The life expectancy in unresectable cases is about 3 months with symptomatic treatment and 6–7 months with additional radiation therapy.
Chemoradiation treatment in the tumor bed with total focal dose up to 60 Gy along with 75 mg/m2 temozolomide (TMZ) and subsequent adjuvant monotherapy with a standard 200 mg/m2 TMZ for 5 days (28 days interval) increases the median time to progression (TTP) from 5.0 to 6.9 months and OS from 12.1 to 14.6 months compared to radiotherapy only (7). According to Absalyamova et al. [2016], various regimens of combined chemoradiation and TMZ led to 18–24 months of recurrence-free survival (RFS) in 11.2% of cases; median TTP was 10.3 months without regard to the type of treatment, and OS was 29.2 months (8).
Second-line therapy with antibodies to vascular endothelial growth factor (VEGF) promotes the increase of RFS from 6.2 to 10 months with median OS of 9.3 months and 36% of 6-month RFS (9). A combination therapy with bevacizumab and irinotecan in the second line increases the likelihood of treatment response to 43%. According to other sources (10), bevacizumab does not significantly improve OS in spite of high vascularization of GBM. Failure to improve the OS of patients with systemic chemotherapy may be caused by systemic toxicity of chemotherapeutic drugs and their insufficient penetration of the blood-brain barrier (BBB) (11). Intra-arterial (IA) administration method is based on the hypothesis that higher concentration of a drug in a certain tumor area increases the probability of tumor cell death. Also, higher doses of chemotherapeutic agents may reduce the toxicity evident in case of systemic approach (12). Mannitol is the most common, effective, and safe method of temporary disruption of BBB, and it has good compatibility with IA chemotherapy. This delivery option of chemotherapeutic drugs improves the treatment outcome in patients with GBM. For instance, Patel et al. [2021] reported that 15 mg/kg bevacizumab led to median progression-free survival of 24 months in 32.5% cases, and median OS of 36 months in 32.1% cases (13). McCrea et al. [2021] used 15 mg/kg bevacizumab with 200 mg/m2 cetuximab, which led to median OS of 311–914 days (14). According to Zawadzki et al. [2021], therapeutic effects of IA bevacizumab resulted in 7–8 weeks of reproducible relief from symptoms (15).
Objective
The prolongation of recurrence-free period is a highly important task in the treatment of patients with GBM. It is a significant prognostic factor for OS and also the basis for objective effectiveness assessment and the choice and optimization of treatment strategy (16). The activation of antitumor immune response may be used to achieve this RFS increase, and cytokine genetic therapy (CGT) deserves special attention in this respect. At least in some cases, concurrent administration of CGT drugs, i.e., recombinant interferon gamma (IFN-γ) and tumor necrosis factor (TNF)—thymosin α1 that have a direct antitumor effect, can be a promising option in the treatment of GBM.
This paper describes a successful clinical case of CGT in a patient with recurrent GBM after a combined treatment, who received IA bevacizumab with hyperosmolar BBB opening. We present this case in accordance with the CARE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-34/rc).
Case presentation
Patient B., a 46-year-old Caucasian male, an office worker with a mainly sedentary lifestyle, does not stick to a particular diet, has no congenital disorders, and reports grade 2 hypertension and chronic acalculous cholecystitis. He has no history of surgeries and there is no data on tumor mutations.
The main symptom of the current disease was a seizure episode in the right limbs with retained awareness that lasted no longer than 1 minute. Repeated episodes were on 7th and 8th day after the manifestation. Magnetic resonance imaging (MRI) revealed a mass in the left parietal lobe.
In the first stage of treatment, microsurgical removal of the neoplasm in the left parietal lobe was performed with neurophysiological monitoring and neuronavigation. Postoperative histology confirmed grade 4 GBM in the left parietal lobe.
A course of linear accelerator (LINAC) radiotherapy in the left parietal lobe of brain (total focal dose 60 Gy) was performed 14 days after surgery, and then a 5-day (28 days interval) course of 320 mg TMZ orally was prescribed. MRI control after the first course of TMZ revealed continued tumor growth. At a case conference, it was decided to repeat surgery in order to revise the wound and remove the tumor. In the postoperative period, 2 courses of second-line therapy with bevacizumab + irinotecan were performed. MRI then revealed a recurrent tumor in the form of hypervascular neoplasm with infiltrating growth pattern on the edge of the bed of the removed tumor in the previously located postoperative cavity in the left parietal lobe. The patient addressed P. Herzen Moscow Oncology Research Institute for examination and treatment. The case was discussed at an oncological team meeting; a massive GBM recurrence in the left parietal area (Figure 1A) was considered unresectable, and repeated radiotherapy was not advised because the total focal dose was already administered and the period after the first radiotherapy was too short.
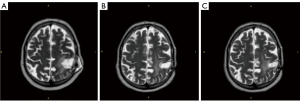
The oncological team decided on mono-therapy with IA bevacizumab with hyperosmolar BBB opening under multimodal anesthesia as a separate treatment option according to the protocol developed and approved by P. Herzen Moscow Oncology Research Institute. In total, the patient received 16 courses of drug administration to the brain arteries with hyperosmolar BBB opening (15 mg/kg bevacizumab).
We catheterized the internal carotid artery under multimodal anesthesia with access through the femoral artery in the X-ray room. Further, we performed intraarterial injection of 15% mannitol solution (120 mL) through an automatic infusion machine (Medrad, Mark 7 Arterion, Bayer) for 40 seconds. Fifteen minutes after the end of mannitol administration, we administered bevacizumab in a dosage of 15 mg/kg for 15 minutes. At the end we removed the endovascular instrument, and achieved hemostasis of the puncture site. The patient was transferred to the post-operative awakening unit.
The treatment resulted in tumor stabilization confirmed by MRI with contrast (Figure 1B) and positron emission tomography (PET), alongside with satisfactory general functional state of patient (80% by Karnofsky index).
When local control was achieved during the treatment with IA bevacizumab with hyperosmolar BBB opening, the patient received 20-day CGT with a combination of 500,000 IU recombinant IFN-γ and 100,000 IU TNF—thymosin α1 every other day. Subcutaneous injections were performed in the outpatient setting by clinical-based health care professionals. Before the course, the patient had signed an informed consent to a non-standard therapy and to the provision of clinical data for scientific research and publication; he was briefed on CGT and its risks and on the voluntary character of TNF-α blood test before and during the treatment.
During 9 courses of CGT, complete tumor regression was supported, which was confirmed by regular monthly MRI with contrast. After 9 courses of CGT, the full effect of tumor regression is still present according to MRI with contrast (Figure 1C) and PET (Figure 2). The survival period after the diagnosis is now 25 months; the patient remains under observation and has no signs of disease progression.

The objective examination of the patient showed that his overall condition is satisfactory; no abnormalities or changes were found throughout the observation period.
The neurological status assessment demonstrated an improvement in the patient’s condition.
Initially, the patient’s consciousness was clear with no meningeal symptoms or cognitive disorders, and the condition was within normal limits. The face was symmetrical and sensitive. Right-sided hemiparesis was up to grade 3 in the arm, grade 2 in the hand, and grade 4–4.5 in the leg. Tendon reflex on the right was brisk. No pathological signs were found.
At the onset of CGT, spastic monoparesis was up to grade 3.5 in the proximal arm and up to grade 3 in the hand. Hypoesthesia was in the upper right limb and Rossolimo’s sign was in the upper right. The patient performed cerebellar tests within the limits of paresis.
The examination after 5 courses of CGT revealed hypoesthesia of the right half of the face with an impression of slight flattening of the right nasolabial fold. Tendon reflexes D>S remained brisk both in the right arm and leg. Spastic upper monoparesis was up to grade 4.5 in the proximal segment and grade 4 in the hand. Hypoesthesia was in the upper right limb and Rossolimo’s sign was in the upper right.
Currently, the patient’s activity is normal with no significant pathological symptoms. He is ambulant and fully capable of self-care (90% by Karnofsky index).
We measured the serum TNF-α bimonthly and noted its raise during the CGT and was stabilized.
The dynamics of changes in the parameters assessed during treatment are shown in Table 1.
Table 1
| CGT stage | Karnofsky index, % | TNF-α, pg/mL | X-ray findings | SUV PET |
|---|---|---|---|---|
| Prior to CGT | 80 | 2.8 | Stabilization | 0.10 |
| 3 courses | 80 | 4.6 | – | – |
| 5 courses | 80 | 14.3 | Complete response | – |
| 7 courses | 90 | 18.9 | – | – |
| 9 courses | 90 | 13.7 | Complete response | 0.17 |
CGT, cytokine genetic therapy; TNF, tumor necrosis factor; SUV, standardized uptake value; PET, positron emission tomography.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The patient did not report any side effects developed during CGT that are characteristic of chemotherapy or target therapy. He was satisfied with the tolerance and the results of treatment and wished to proceed with the recommended CGT program.
Safety
CGT was well-tolerated without pronounced adverse events (AEs). The registered side effects (flu-like syndrome) were of grade AE 1 on CTCAE 5.0 scale and did not require therapy adjustment or cancellation.
Further observation
The patient currently continues CGT program with the previously prescribed doses under regular MRI control to confirm no signs of continued growth.
Figure 3 shows the timeline of events.

Discussion
Key findings
The survival time from the diagnosis is currently 25+ months, while RFS is 1 month on the first line and 2 months on the second line of therapy. This is significantly lower than the literature values suggesting a highly aggressive tumor process. The repeated surgery in less than 6 months after the first surgery additionally confirms the conclusion because this pattern is associated with a shorter survival period (7).
Strengths and limitations
The RFS was 9.5+ months with IA infusions and corresponded to previously described results for this type of treatment (14). The RFS with CGT was 9+ months. The time from the diagnosis is currently 25+ months. A combination of IA infusions of antitumor drugs and subsequent CGT led to RFS of 18.5+ months, which exceeds RFS for 1 and 2 lines of standard chemotherapy and is similar to the results of GBM patients who receive IA chemotherapy. Although the patient in this observation did not receive injections of cytostatic drugs and/or target drugs for 9 months, the tumor growth was not radiologically registered and complete disease regression was achieved. This fact is undoubtedly a strong side of the described case.
The RFS increase may be due to activated antitumor immune response manifested specifically by a higher blood TNF-α level on the background of CGT; however, other mechanisms may also be in play.
It is already known that recombinant IFN-γ in CGT can promote reduction in the number of endothelial cells and destruction of blood vessels and then tumor necrosis (17). It is also possible that administered recombinant IFN-γ makes BBB endothelium more permeable and therefore supports antitumor action of CGT in the sites of GBM growth and compensates for the local immune suppression induced by the tumor. The disturbed proliferation of neuroepithelial cells is a possible cause of glial tumors, and CGT drugs may have a direct antiproliferative effect on them. CGT may also regulate dysfunctions in microglia and correct changes in cytokine milieu that usually accompany GBM growth.
Anyway, to prove the fixed effects wide-scheduled multi-center randomized controlled studies should take place, in which the separate roles of both bevacizumab and cytokine drugs would be defined.
Comparison with similar research works
According to de Vries et al. [1996], TNF-α destructs BBB as a result of the decrease of transendothelial electrical resistance in endothelial cells of brain microvessels (18), which can be connected with possible local effect of CGT.
Tyrinova [2019] demonstrated that the cytotoxic activity of dendrite cells against GBM cells induced by IFN-α is caused by defects in TNF-α/TNF-R1 dependent mechanism of lysis, which are in turn caused by the impaired expression of membrane-bound form of TNF-α in dendrite cells (19). Cultures of dendrite cells of GBM patients treated with exogenous interleukin-2 and extracellular two-chain deoxyribonucleic acid increased the expression of membrane-bound form of TNF, and their cytotoxic activity against tumor cells improved. These effects may be present during CGT with TNF—thymosin α1. Targeted experiments are needed to clarify the mechanisms of action of CGT in GBM patients.
Explanations of findings
Consistent IA antitumor therapy with hyperosmolar BBB opening and CGT was the most effective treatment option for the described GBM patient and led to a complete and prolonged regression of the tumor.
Implications and actions needed
CGT as a part a complex antitumor treatment contributed to the maintenance of high quality of life and better antitumor immune response.
Conclusions
The search for new options of conservative adjuvant therapy of GBM that will bring better objective results of treatment and increase the survival rate remains an important clinical problem. CGT is an effective and low-toxic approach to the management of malignant tumors in adults. It can increase RFS and can successfully supplement traditional oncological treatment and improve prognosis in patients with GBM who receive chemotherapy. It can also be used to address the problem of intolerance to cytostatic drugs and their high toxicity.
Acknowledgments
We are grateful to the patient for the readiness to share the case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-34/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zolotova SV, Khokhlova EV, Belyashova AS, et al. Investigation of the metabolic features of primary glioblastomas by Tc-MIBI SPECT/CT and evaluation of their effect on disease prognosis. Zh Vopr Neirokhir Im N N Burdenko 2019;83:17-26. [Crossref] [PubMed]
- Silantyev AS, Falzone L, Libra M, et al. Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics. Cells 2019;8:863. [Crossref] [PubMed]
- Komori T. Pathology and genetics of diffuse gliomas in adults. Neurol Med Chir (Tokyo) 2015;55:28-37. [Crossref]
- Reifenberger G, Wirsching HG, Knobbe-Thomsen CB, et al. Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat Rev Clin Oncol 2017;14:434-52. [Crossref] [PubMed]
- Gessler F, Bernstock JD, Braczynski A, et al. Surgery for Glioblastoma in Light of Molecular Markers: Impact of Resection and MGMT Promoter Methylation in Newly Diagnosed IDH-1 Wild-Type Glioblastomas. Neurosurgery 2019;84:190-7. [Crossref] [PubMed]
- Müller S, Agnihotri S, Shoger KE, et al. Peptide vaccine immunotherapy biomarkers and response patterns in pediatric gliomas. JCI Insight 2018;3:e98791. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Absalyamova OV, Kobyakov GL, Ryzhova MV, et al. Outcomes of application of modern first-line chemotherapy regimens in complex treatment of glioblastoma patients. Zh Vopr Neirokhir Im N N Burdenko 2016;80:5-14. [Crossref] [PubMed]
- Greenberg MS. Handbook of neurosurgery. 8th edition. New York: Thieme; 2016. ISBN: 9781626232419.
- Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699-708. [Crossref] [PubMed]
- Kopecka J, Riganti C. Overcoming drug resistance in glioblastoma: new options in sight? Cancer Drug Resist 2021;4:512-6. [Crossref] [PubMed]
- Pinkiewicz M, Pinkiewicz M, Walecki J, et al. A systematic review on intra-arterial cerebral infusions of chemotherapeutics in the treatment of glioblastoma multiforme: The state-of-the-art. Front Oncol 2022;12:950167. [Crossref] [PubMed]
- Patel NV, Wong T, Fralin SR, et al. Repeated superselective intraarterial bevacizumab after blood brain barrier disruption for newly diagnosed glioblastoma: a phase I/II clinical trial. J Neurooncol 2021;155:117-24. [Crossref] [PubMed]
- McCrea HJ, Ivanidze J, O'Connor A, et al. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: results of a phase I trial. J Neurosurg Pediatr 2021;28:371-9. [Crossref] [PubMed]
- Zawadzki M, Walecki J, Kostkiewicz B, et al. Follow-up of intra-arterial delivery of bevacizumab for treatment of butterfly glioblastoma in patient with first-in-human, real-time MRI-guided intra-arterial neurointervention. J Neurointerv Surg 2021;13:1037-9. [Crossref] [PubMed]
- Sklyar SS, Matsko MV. Influence of clinical and molecular genetic characteristics on the first relapse-free period in patients with glioblastoma in the era of modern chemoradiotherapy. Russian Journal for Personalized Medicine 2022;2:23-34. [Crossref]
- Kammertoens T, Friese C, Arina A, et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature 2017;545:98-102. [Crossref] [PubMed]
- de Vries HE, Blom-Roosemalen MC, van Oosten M, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol 1996;64:37-43. [Crossref] [PubMed]
- Tyrinova TV. Cytotoxic activity of dendrite cells against glioblastoma cells: mediators, regulatory mechanisms, and the possibility of directed correction. Synopsis of a thesis for Doctorate in Biology. Novosibirsk 2019;39.


