
A meta-analysis and systematic review of randomized controlled trials in combination gemcitabine with erlotinib in the pancreatic cancer
Highlight box
Key findings
• Gemcitabine and erlotinib (Gem-Erlo) was more effective than gemcitabine alone in treating pancreatic cancer (PaC).
What is known and what is new?
• Some controlled clinical trials (RCTs) have reported on the efficacy of Gem-Erlo in the treatment of PaC, but conclusions have been inconsistent and no consensus has been reached.
• Through a meta-analysis, we concluded that Gem-Erlo demonstrated improvement in disease control rate compared to single-agent gemcitabine. Median overall survival, median progression-free survival, objective response rate, and 1-year survival were higher in the Gem-Erlo group than in the treatment-alone group, although not statistically difference.
What is the implication, and what should change now?
• Gem-Erlo provides better benefits for PaC patients compared to gemcitabine alone. However, due to the small sample size of our study, further studies are required to comprehensively investigate the efficacy and adverse events of Gem-Erlo in the treatment of PaC.
Introduction
Pancreatic cancer (PaC), recognized as the “king of cancer”, is characterized by difficulties in early diagnosis, low treatment efficiency, easy recurrence and metastasis, and short survival period (1-3). For early-stage PaC, Surgical resection is the best treatment and the only effective way to achieve long-term survival. However, most patients have missed the optimal period of surgical treatment at the time of diagnosis, so chemotherapy has become the main strategy for PaC clinical treatment (2,4,5). Gemcitabine is an extensively utilized cytosine nucleoside antitumor medication. It is a fundamental drug in PaC and is considered the first line chemotherapy (6,7). However, in clinical application, gemcitabine shows only about 20% clinical effectiveness because of its high drug resistance. The 1-year survival rate of gemcitabine is only 17% to 23% in patients with metastasis (8,9). Therefore, the combination use of gemcitabine with other chemotherapeutic drugs (5-fluorouracil, cisplatin, oxaliplatin, irinotecan, pemetrexed and capecitabine) has been carried out (10-15).
Erlotinib is an epidermal growth factor receptor (EGFR) inhibitor, approved by the U.S. Food and Drug Administration (FDA) for use in PaC in 2004, but has limited therapeutic benefit as a single agent (16-18). In 2005, the FDA approved the usage of Gem-Erlo for PaC treatment (19). A large phase III trial demonstrated that Gem-Erlo is more effective than gemcitabine alone (20). But there is also controversy in the advantages of Gem-Erlo on PaC treatment recent years (21-24). In this case, our team chose to use a meta-analysis assessing the clinical meaning of gemcitabine only or combining with erlotinib in PaC. A comprehensive search of relevant randomized controlled clinical trials (RCTs) was conducted in PubMed, Embase, Web of Science, and Cochrane Library. The clinical parameters used for this comparison were disease control rate (DCR), objective response rate (ORR), median progression-free survival (median PFS), median overall survival (median OS) and 1-year survival rate. Our results will provide useful references for comparing the effectiveness and safety of Gem-Erlo versus gemcitabine alone in the treatment of PaC. It is time to take measures to improve the dismal results of gemcitabine. We present this article in accordance with the PRISMA reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-45/rc).
Methods
The meta-analysis was registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/), with the registration number CRD42023452611, which outlines the detailed methodology used in this study.
Search strategies
The main sources of literature searched were English-language literature from PubMed, Web of Science, Embase, and Cochrane Library databases. We systematically searched all eligible studies from database creation through July 2023 using gemcitabine, erlotinib, and pancreatic cancer as search terms. We also searched relevant conference abstracts and included literature that met the criteria. The detailed search method is shown in Supplementary file (Appendix 1). At the same time, we searched the references included in the study to prevent the omission of studies.
Study selection
Two independent reviewers (L.Y. and W.L.) screened the potentially relevant articles in an unblinded, standardized manner. Initial screening was done first by reading the abstract and title, and then by the full text when necessary to determine whether they met the inclusion and exclusion criteria. All disagreements regarding study selection among two researchers were resolved through consensus, and if necessary, a third author was consulted for a decision.
Inclusion criteria
Only studies that met the population, intervention, comparison, outcomes and study design (PICOS) principles were included in this meta-analysis, as shown below:
- Population (P): PaC patients;
- Intervention (I): treatment with gemcitabine plus erlotinib;
- Comparison (C): control or placebo group treatment with gemcitabine alone;
- Outcomes (O): median OS; median PFS; DCR; ORR; 1-year survival rate;
- Study design (S): RCTs.
Exclusion criteria
After automatically and manually removal of duplicates in Endnote, the titles and abstracts were independently reviewed by two researchers (L.Y. and W.L.). Case reports, letters, clinical records, reviews, meta-analyses screened, non-English published Literatures, incomplete or unavailable data were excluded. In addition, studies not relevant to the title of article (such as studies using animal models and interventions other than Gem-Erlo) are excluded. Any disagreement between the two fellows is resolved through discussion or consultation with a third author (J.D.).
Data extraction
Two researchers (L.Y. and W.H.) independently extracted the following information from the literature that met the inclusion criteria: (I) the first author’s name and the year of publication; (II) the study design, number of patients treated by Gem-Erlo group and gemcitabine alone group, age (median and range); (III) disease status before treatment, specific dosage, and administration interval of Gem-Erlo regimen; (IV) ORR, DCR, 1-year survival rate, median PFS, median OS, and the incidence of grade 3/4 AEs. Any disagreements were resolved by consulting a third author (A.B.Z.). To explore sources of heterogeneity between included studies, we also conducted subgroup analyses and sensitivity analyses.
Assessment of the risk of bias in the included studies
The quality of the literature included in this study was assessed by two researchers (L.Y. and J.Z.) using the “risk of bias assessment” tool (version 1.0) of the Cochrance systematic evaluation. The evaluation metrics cover the following seven areas: generation of randomized sequences, allocation concealment, blinding of investigators and subjects, blinding of outcome evaluators, completeness of outcome information, selective reporting of study results and other sources of bias. Each item can be described as “low risk of bias”, “high risk of bias”, or “unclear risk”. Eventually, the above entry evaluations were synthesized to produce a risk of bias assessment map and quality results for each literature. In case of disagreement between the two researchers, a third researcher (M.Q.) will then make a separate assessment and the assessment will be decided by mutual agreement among three researchers.
Data analysis
The meta-analysis was performed using Review Manager 5.3 software. The primary outcome we extracted from the included literature was DCR, the second end points included median OS, median PFS, ORR, and 1-year survival rate. AEs documented in the original study were extracted in our analyses. The data used for meta-analysis were extracted directly from the original study or calculated indirectly on the basis of the original study by conversion tools. When the level of heterogeneity in the meta-analysis was P>0.1, or P≤0.1 but I2≤50%, a fixed-effects model and weighted mean difference (WMD) was applied for statistical analysis. Conversely, due to the significant heterogeneity (P≤0.1, I2>50%) between the studies, a random effects model and standardized mean difference (SMD) was performed for combined analysis. The risk ratio (RR) or odds ratio (OR) was used to compare toxicities. All results extracted are presented with 95% confidence interval (CI). Statistical significance was P≤0.05. Statistical heterogeneity between studies was evaluated by Q test and I2 statistics. If there were significant differences between studies, we conducted subgroup analyses or sensitivity analyses in order to identify sources of heterogeneity. Funnel plot analyses were not conducted to assess publication bias because of the limited number of literatures included.
Results
Study selection
A flowchart of the qualified and detailed literature search is presented in Figure 1. A total of 1,172 articles were retrieved from four databases, including 279 in PubMed, 65 in Cochrane Library, 643 in Embase, and 185 in Web of Science. Six hundred and four were retained after removing duplicate literature. Five hundred and twenty-two papers were removed by reading the titles and abstracts, of which, 153 without relevant topics were excluded, 53 were reviews or meta-analyses, 20 were animal experiments, 15 were non-English language documents, and 281 were conference abstracts without outcome. After reading the full texts, 75 papers were left, 48 were not RCTs and 27 were clinical records and cases with no results. Ultimately, seven eligible RCTs were included in the meta-analysis, two of which were conference abstracts.
Characteristics of the studies
Literature was screened based on the above inclusion and exclusion criteria, and ultimately seven RCTs were included (20-26). All studies were published from 2007 to 2022. The study enrolled 2,152 patients with PaC, including two studies of patients with postoperative resected PaC (21,22), one study of locally advanced PaC (23), two studies of advanced PaC (20,25), one study of locally advanced or metastatic PaC (26), and one study of patients with metastatic PaC (24). The median ages of patients included in the study were similar, ranging from approximately 63–70 years old. In addition, seven studies compared Gem-Erlo to gemcitabine alone, and gemcitabine was administered using the usual dosage: 1,000 mg/m2, and erlotinib was administered at a dose of 100 or 150 mg/day. The basic characteristics of all included studies are presented in Table 1.
Table 1
| Study | Study design | Study period | Initially status | Exp: median age [range] (years) | Con: median age [range] (years) | Patient (Exp/Con) | Criteria for AEs | Follow-up (months) | Main outcome measures | Exp: treatment regimen | Con: treatment regimen |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hammel et al. (23) | RCT | 2008–2011 | Locally advanced | 63.0 [58.0–71.0] | 64.0 [57.0–70.0] | 219/223 | ND | 36.7 | Median OS, median PFS | Gem 1,000 mg/m2, weekly ×3 weeks for 4 weeks + Erlo 100 or 150 mg/d | Gem 1,000 mg/m2, weekly ×3 weeks for 4 weeks |
| Moore et al. (20) | RCT | 2001.10–2003.01 | Advanced | 63.7 [37.9–84.4] | 64.0 [36.1–92.4] | 285/284 | NCI CTC | ND | Median OS, median PFS, DCR | Gem 1,000 mg/m2, weekly ×7 weeks for 8 weeks, then weekly ×3 every 4 weeks + Erlo 100 or 150 mg/d | Gem 1,000 mg/m2, weekly ×7 weeks for 8 weeks, then weekly ×3 every 4 weeks + placebo 100 or 150 mg/d |
| Wang et al. (24) | RCT | 2005.07–2012.06 | Metastatic | 70 [33–91] | 70 [33–91] | 44/44 | NCI CTC | 7.2 | Median OS, median PFS, ORR, DCR | Gem 1,000 mg/m2, weekly ×7 weeks for 8 weeks, then weekly ×3 every 4 weeks + Erlo 100 mg/d | Gem 1,000 mg/m2, weekly ×7 weeks for 8 weeks, then weekly ×3 every 4 weeks |
| Abrams et al. (21) | RCT | 2009.11.17–2014.02.28 | Post head pancreatectomy | ND | ND | 159/163 | NCI CTC | 23.8 | Median OS | Gem 1,000 mg/m2, weekly ×3 weeks for 4 weeks + Erlo 100 mg/d | Gem 1,000 mg/m2, weekly ×3 weeks for 4 weeks |
| Sinn et al. (22) | RCT | 2008.04–2013.07 | Post-pancreatectomy | 63 [28–82] | 65 [24–82] | 219/217 | NCI CTC | 54 | Median OS | Gem 1,000 mg/m2, weekly ×3 weeks for 4 weeks + Erlo 100 mg/d | Gem 1,000 mg/m2, weekly ×3 weeks for 4 weeks |
| Takahashi et al. (25) | RCT | 2009.01–2013.03 | Advanced | ND | ND | 102/202 | ND | ND | ORR, DCR | ND | ND |
| Cho et al. (26) | RCT | ND | Locally advanced or metastatic | ND | ND | 44/47 | ND | ND | Median PFS, ORR, DCR | ND | ND |
RCT, randomized controlled trial; ND, non-determined; Exp, experimental group; Con, control group; AEs, adverse events; NCI CTC, National Cancer Institute Common Terminology Criteria; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate; Gem, gemcitabine; Erlo, erlotinib.
Risk assessment of bias
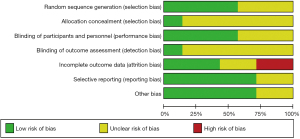
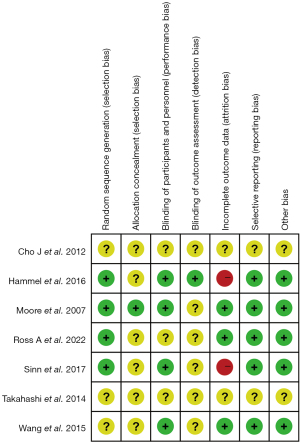
The results of the risk of bias assessment for the seven studies according to the Cochrane tool are shown in Figures 2,3. We found that four studies had a low risk of random sequence generation (20-23), and the other three studies did not mention information related to random sequence generation (unclear risk of bias) (24-26). Only one of the included studies had reference to allocation concealment (low risk of bias) (20). For the assessment of blinding of measurers and patients, one study was at high risk of bias (24), three studies were at low risk (20,22,23), and the other three did not account for relevant information (risk of bias unclear) (21,25,26). Only one study accounted for outcome assessment blinding (low risk of bias) (23). We also assessed the completeness of the results for bias, with three studies having a low risk of bias (20,21,24), two studies having incomplete information about the results (high risk of bias) (22,23), and the remaining two studies not being able to judge the completeness of the results because of a lack of full-text information (25,26). Selective reporting bias and other biases were absent in five studies and were not reported in the other two. Funnel plot analysis was not performed due to insufficient number of included studies.
Meta-analysis
Seven eligible articles were meta-analyzed using a random effects model, with DCR, ORR, median OS, median PFS, and 1-year survival rate as outcome metrics to evaluate the effectiveness of Gem-Erlo in the treatment of PaC, and AEs as a safety indicator.
Primary indicators
DCR
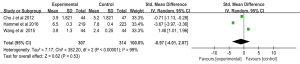
The DCR was reported in four studies of 462 patients in the Gem-Erlo group and 564 patients in the control group (20,24-26). Significant heterogeneity was detected between the studies [Q=8.73; data frame (df) =3; I2=66%; P=0.03], therefore, a random effects model was used for the meta-analysis of the DCR. Pooled analysis showed that the patients treated with Gem-Erlo had significantly higher DCR compared with the patients treated with gemcitabine alone (OR =1.74, 95% CI: 1.03 to 2.92; P=0.04) (Figure 4).

Secondary indicators
The median OS
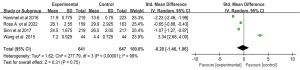
The median OS was reported in four studies of 641 patients in the Gem-Erlo group and 647 patients in the control group (21-24). The median OS ranged from 4.4–29.9 months. Pooled analysis showed significant heterogeneity between groups (Q=277.79; df =3; I2=99%; P<0.001), therefore, a random effects model was used for the meta-analysis of the median OS. The SMD for the median OS was −0.20; 95% CI: −1.46 to 1.06; P=0.75, which suggests that the Gem-Erlo group no significantly prolonged the OS, compared with the control group (Figure 5).
The median PFS
The median PFS was reported in three studies of 307 patients in the Gem-Erlo group and 314 patients in the control group (23,24,26). The median PFS ranged from 2.4–7.8 months. Pooled analysis showed significant heterogeneity between groups (Q=352.20; df =2; I2=99%; P<0.001), therefore, a random effects model was used for the meta-analysis of the median PFS. The SMD for the median PFS was −0.97; 95% CI: −4.01 to 2.07; P=0.53, which suggests that Gem-Erlo group no significantly improved the median PFS of the patients, compared with the control group (Figure 6).
ORR
The ORR was reported in four studies of 475 patients in the Gem-Erlo group and 577 patients in the control group (20,24-26). Significant heterogeneity was detected between the studies (Q=1.04; df =3; I2=0%; P=0.79), therefore, a fixed-effects model was used for the meta-analysis of the ORR. Pooled analysis showed that the ORR of the patients treated with gemcitabine alone was lower compared with the patients treated with Gem-Erlo (OR =1.29; 95% CI: 0.84 to 1.97), but the difference was not significant (P=0.25) (Figure 7).
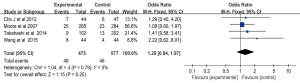
One-year survival rate
The 1-year survival rate was reported in three studies of 548 patients in the Gem-Erlo group and 548 patients in the control group (20,22,26). There was no significant heterogeneity between studies (Q=2.56; df =2; I2=22%; P=0.28), therefore, a fixed-effects model was used for the meta-analysis of the 1-year survival rate. Pooled analysis showed that the 1-year survival rate of the patients treated with Gem-Erlo was higher compared with the patients treated with gemcitabine alone (OR =1.18; 95% CI: 0.88 to 1.57), but the difference was not significant (P=0.26) (Figure 8).

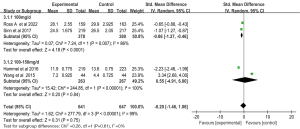
Subgroup of the median OS
Pooled analysis of the median OS presented there is a significant heterogeneity between groups (I2=99%; P<0.001). To explore the sources of heterogeneity, we conducted subgroup analyses of dose and whether adjuvant therapy was administered postoperatively of the median OS.
Dosage subgroup of the median OS
Subgroup analysis with random effects model that Gem-Erlo group significantly increased the median OS in the 100 mg/d subgroup (SMD =−0.86; 95% CI: −1.27 to −0.46; P<0.001; heterogeneity test P=0.007; I2=86%). However, no statistically significant differences were observed in the 100–150 mg/d subgroup (SMD =0.55; 95% CI: −4.91 to 6.00; P=0.84). Significant heterogeneity between the two dosages was founded (heterogeneity test P<0.001; I2=99%) (Figure 9).
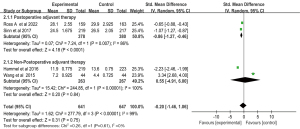
Postoperative adjuvant therapy subgroup of the median OS
Subgroup analysis with random effects model that Gem-Erlo group significantly increased the median OS in the postoperative adjuvant therapy subgroup (SMD =−0.86; 95% CI: −1.27 to −0.46; P<0.001; heterogeneity test P=0.007; I2=86%). However, no statistically significant differences were observed in the non-postoperative adjuvant therapy subgroup (SMD =0.55; 95% CI: −4.91 to 6.00; P=0.84). Significant heterogeneity between the two dosages was founded (heterogeneity test P<0.001; I2=99%) (Figure 10).
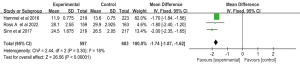
Sensitivity analysis
In order to explore the stability of the meta-analysis results and source of heterogeneity. The sensitivity analysis of median OS was carried out, Forest plots of median OS showed heterogeneity test P=0.75; I2=99%. The small number of patients studied by Wang et al. (24) may be a source of heterogeneity. Sensitivity analysis of the median OS showed no significant heterogeneity between the two groups (heterogeneity test P=0.30; I2=18%), when this literature was excluded, and the Gem-Erlo group significantly prolonged the median OS compared to the gemcitabine alone group (MD =−1.74; 95% CI: −1.87 to −1.62; P<0.001) (Figure 11).
Adverse events (AEs)
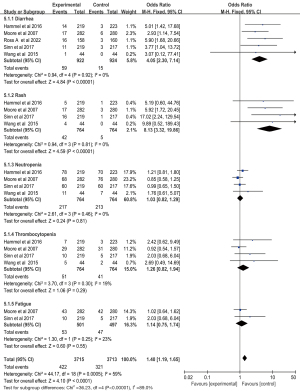
The results of the meta-analysis of the grade 3/4 AEs are presented in Figure 12. Main AEs were analyzed. Neutropenia (60.8%) was the most frequent grade 3/4 AEs between groups. As compared with gemcitabine alone group, Gem-Erlo group significantly increased the incidence of diarrhea (OR =4.05; 95% CI: 2.30 to 7.14; P<0.001), rash (OR =8.13; 95% CI: 3.32 to 19.86; P<0.001), while showing no difference in the incidence of neutropenia (OR =1.03; 95% CI: 0.82 to 1.29; P=0.81), thrombocytopenia (OR =1.26; 95% CI: 0.82 to 1.94; P=0.29), fatigue (OR =1.14; 95% CI: 0.75 to 1.74; P=0.55). After data pooling of the five AEs, we found that the Gem-Erlo group had higher AEs compared with that of gemcitabine alone group (OR =1.40; 95% CI: 1.19 to 1.65; P<0.001).
Discussion
PaC is typically diagnosed during its intermediate to advanced stages, resulting in a limited proportion of patients (15% to 20%) being suitable candidates for surgical intervention. However, the incidence of recurrence and metastasis remains notably elevated (27,28). It was reported that gemcitabine can prolong patients’ survival of less than 6 months, but cannot improve the prognosis of patients with intermediate to advanced and post-radical PaC (29,30). In November 2005, the U.S. FDA approved gemcitabine + erlotinib for use in patients with locally advanced, advanced or distant metastases. Although positive findings were reported, there was still no consensus on whether Gem-Erlo provides a significant advantage over gemcitabine alone in treating PaC (20-24). Our meta-analysis and systematic evaluation showed that the total OR of the DCR for the primary outcome indicator was 1.74, the 95% CI was 1.03 to 2.92, Gem-Erlo had a significantly higher DCR than gemcitabine alone (P<0.05). Moore et al. (20) and Wang et al. (24) also reported that the combination treatment of Gem-Erlo increased DCR compared to gemcitabine alone. According to a review conducted by Rivera et al., Gem-Erlo was found to have a better DCR than gemcitabine alone for advanced PaC (59% vs. 49.4%) (31). Meanwhile, our study found the Gem-Erlo group had higher ORR compared to the gemcitabine alone group, although the difference in ORR results was not statistically significant. In the retrospective study by Lim et al., the results also showed a higher ORR in the Gem-Erlo group than in the gemcitabine alone group (15.9% vs. 12.7%) (32). This indicates that Gem-Erlo improved the number of cases in remission and stable lesions than gemcitabine alone. And a study was a clinical trial of gemcitabine in combination with albumin-bound paclitaxel (nab-paclitaxel) (33). The study indicated that both the OS and the PFS were increased by gemcitabine plus nab-paclitaxel. This implies that the limited sample size may be the cause of the lack of a statistically significant difference in the median OS and PFS in our investigation. Future high-quality, large-sample, multicenter clinical trials are required to confirm Gem-Erlo’s effectiveness for PaC survival.
In addition, our results showed no significant difference in median OS between the two groups and high heterogeneity (I2=99%). Heterogeneity between included studies may have contributed to the lack of significant benefit in median OS. We used a random-effects model to perform subgroup analyses based on dose and postoperative use of adjuvant therapy for the median OS. Subgroup analysis showed that heterogeneity among studies all decreased. In order to identify the sources of heterogeneity, sensitivity analyses were conducted on the median OS. The results showed that the study by Wang et al. (24), was the source of heterogeneity, which could be attributed to the small sample size of their included studies. Interestingly, we discovered a greater 5-year survival rate in patients treated with Gem-Erlo compared to gemcitabine alone in the study, despite the fact that there was no significant difference in 1-year survival rate (22). Our work provides a solid foundation for initiating follow-up studies, despite the constraint that some of the included studies were unable to extract patient 2- and 5-year survival rate data. The link between chemotherapeutic agents and the efficacy of treatment for patients with PaC has been further solidified, which is expected to accelerate the development of clinical chemotherapeutic agents to guide current therapy.
In order to assess the safety of the combination group, we analyzed the most frequent AEs that associated with chemotherapy for PaC, including diarrhea, dermatillomania, neutrophil and thrombocytopenia rates. The data showed that the incidence of stage 3/4, rash and diarrhea was significantly higher in the Gem-Erlo group than the gemcitabine monotherapy group, but it was under an acceptable level. The addition of immune-boosting drugs to the clinic may reduce the incidence of these AEs. There were no significant differences in fatigue, neutrophil and thrombocytopenia reduction rates. Studies reported that patients with more severe rashes had significantly higher PFS and OS (20,34). The incidence of rash is positively correlated with therapeutic response rate and survival rate in patients treated with Gem-Erlo (35,36). As can be inferred from the above, the higher incidence of AEs in Gem-Erlo may not be entirely a bad thing for patients’ survival.
In China, the incidence rate of PaC ranks the seventh and mortality rate ranks the sixth among malignant tumors, and will become the second most deadly tumor in China in 2030 (27,37). This study provides objective evidences to the efficacy and AEs of Gem-Erlo in PaC management for Chinese patients. However, there are some limitations. First the small sample size may have an impact on the accuracy of the results. Second, two studies were abstracts (25,26), and we were unable to obtain detailed information about the patients, treatment regimens, outcomes, and occurrence of AEs, which would have influence us for a deeper analysis. Third, the duration of follow-up in the included studies was inconsistent, with some studies having no follow-up or no records. Last but not least, an inevitable constraint that may affect our results was the heterogeneity of the different trial populations and the research limitations. Therefore, we should be careful when interpreting our results. Factors such as the patient’s financial condition, mental status and nutritional status can also affect the outcome and prognosis of Gem-Erlo treatment. Because of these problems, further multicenter, large-sample, long follow-up randomized controlled trials are needed to determine the efficacy and safety of Gem-Erlo for PaC.
Conclusions
In summary, Gem-Erlo demonstrated improvement in DCR compared to single-agent gemcitabine. There was no significant improvement in median PFS, median OS, ORR and 1-year survival, but there is a correlation between the prognosis of PaC and the occurrence of AEs. Due to heterogeneous populations and study limitations, further studies are required to comprehensively investigate the efficacy and AEs of Gem-Erlo in the treatment of PaC.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-45/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-45/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Okusaka T. Treatment for postoperative recurrence of pancreatic cancer: a narrative review. Chin Clin Oncol 2022;11:19. [Crossref] [PubMed]
- Kishi Y. Let’s come together to fight with pancreatic cancer. Chin Clin Oncol 2023;12:1. [Crossref] [PubMed]
- Springfeld C, Jäger D, Büchler MW, et al. Chemotherapy for pancreatic cancer. Presse Med 2019;48:e159-74. [Crossref] [PubMed]
- Okusaka T, Furuse J. Recent advances in chemotherapy for pancreatic cancer: evidence from Japan and recommendations in guidelines. J Gastroenterol 2020;55:369-82. [Crossref] [PubMed]
- Jain A, Bhardwaj V. Therapeutic resistance in pancreatic ductal adenocarcinoma: Current challenges and future opportunities. World J Gastroenterol 2021;27:6527-50. [Crossref] [PubMed]
- Taieb J, Prager GW, Melisi D, et al. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open 2020;5:e000587. [Crossref] [PubMed]
- Jia X, Du P, Wu K, et al. Pancreatic Cancer Mortality in China: Characteristics and Prediction. Pancreas 2018;47:233-7. [Crossref] [PubMed]
- Borazanci E, Von Hoff DD. Nab-paclitaxel and gemcitabine for the treatment of patients with metastatic pancreatic cancer. Expert Rev Gastroenterol Hepatol 2014;8:739-47. [Crossref] [PubMed]
- Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002;20:3270-5. [Crossref] [PubMed]
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24:3946-52. [Crossref] [PubMed]
- Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509-16. [Crossref] [PubMed]
- Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004;22:3776-83. [Crossref] [PubMed]
- Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer 2002;94:902-10. [Crossref] [PubMed]
- Miller VA, Kris MG. Docetaxel (Taxotere) as a single agent and in combination chemotherapy for the treatment of patients with advanced non-small cell lung cancer. Semin Oncol 2000;27:3-10. [PubMed]
- Li J, Yuan S, Norgard RJ, et al. Epigenetic and Transcriptional Control of the Epidermal Growth Factor Receptor Regulates the Tumor Immune Microenvironment in Pancreatic Cancer. Cancer Discov 2021;11:736-53. [Crossref] [PubMed]
- Tzeng CW, Frolov A, Frolova N, et al. Epidermal growth factor receptor (EGFR) is highly conserved in pancreatic cancer. Surgery 2007;141:464-9. [Crossref] [PubMed]
- Jimeno A, Tan AC, Coffa J, et al. Coordinated epidermal growth factor receptor pathway gene overexpression predicts epidermal growth factor receptor inhibitor sensitivity in pancreatic cancer. Cancer Res 2008;68:2841-9. [Crossref] [PubMed]
- Burris H 3rd, Rocha-Lima C. New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist 2008;13:289-98. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Abrams RA, Winter KA, Safran H, et al. Results of the NRG Oncology/RTOG 0848 Adjuvant Chemotherapy Question-Erlotinib+Gemcitabine for Resected Cancer of the Pancreatic Head: A Phase II Randomized Clinical Trial. Am J Clin Oncol 2020;43:173-9. [Crossref] [PubMed]
- Sinn M, Bahra M, Liersch T, et al. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol 2017;35:3330-7. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Wang JP, Wu CY, Yeh YC, et al. Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget 2015;6:18162-73. [Crossref] [PubMed]
- Takahashi H, Kuwahara A, Okuyama H, et al. Efficacy and possible biomarker of gemcitabine and erlotinib for advanced pancreatic cancer. Ann Oncol 2014;25:v65. [Crossref]
- Cho JY, Lim JY, Lee SJ, et al. 722P - A Pilot Trial of Gemcitabine in Combination with Capecitabine or Erlotinib Compared to Gemcitabine alone in Patients with advanced Pancreatic Cancer. Ann Oncol 2012;23:ix240. [Crossref]
- Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 2018;15:333-48. [Crossref] [PubMed]
- Groot VP, Rezaee N, Wu W, et al. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg 2018;267:936-45. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Xie J, Liu JH, Liu H, et al. Tanshinone IIA combined with adriamycin inhibited malignant biological behaviors of NSCLC A549 cell line in a synergistic way. BMC Cancer 2016;16:899. [Crossref] [PubMed]
- Rivera F, López-Tarruella S, Vega-Villegas ME, et al. Treatment of advanced pancreatic cancer: from gemcitabine single agent to combinations and targeted therapy. Cancer Treat Rev 2009;35:335-9. [Crossref] [PubMed]
- Lim JY, Cho JH, Lee SJ, et al. Gemcitabine Combined with Capecitabine Compared to Gemcitabine with or without Erlotinib as First-Line Chemotherapy in Patients with Advanced Pancreatic Cancer. Cancer Res Treat 2015;47:266-73. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Aranda E, Manzano JL, Rivera F, et al. Phase II open-label study of erlotinib in combination with gemcitabine in unresectable and/or metastatic adenocarcinoma of the pancreas: relationship between skin rash and survival (Pantar study). Ann Oncol 2012;23:1919-25. [Crossref] [PubMed]
- Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231-7. [Crossref] [PubMed]
- Park S, Chung MJ, Park JY, et al. Phase II Trial of Erlotinib Plus Gemcitabine Chemotherapy in Korean Patients with Advanced Pancreatic Cancer and Prognostic Factors for Chemotherapeutic Response. Gut Liver 2013;7:611-5. [Crossref] [PubMed]
- Wang Y, Cui J, Wang L. Patient-derived xenografts: a valuable platform for clinical and preclinical research in pancreatic cancer. Chin Clin Oncol 2019;8:17. [Crossref] [PubMed]



