
Real-world evidence of survival outcomes in breast cancer subtypes after neoadjuvant chemotherapy in a Brazilian reference center
Highlight box
Key findings
• The pathological complete response (pCR) rate among breast cancer (BC) patients receiving neoadjuvant chemotherapy (NAC) was 22.7%.
• pCR was linked to higher overall survival (OS) and disease-free survival (DFS), especially in triple negative and luminal B subtypes.
• pCR did not correlate with improved OS or DFS in the luminal A subtype.
What is known and what is new?
• NAC is increasingly used for operable BC to treat aggressive disease.
• pCR is generally associated with better survival outcomes.
• Data from a Brazilian public reference center show a pCR rate of 22.7%, with survival benefits varying by BC subtype.
What is the implication, and what should change now?
• The study highlights the need to consider BC subtypes when evaluating NAC effectiveness and pCR prognostic value.
• Treatment protocols should incorporate subtype-specific strategies to optimize outcomes for BC patients receiving NAC.
Introduction
Breast cancer (BC) remains one of the most prevalent cancers among women, with an estimated annual incidence of 47.8 new cases per 100,000 individuals (1). In Brazil, a developing country, projections for 2023 suggest that approximately 73,000 new cases of BC will be diagnosed and nearly 18,000 women may succumb to the disease (2,3). Despite significant advances in treatment, such as better chemotherapy and targeted therapies (4), identification of high-risk patients (e.g., carriers of BRCA mutations) (5) and prophylactic strategies including bilateral oophorectomy and mastectomy, BC continues to impose a significant clinical, social and economic burden, with an annual mortality rate of approximately 13.6 per 100,000 (1).
Neoadjuvant chemotherapy (NAC) for BC has evolved significantly, transitioning from being reserved for inoperable tumors to treating operable BC such as HER2 positive (6-10) and triple-negative breast cancers (TNBCs) (11). A pathological complete response (pCR) following NAC correlates with a favorable prognosis, even exceeding the outcomes of immediate surgery (9,11). Furthermore, NAC is used to facilitate breast and axillary conservation (12-15) and inform adjuvant treatment decisions after incomplete response (16,17).
The achievement of a pCR is considered a significant prognostic factor. Extensive research, including various randomized controlled trials (RCTs) and meta-analyses, has shown that patients who achieve pCR after NAC tend to have better overall survival (OS) and disease-free survival (DFS) results, this being consistent between different subtypes of BC (12).
In the realm of real-world data (RWD), OS is widely recognized by oncologists as a definitive measure due to its objectivity and compatibility with data derived from clinical trials (18-20). Furthermore, the American Society of Clinical Oncology (ASCO) has issued guidelines for classifying evidence levels, usually placing RWD at level III or IV, denoting moderate to low evidence strength. Despite this, ASCO acknowledges the importance of RWD in clinical oncology research. It is especially noted for its utility in research areas that remain unaddressed by clinical trials or where trial conduction is deemed unfeasible (21,22).
These therapeutic innovations, while promising, are based on evidence from controlled and randomized clinical trials, which may not fully represent the typical patient population encountered in daily clinical practice due to strict inclusion and exclusion criteria (19). The shift to precision oncology (21) has further narrowed the population eligible for clinical trials, increasing the discrepancy between trial participants and the general cancer population treated in real-world settings.
Interestingly, clinical trials recruit only about 5% of the total cancer population, highlighting the need for studies that include a broader range of patients (19). Specifically, in Brazil, participation in global cancer research remains low, and the 2021 Interfarma report indicates involvement in only 2.4% of global cancer studies (20). This underscores the importance of RWD to enhance our understanding of the effectiveness of treatment in standard healthcare settings (22).
Taking into account these aspects, this trial aims to evaluate RWD to improve our understanding of pCR and its correlations with OS and DFS among BC patients who have received NAC at a leading public reference center in Brazil. Furthermore, this study has investigated the connections between pCR and various BC subtypes, in order to shed light on the nuanced effects of NAC in different demographics of patients and cancer characteristics. We present this article in accordance with the STROBE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-54/rc).
Methods
Study design and data source
This study used a retrospective cohort based on RWD of a reference center for the treatment of female cancers in Brazil (Women’s Health Hospital). As it was an analysis of the secondary database of the institution, the present study was approved by the ethics committee. The ISPE/ISPOR recommendations for the development of an exploratory study in the real world were followed (23). Data from women diagnosed and treated in the aforementioned healthcare setting were considered for this study from January 2011 to December 2020.
Ethics
This study was submitted and approved by the Research Ethics Committee of the Women’s Health Hospital (No. CAAE 39097520.4.2001.0069). This was a retrospective study and the requirement for informed consent was waived due to this. This study was carried out according to the principles of the Declaration of Helsinki (as revised in 2013), including the protection of patient confidentiality.
Follow-up, inclusion, and exclusion criteria
Each patient was followed from diagnosis until their last hospital visit (if alive) or death. Women older than 18 years with a diagnosis of nonmetastatic BC treated with NAC were included in this study. Patients with inflammatory BC, stage IV cancer at diagnosis, missing data, or who participated in clinical trials were excluded.
Outcomes and clinical data
The present RWD study had an exploratory focus; no treatment superiority or other specific hypothesis testing was evaluated. The primary objective of this study was to find an association between pCR and the clinical outcomes of OS and DFS. Furthermore, an exploratory analysis was performed via the correlation of BC subtypes and differences in OS and DFS associated with pCR.
The clinical data from this study comprised demographic variables (age, weight, body mass index, etc.), cancer-related variables (staging, type of BC, Ki-67 index and cancer subtypes, such as triple negative, luminal A, etc.) and treatment-related variables (type of NAC used). The morphology of neoplasms was coded according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) (24,25). Sarcomas and other morphological types were classified as ‘other subtypes’. Histological grade was classified as GI, GII or GIII, according to the Bloom and Richardson classification (26). The expression of estrogen receptor (ER) and progesterone receptor (PR) by immunohistochemistry was considered positive when 1%. HER2 expression was considered positive when reported as 3+ or confirmed by immunofluorescence technique. The classification of the tumor phenotype was carried out according to the recommendations of the St. Gallen Consensus of 2023 (27). Differentiation between luminal phenotypes was carried out according to Ki-67 expression, with luminal A considered when Ki-67 <14% (28,29).
NAC
Patients received NAC according to institutional protocols and based on availability and access to medications. Access to medications for the treatment of HER2+ patients is not universal in Brazil. The treatment regimens used included 4 cycles of doxorubicin + cyclophosphamide followed by 4 cycles of docetaxel for all patients. HER2 positive patients may have used the same regimen with or without trastuzumab (30), and TNBC patients may have received carboplatin-containing regimens.
pCR definition
We defined pCR as no residual invasive tumor or ductal carcinoma in situ (DCIS) in the primary tumor bed or ipsilateral axillary lymph nodes (ypT0 ypN0) (31).
Analyses
The primary outcome of this study was to evaluate the relationship between clinical data and pCR. To accomplish this, patients were divided into pCR and non-pCR groups; our aim was to explore if there was an association between clinical information and outcome using Fisher or Chi-squared tests for categorical covariates. Student’s t-test was used to assess differences between groups of parametrically distributed data (e.g., age).
To statistically compare the effectiveness of each NAC regimen, we used a forest plot analysis. This approach provided a visual and quantitative comparison of odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) across different treatment schemes. The ORs were calculated to assess the probability of achieving pCR in each chemotherapy scheme compared to the aggregated data from all other schemes.
To assess OS and DFS, the Kaplan-Meier method and the log rank test were used to assess differences in the pCR and non-pCR subgroups. Both OS and DFS curves considered 5 years as the cut-off point for right-censoring. To define the duration of OS and DFS, the diagnosis date was used as the initial time and the death and/or recurrence date was used as the final event time. All inferential analyzes considered a P value <0.05 as a statistically significant cutoff.
The present study required an estimate of the number of patients in whom pCR would be achieved given a population of BC patients exposed to NAC. As Brazil has a limited budget healthcare system, in which innovative drugs are not available in the public system, we searched the results of the National Surgical Adjuvant Breast and Bowel Project Protocols B-27 (NSABP) study (10) for evidence related to the use of NAC in Brazil. In that study, of the patients who received NAC, 26.3% of the patients in the non-pCR group died (n=490 deaths of 1,857 patients), while 10.3% of the patients in the pCR group died (n=42 of 397 patients) at the median follow-up of eight years. That is, of the 2,254 patients, 17.6% (n=397/2,254) had pCR. Considering a power of 90% and a two-tailed alpha of 5%, at least 30 pCR events were required to find a significant difference in pCR in our study. For a sample size considering survival in patients with pCR, at least 40 death events were required to find differences in survival between groups of patients without pCR and pCR. Therefore, we considered a sample of at least 1,500 patients sufficient for the present study using a 5-year follow-up horizon.
Results
Sample characteristics and NAC
From 2011 to 2020, 2,862 of the 8,002 patients in the database received NAC; 1,201 were excluded from this study due to having inflammatory cancer (n=325), participating in the randomized clinical trial (RCT) (n=375), inoperable BC (n=380) and patients with progression to metastatic disease (n=121). In general, 1,601 patients who underwent surgical BC and NAC treatment were included in this study (Figure 1). These women had clinical disease in the majority stage IIIA (35%), with a mean age of 49±10.7 years (a minimum of 20 years and a maximum of 84 years) and a mean body mass index of 28.06±5.6 kg/m2; most were premenopausal (61.2%). In total, 19.5% of the patients had a first-degree relative with a history of BC.
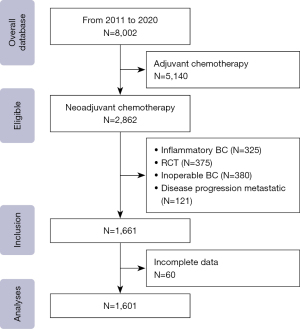
The predominant histological type was non-special invasive carcinoma (94.2%); 49.4% of the patients presented grade 2 histological findings.
Most of the patients did not present with angiolymphatic or perineural invasion (89.5% and 96.6%, respectively). Lymphocytic infiltration was present in 98.3% of the patients. The proliferation index (Ki-67 index 14) was high in 86.7% of patients.
The predominant histological type was non-special invasive carcinoma (94.2%); 49.4% of the patients presented grade 2 histological findings (Table 1).
Table 1
| Characteristics | pCR (n=364) | Non-pCR (n=1,237) | P value* |
|---|---|---|---|
| Age, years | 49±10.84 | 49±10.76 | >0.99 |
| Weight, kg | 70.8±15.19 | 71±14.63 | 0.82 |
| Height, m | 1.59±0.06 | 1.59±0.07 | – |
| Body mass index, kg/m2 | 28±5.73 | 28±5.52 | >0.99 |
| Menopausal status | 0.32 | ||
| Premenopause | 214 (58.8) | 766 (61.9) | |
| Menopause | 151 (41.4) | 470 (38.0) | |
| Breast cancer family history | |||
| First degree | 42 (11.5) | 119 (9.6) | 0.28 |
| Second degree | 27 (7.4) | 124 (10.0) | 0.13 |
| No history | 295 (81.0) | 994 (80.4) | 0.77 |
| Histological type | |||
| IDC | 346 (95.1) | 1,163 (94.0) | 0.45 |
| ILC | 14 (3.8) | 51 (4.1) | 0.81 |
| Other | 4 (1.1) | 23 (1.9) | 0.48 |
| Histological grade (SBR) | |||
| G1 | 93 (25.5) | 326 (26.4) | 0.75 |
| G2 | 178 (48.9) | 616 (49.8) | 0.76 |
| G3 | 93 (25.5) | 295 (23.8) | 0.50 |
| Angiolymphatic invasion | 0.53 | ||
| Presence | 35 (9.6) | 133 (10.8) | |
| Absence | 329 (90.4) | 1,104 (89.2) | |
| Perineural invasion | 0.78 | ||
| Presence | 15 (4.1) | 55 (4.4) | |
| Absence | 349 (95.9) | 1,182 (95.6) | |
| Ki-67 index | 0.57 | ||
| ≤14 | 45 (12.4) | 167 (13.5) | |
| >14 | 319 (87.6) | 1,070 (86.5) | |
| Estrogen receptor | 0.44 | ||
| Positive | 196 (53.8) | 694 (56.1) | |
| Negative | 168 (46.2) | 543 (43.9) | |
| Progesterone receptor | 0.64 | ||
| Positive | 173 (47.5) | 605 (48.9) | |
| Negative | 191 (52.5) | 632 (51.1) | |
| HER2 | 0.35 | ||
| Positive | 92 (25.3) | 284 (23.0) | |
| Negative | 272 (74.7) | 953 (77.0) | |
| Subtypes | |||
| Luminal A | 34 (9.3) | 131 (10.6) | 0.49 |
| Luminal B | 126 (34.6) | 437 (35.3) | 0.80 |
| HR+/HER2 | 43 (11.8) | 146 (11.8) | 0.99 |
| TNBC | 112 (30.8) | 385 (31.1) | 0.89 |
| HER2 | 49 (13.5) | 138 (11.2) | 0.22 |
| Clinical staging AJCC (TNM) | <0.001 | ||
| I | 10 (2.7) | 32 (2.6) | |
| IIA | 18 (4.9) | 75 (6.1) | |
| IIB | 85 (23.4) | 266 (21.5) | |
| IIIA | 193 (53.0) | 490 (39.6) | |
| IIIB | 56 (15.4) | 346 (28.0) | |
| IIIC | 2 (0.5) | 28 (2.3) |
Data are presented as mean ± SD or n (%). *, Chi-squared test. pCR, pathological complete response; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; SBR, Scarff-Bloom-Richardson; TNBC, triple-negative breast cancer; AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; SD, standard deviation.
Regarding tumor subtypes, luminal A, luminal B, HER2, and triple negative were present in 10.3%, 35.2%, 23.4% and 31.1% of patients, respectively, and a significant portion of the sample had axillary involvement, with N1 and N2 in 44.6% and 31.6% of patients, respectively.
The NAC schemes were distributed among five different combinations, the most commonly used among all patients was AC-T (40.6%) and its variants, with the addition of trastuzumab in patients positive for HER2 (10.9%).
Response to NAC
In our investigation of the effectiveness of NAC, we evaluated patient outcomes post-treatment when approaching potentially curative surgery. Our cohort consisted of 1,601 individuals, among whom 364 (22.7%) achieved a complete response, with the absence of tumor cells in both breast tissue and lymph nodes (pCR denoted ypT0 ypN0). When considering cases with residual noninvasive cancer cells present (labeled as DCIS), the aggregate of patients exhibiting complete response increases marginally to 392 (24.5%), a statistic not considered significant (P=0.56) (Table 1).
We noted the variability in complete response rates among different BC subtypes, although the disparity was not statistically substantial. Furthermore, patients diagnosed with advanced stages of cancer, particularly stages IIIA and IIIB, showed a higher probability of complete response (statistical significance P<0.001), despite a smaller patient population in these stages (Figure 2A,2B).

In our analysis of NAC regimens, we evaluated the effectiveness of different schemes to achieve pCR. We investigated the results of five NAC schemes: AC-T, AC-TPlatin, TAC, FAC, and AC-TH. Our findings revealed varied response rates: AC-T led to a pCR in 148 out of 656 cases (22.6%), AC-TPlatin in 79 out of 298 cases (26.5%), TAC in 60 out of 325 cases (18.5%), FAC in 27 out of 96 cases (28.1%), and AC-TH in 50 out of 226 cases (22.1%). These results provided a foundational understanding of how each chemotherapy regimen performs in terms of inducing complete tumor regression before surgical intervention (Table 2).
Table 2
| Neoadjuvant chemotherapy | pCR (n=364), n (%) | Non-pCR (n=1,237), n (%) | P value* |
|---|---|---|---|
| AC-T | 148 (40.7) | 508 (41.1) | <0.001 |
| AC-TPlatin | 79 (21.7) | 219 (17.7) | <0.001 |
| TAC | 60 (16.5) | 265 (21.4) | <0.001 |
| FAC | 27 (7.4) | 69 (5.6) | <0.001 |
| AC-TH | 50 (13.7) | 176 (14.2) | <0.001 |
*, Chi-squared test. pCR, complete pathological response; A, anthracycline; C, cyclophosphamide; T, taxane; Platin, carboplatin; FAC, 5-fluoracil/adriamycin/cyclophosfamide; H, trastuzumab.
Subsequent statistical analysis, visualized through a forest plot, presented the ORs and 95% CIs for achieving pCR in each chemotherapy scheme. Specifically, AC-T had an OR of 0.99 (95% CI: 0.78 to 1.25, P<0.001), suggesting that there were no significant differences from the overall population response rates. AC-TPlatin showed an OR of 1.30 (95% CI: 0.97 to 1.73, P<0.001), indicating a nonsignificant chance of achieving pCR. The TAC exhibited an OR of 0.73 (95% CI: 0.54 to 0.99), suggesting a significantly lower chance of achieving pCR compared to the overall population. FAC presented an OR of 1.36 (95% CI: 0.86 to 2.16, P<0.001), which implies a not significant difference in pCR. Lastly, AC-TH had an OR of 0.93 (95% CI: 0.66 to 1.30, P<0.001), showing no significant variance from the general population (Figure 3).
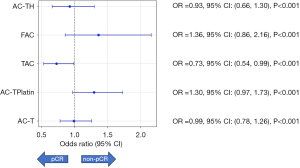
The statistical significance was underlined by P values, and all schemes except TAC did not show a significant deviation from the expected rates of pCR considering conventional thresholds. The TAC scheme was notably notable for its significantly lower odds of achieving a pCR, as highlighted by its OR and associated P value. This analysis underscores the variations in effectiveness among different NAC regimens and emphasizes the need for personalized treatment approaches based on the specific characteristics and response rates of the cancer being treated.
Secondary outcomes (OS and DFS)
In general, locoregional recurrence was observed in 1.9% of pCR patients; 9% of patients without pCR exhibited multiple metastases. In the sample studied, 25% of patients with pCR died; however, a death event was only present in 21% of patients who did not show pCR during the 5-year follow-up (Table 3).
Table 3
| Variables | pCR | Non-pCR | P value* |
|---|---|---|---|
| Recurrence, n (%) | 0.187 | ||
| Locoregional recurrence | 7 (1.9) | 13 (1.1) | |
| Breast | 4 (1.1) | 6 (0.5) | |
| Axillary | 3 (0.8) | 7 (0.6) | |
| Wall chest | 1 (0.3) | 1 (0.1) | |
| No recurrence | 357 (98.1) | 1,224 (98.9) | |
| Distant metastasis, n [%] | <0.001 | ||
| Total | 23 [6] | 244 [20] | |
| Bone | 6 [2] | 37 [3] | |
| Lung | 7 [2] | 61 [5] | |
| Central nervous system | 3 [1] | 17 [1] | |
| Multiple | 7 [2] | 115 [9] | |
| No metastasis | 341 [94] | 993 [80] | |
| Deaths, n (%) | 92 (25.3) | 263 (21.3) | 0.10 |
| Breast cancer | 72 (19.7) | 231 (19.5) | |
| Cardiovascular disease | 5 (1.4) | 8 (0.6) | |
| Stroke | 5 (1.4) | 7 (0.5) | |
| Sepsis | 10 (2.8) | 9 (0.7) |
*, Chi-squared test. pCR, pathologic complete response.
Using the Kaplan-Meier method, survival at 60 months did not reach the median, and more than 50% of the patients were alive. DFS was 90.4% in the pCR group and 66.7% in the non-pCR group, with a log rank P<0.001. OS was 89.0% in the pCR group and 61.0% in the non-pCR group, with a logarithmic rank P<0.001 (Figure 4A,4B).

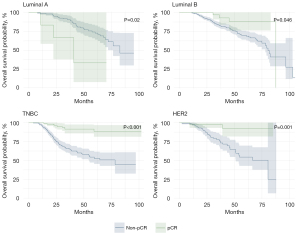
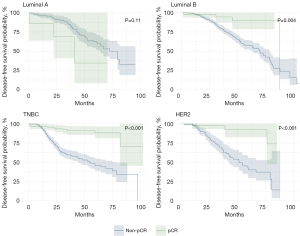
The analyses of pCR status based on OS and DFS by tumor subtype are shown in Figures 5,6. Patients with luminal A had higher OS when they did not reach pCR (pCR 35% vs. non-pCR 35%, P=0.02). Luminal B, TNBC, and HER2+ patients had better OS when they achieved pCR, as follows: luminal B (pCR 90% vs. non-pCR 12.5%, P=0.046), TNBC (pCR 92% vs. non-pCR 45%, P<0.001) and HER2+ (pCR 92% vs. non-pCR 25%, P=0.001). The same was observed for DFS; there was no significant difference in luminal A patients (pCR 35% vs. non-pCR 34%, P=0.11). Luminal B patients, TNBC and HER2+ also showed better DFS when receiving pCR, as follows: luminal B (pCR 86% vs. non-pCR 12.5%, P=0.004), TNBC (pCR 77% vs. non-pCR 32.5%, P<0.001) and HER2+ (pCR 73% vs. no pCR 12.5%, P<0.001).
Discussion
Our study is pioneering in evaluating the impact of various definitions of pCR on the outcomes of RWD among a substantial cohort of BC patients from a single reference center in Brazil. These patients received NAC regimens based on anthracycline and taxanes, along with combinations including neoadjuvant trastuzumab and platinum-based treatments. In addition, this research aims to elucidate the prognostic significance of the various subtypes of BC.
pCR after NAC may be an important landmark. In clinical trials, a systematic review of met analyzes suggested that achieving pCR was associated with a 64% increased survival chance (hazard ratio =0.36) (17,23,31). On the contrary, a larger systematic review, with a meta-analysis that aimed to evaluate pCR as an endpoint of OS and DFS, suggested that there was a weak association between pCR and OS/DFS (32-34).
In general, our study was consistent with the literature on the accepted range of pCR rates associated with patients exposed to BC by NAC (20–40%), as well as systematic reviews (32-34). The current study highlighted the importance of RWD in evaluating results relevant to daily practice; we found that pCR was a relevant predictor of OS and DFS.
Importantly, few covariates were associated with pCR. This was an interesting finding of this study, which was aligned with another real-world assessment of pCR in TNBC patients who received NAC (32,34-36). Many authors believe that better biomarkers are required to better characterize BC and assess associations between pCR and OS/DFS. Despite the emergence of new targeted therapies (34), the impact of NAC on pCR and OS has been poorly investigated in the literature (36,37).
Our research indicated a pCR rate of 22.7%, reflecting the results of various institutions that do not segment data by subtypes, with pCR rates that fluctuate between 13.0% and 26.1% in all subtypes in RCT data after anthracycline- and taxane-based chemotherapy treatments (38-40). Interestingly TAC regiments led to only 16.5% pCR rate, that can be explained because patients with more aggressive disease or larger tumors were prescribed TAC to achieve a better response, a potential bias. Moreover, most of the patients who received the TAC regimen were HER2 positive at a time when we did not have access to neoadjuvant trastuzumab. A retrospective analysis of the Public Health System (PHS) in Chile that included 439 patients reported a pCR rate of 24.1% (41). In contrast, a separate investigation in China with a cohort of 240 patients observed a pCR rate of 12.5% (42). Rezende et al. (43) analyzed 310 women in the public healthcare system in Brazil who underwent NAC, documenting a pCR rate of 13.9%. Additionally, a retrospective evaluation involving 1,639 patients in Mexico showed a pCR rate of 28.4% (44). The consistent use of anthracyclines and taxanes as the primary components of NAC in both RCTs and RWD reflects consistent trends.
Moreover, Cortazar et al. (33) documented that pCR exhibited the weakest correlations with hormone-receptor positive, low-grade, and HER2-positive tumors. Correspondingly, von Minckwitz et al. (40) observed that pCR correlations were strongest with more aggressive subtypes of BC, while demonstrating weak prognostic significance in patients with luminal subtypes A or B. These results are in alignment with our study’s conclusions.
Importantly, throughout the duration of this study, double blockade (trastuzumab and pertuzumab) was not available to treat HER2 positive patients, and there was no possibility of using immunotherapy for triple negative patients. pCR was achieved in approximately 11% of patients with the HER2 positive subtype; an explanation for this is that the institutions had not received trastuzumab before 2016 in the adjuvant or neoadjuvant setting. In other words, the use of anti-HER therapy was low in our study population; this article may be valid for countries with low access to such monoclonal antibodies (35-38). Recognized adjuvant treatments that reduce relapse and even mortality, such as adjuvant capecitabine for triple negative disease and TDM-1 for HER2 positive disease with incomplete response, were also not available in the setting of this study (16,17,38-43).
Another important finding of our study was that the Ki-67 index did not show an important statistical correlation with pCR. The Ki-67 index is a nuclear antigen expressed in the G1, S and G2 phases (but not in the resting state of the cell cycle). As a relevant biomarker for BC proliferation, the optimal cut-off point of the Ki-67 index has been reported heterogeneously through many studies; in most cases, the literature uses empirical categorization (>14%, >15% or even >30% Ki-67 expression) (39,45,46). Nishimura et al. [2010] (46) suggested that in a locally advanced subset of patients exposed to NAC, mean Ki-67 levels were 63% in the pCR group and 45% in the non-pCR group. There were no patients with non-pCR status who showed Ki-67 levels higher than 25% (47,48). More interestingly, Nishimura et al. suggested that decreased Ki-67 values after NAC might have a predictive role for pCR, as they showed that DFS was higher in patients with a Ki-67 index <12% on the 4,000th day after NAC (46). This study illustrated that the Ki-67 index should not be used separately from other biomarkers and that its value could be potentialized if explored as a dynamic variable, not as a static BC marker (28,41).
The analysis of the status of pCR in relation to OS and DFS across different tumor subtypes, as shown in Figures 5,6, presents notable findings. Interestingly, patients with luminal A showed a higher OS rate when they did not achieve pCR (35% for both pCR and non-pCR, P=0.02), which is a unique outcome among the studied groups. This could suggest that, for patients with luminal A, pCR is not a significant prognostic marker of OS, possibly due to the inherently less aggressive nature of luminal A tumors or other factors not directly related to tumor response to chemotherapy. This notion aligns with findings from Cortazar et al. (33) and is supported by research presented by von Minckwitz et al. (40), indicating that pCR may not significantly impact luminal A outcomes due to their specific tumor biology (49,50).
On the contrary, patients with the luminal B, TNBC, and HER2+ subtypes demonstrated significantly better OS rates after achieving pCR. Specifically, luminal B patients had an OS rate of 90% with pCR compared to 12.5% without, TNBC patients had an OS of 92% with pCR versus 45% without, and HER2+ patients showed an OS rate of 92% with pCR compared to 25% without. These differences underscore the importance of achieving pCR in these subtypes, indicating that pCR is a strong prognostic factor for better survival outcomes. The substantial benefits of pCR in these more aggressive subtypes are well documented in the literature, including meta-analyses and studies that highlight the prognostic value of pCR, particularly in patients with TNBC and HER2+ (49,50).
The DFS results mirrored these patterns; luminal A patients did not show a significant difference in DFS between pCR and non-pCR (35% vs. 34%, P=0.11), again suggesting that pCR might not be a critical endpoint for this subgroup. However, for patients with luminal B, TNBC, and HER2+, a significant improvement in DFS was observed for those achieving pCR. The DFS rates were 86% versus 12.5% for luminal B, 77% versus 32.5% for TNBC and 73% versus 12.5% for HER2+ for pCR versus non-pCR, respectively. The improvements in DFS for these subtypes reinforce the prognostic significance of pCR and are consistent with existing research that underscores the variable impact of pCR in different BC subtypes (51).
These findings suggest that while pCR is an important indicator of prognosis for patients with luminal B, TNBC, and HER2+, reflecting significantly better survival outcomes, this is not the case for patients with luminal A. This could indicate different tumor biology between subtypes and the varying effectiveness of chemotherapy, thus necessitating subtype-specific approaches in treatment and prognosis evaluation. It also raises questions about the biological mechanisms that underpin these variations and points to the need for personalized treatment strategies that consider individual tumor characteristics beyond mere subtype classification, as underscored by existing studies (46,49-51).
This study incorporated RWD related to NAC BC treatment. The relevance of pCR to OS and DFS of women with BC from a developing country eligible for NAC in an uncontrolled environment (not in a strictly controlled trial) can help us better understand our population. We highlighted the risk associated with the lack of proven therapies in Brazil’s PHS, which could directly affect the probability of achieving pCR (especially in patients with HER2 positive and triple negative diseases); as presented in this study, this could lead to a worse prognosis.
Finally, this study had limitations, similar to other RWD studies. Our data set was not initially designated to study the influence of pCR on OS/DFS; it was designed to monitor patients treated according to local preferences and their daily routine. This problem was attenuated by the fact that pCR, OS, and DFS are objective outcomes and relevant indicators of BC treatment. Similarly, other confounding observational studies and selection bias might be important sources of limitations. Additionally, due to the type of study, surrogacy could not be demonstrated.
This study provides valuable insights into the effectiveness of NAC in a real-world setting, specifically within a Brazilian public reference center. By demonstrating a pCR rate of 22.7% across various BC subtypes and stages, this research highlights the potential of NAC to improve survival outcomes in aggressive BC cases. The findings underscore the importance of NAC in achieving better OS and DFS, particularly in subtypes such as triple negative and luminal B. These results can guide oncologists in developing countries to optimize NAC protocols, adapt treatment strategies based on subtype-specific responses, and ultimately enhance the efficacy of BC treatment without necessitating significant additional resources. This approach could help improve patient outcomes and resource utilization in settings with limited healthcare budgets.
Conclusions
Our study demonstrated that the data on pCR rates in patients undergoing NAC for BC treatment in real life are consistent with the data from clinical trials and that pCR was correlated with increases in OS and DFS in an RWE study. Furthermore, it showed that despite the lack of access to adequate treatments in patients with HER2 and TNBC, and that the pCR rates of these subtypes were lower than those of the RCTs, the OS and DFS of patients with pCR were also better.
Acknowledgments
We thank all the patients who were included, even anonymously, in the study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-54/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-54/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-54/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-54/coif). A.M. reports the consulting fees and payment for lectures from Roche, Astrazeneca, Daiichi Sankio, Novartis, and Merck; support for attending meetings from Roche, and participation on a Data Safety Monitoring Board or Advisory Board from Eli Lilly, Merck. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was submitted and approved by the Research Ethics Committee of the Women’s Health Hospital (No. CAAE 39097520.4.2001.0069), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Today. [Internet] Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/39-all-cancers-fact-sheet.pdf (accessed on 19 January 2023).
- Santos MO, Lima FCDS, Martins LFL, et al. Estimativa de Incidência de Câncer no Brasil, 2023-2025. Rev Bras Cancerol 2023;69:e-213700. [Crossref]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med 2021;384:2394-405. [Crossref] [PubMed]
- Pashayan N, Antoniou AC, Ivanus U, et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol 2020;17:687-705. [Crossref] [PubMed]
- Visvanathan K, Levit LA, Raghavan D, et al. Untapped Potential of Observational Research to Inform Clinical Decision Making: American Society of Clinical Oncology Research Statement. J Clin Oncol 2017;35:1845-54. [Crossref] [PubMed]
- Wang M, Hou L, Chen M, et al. Neoadjuvant Chemotherapy Creates Surgery Opportunities For Inoperable Locally Advanced Breast Cancer. Sci Rep 2017;7:44673. [Crossref] [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [Crossref] [PubMed]
- Omarini C, Guaitoli G, Pipitone S, et al. Neoadjuvant treatments in triple-negative breast cancer patients: where we are now and where we are going. Cancer Manag Res 2018;10:91-103. [Crossref] [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-84. [Crossref] [PubMed]
- Golshan M, Loibl S, Wong SM, et al. Breast Conservation After Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer: Surgical Results From the BrighTNess Randomized Clinical Trial. JAMA Surg 2020;155:e195410. [Crossref] [PubMed]
- Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Chen AM, Meric-Bernstam F, Hunt KK, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 2004;22:2303-12. [Crossref] [PubMed]
- Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609-18. [Crossref] [PubMed]
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147-59. [Crossref] [PubMed]
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 2019;380:617-28. [Crossref] [PubMed]
- Antonini M, Mattar A, Pannain GD, et al. Integrative review of clinical trials and meta-analysis of the main studies of neoadjuvant chemotherapy in the treatment of breast cancer in the past 30 years. Mastology 2023;33: [Crossref]
- Li M, Chen S, Lai Y, et al. Integrating Real-World Evidence in the Regulatory Decision-Making Process: A Systematic Analysis of Experiences in the US, EU, and China Using a Logic Model. Front Med (Lausanne) 2021;8:669509. [Crossref] [PubMed]
- Interfarma. (Internet). Available online: https://www.interfarma.org.br/wp-content/uploads/2021/04/the-importance-of-clinical-research-to-brazil-interfarma1.pdf (accessed on 19 January 2023).
- Regier DA, Pollard S, McPhail M, et al. A perspective on life-cycle health technology assessment and real-world evidence for precision oncology in Canada. NPJ Precis Oncol 2022;6:76. [Crossref] [PubMed]
- Rivera DR, Lee JSH, Hsu E, et al. Harnessing the Power of Collaboration and Training Within Clinical Data Science to Generate Real-World Evidence in the Era of Precision Oncology. Clin Pharmacol Ther 2019;106:60-6. [Crossref] [PubMed]
- Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies--report of the ISPOR Task Force on Retrospective Databases. Value Health 2003;6:90-7. [Crossref] [PubMed]
- Fritz A, Percy C, Jack A., et al. International classification of diseases for oncology (ICD-O), 3rd edn. Geneva: World Health Organization; 2000.
- Fritz A, Percy C, Jack A, et al. International Classification of diseases for oncology (ICD-O), first revision of 3rd edn. Geneva: World Health Organization; 2013.
- BLOOM HJ. RICHARDSON WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 1957;11:359-77. [Crossref] [PubMed]
- Curigliano G, Burstein HJ, Gnant M, et al. Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann Oncol 2023;34:970-86. [Crossref] [PubMed]
- Cirqueira MB, Moreira MA, Soares LR, et al. Effect of Ki-67 on Immunohistochemical Classification of Luminal A to Luminal B Subtypes of Breast Carcinoma. Breast J 2015;21:465-72. [Crossref] [PubMed]
- Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736-47. [Crossref] [PubMed]
- Barrios CH, Reinert T, Werutsky G. Access to high-cost drugs for advanced breast cancer in Latin America, particularly trastuzumab. Ecancermedicalscience 2019;13:898. [Crossref] [PubMed]
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778-85. [Crossref] [PubMed]
- Conforti F, Pala L, Sala I, et al. Evaluation of pathological complete response as surrogate endpoint in neoadjuvant randomised clinical trials of early stage breast cancer: systematic review and meta-analysis. BMJ 2021;375:e066381. [Crossref] [PubMed]
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. [Crossref] [PubMed]
- Spring LM, Fell G, Arfe A, et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin Cancer Res 2020;26:2838-48. [Crossref] [PubMed]
- Delgado-Bocanegra RE, Millen EC, Nascimento CMD, et al. Intraoperative imprint cytology versus histological diagnosis for the detection of sentinel lymph nodes in breast cancer treated with neoadjuvant chemotherapy. Clinics (Sao Paulo) 2018;73:e363. [Crossref] [PubMed]
- Shin HC, Han W, Moon HG, et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann Surg Oncol 2013;20:2582-9. [Crossref] [PubMed]
- Kim MK, Han W, Moon HG, et al. Nomogram for predicting breast conservation after neoadjuvant chemotherapy. Cancer Res Treat 2015;47:197-207. [Crossref] [PubMed]
- Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2003;21:4165-74. [Crossref] [PubMed]
- Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672-85. [Crossref] [PubMed]
- von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747-56. [Crossref] [PubMed]
- Acevedo F, Petric M, Walbaum B, et al. Better overall survival in patients who achieve pathological complete response after neoadjuvant chemotherapy for breast cancer in a Chilean public hospital. Ecancermedicalscience 2021;15:1185. [Crossref] [PubMed]
- Wang J, Sang D, Xu B, et al. Value of Breast Cancer Molecular Subtypes and Ki67 Expression for the Prediction of Efficacy and Prognosis of Neoadjuvant Chemotherapy in a Chinese Population. Medicine (Baltimore) 2016;95:e3518. [Crossref] [PubMed]
- Resende U, Cabello C, Ramalho SOB, et al. Prognostic assessment of breast carcinoma submitted to neoadjuvant chemotherapy with pathological non-complete response. BMC Cancer 2019;19:601. [Crossref] [PubMed]
- Villarreal-Garza C, Bargallo-Rocha JE, Soto-Perez-de-Celis E, et al. Real-world outcomes in young women with breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 2016;157:385-94. [Crossref] [PubMed]
- Guarneri V, Piacentini F, Ficarra G, et al. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol 2009;20:1193-8. [Crossref] [PubMed]
- Nishimura R, Osako T, Okumura Y, et al. Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancer as a predictor for chemosensitivity and for prognosis. Breast Cancer 2010;17:269-75. [Crossref] [PubMed]
- Antonini M, Mattar A, Bauk Richter FG, et al. Real-world evidence of neoadjuvant chemotherapy for breast cancer treatment in a Brazilian multicenter cohort: Correlation of pathological complete response with overall survival. Breast 2023;72:103577. [Crossref] [PubMed]
- de Paula BHR, Kumar S, Morosini FM, et al. Real-world assessment of the effect of impact of tumor size on pathological complete response rates in triple negative breast cancer after neoadjuvant chemotherapy. Chin Clin Oncol 2020;9:78. [Crossref] [PubMed]
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796-804. [Crossref] [PubMed]
- Huang M, O'Shaughnessy J, Zhao J, et al. Association of Pathologic Complete Response with Long-Term Survival Outcomes in Triple-Negative Breast Cancer: A Meta-Analysis. Cancer Res 2020;80:5427-34. [Crossref] [PubMed]
- Zhu S, Lu Y, Fei X, et al. Pathological complete response, category change, and prognostic significance of HER2-low breast cancer receiving neoadjuvant treatment: a multicenter analysis of 2489 cases. Br J Cancer 2023;129:1274-83. [Crossref] [PubMed]


