
Short-term outcomes in obese and non-obese patients undergoing transperitoneal laparoscopic adrenalectomy for benign or malignant adrenal diseases: an updated systematic review and meta-analysis
Highlight box
Key findings
• Transperitoneal laparoscopic adrenalectomy (TLA) is the most frequently chosen approach in adrenal surgery.
• At present, impact of obesity on patient intraoperative and postoperative outcomes following laparoscopic adrenalectomy is frequently under discussion.
What is known and what is new?
• According to some studies, obesity is suggested to play a detrimental role on patient outcomes following adrenal surgery. However, other studies underlined lack of association between obesity and onset of postoperative complications (obesity paradox).
• By including just comparative studies of non-obese and obese adult patients undergoing TLA for benign or malignant adrenal disorders, our meta-analysis showed that TLA did not record any statistically different short-term outcomes (operative time, intraoperative complications, estimated blood loss, transfusions, conversion to open surgery, overall postoperative complications, major postoperative complications, length of hospital stay) between the two populations.
What is the implication, and what should change now?
• We can say that obesity does not impact TLA safety and effectiveness. Given the significant biases among meta-analyzed studies, elucidation of results is strongly needed.
• Confirmation of our results must go through additional randomized, possibly multi-center trials.
Introduction
Adrenalectomy represents the definitive treatment for multiple functional and/or organic adrenal disorders (1,2). Major indications for surgery are hormonally inactive/non-functioning tumors (36.8–39.6%), catecholamine-secreting tumors/pheochromocytoma (18.9–27.4%), aldosterone-producing tumors/aldosteronoma (11.8–17.9%), glucocorticosteroid-secreting tumors/Cushing’s syndrome (15.4–25.2%), virilizing/sex hormone-secreting tumors (1.1–1.2%), and adrenal gland metastases (4.6%) (1).
Although open adrenalectomy still maintains some important indications shared among experts and many scientific societies (e.g., adrenocortical carcinoma), minimally invasive surgery is considered the gold standard for the treatment of most of abovementioned surgical adrenal disorders, as stressed by guidelines issued by European Society of Endocrinology, European Society of Endocrine Surgeons, and American Association of Endocrine Surgeons (1,3-11). In 1992, Gagner et al. described the first cases of laparoscopic adrenalectomy and their encouraging results (12). Since then, many studies have been published on this topic, all of them highlighting the substantial advantages of minimally invasive surgery, if compared to the conventional one; among them, lower rates of postoperative morbidity and mortality, less frequent occurrences of overall and major complications, milder postoperative pain, shorter length of hospitalization, better cosmetic results must be borne in mind (1,5-7).
Laparoscopic procedures can be performed through either a transperitoneal approach or a retroperitoneal one (13-18). At present, transperitoneal laparoscopic adrenalectomy (TLA) is the most frequently chosen approach, as it allows the best overall view of adrenal lodge and surrounding area, thus providing adequate working space even for larger lesions (7,19). Exploration of abdominal cavity represents an additional advantage of transabdominal approach, as it allows the treatment of other associated abdominal disorders during surgery (7,19). Furthermore, in case of difficult dissection or intraoperative hemorrhage, this method allows prompt conversion to open surgery (7,19).
At present, impact of obesity on patient outcomes following general abdominal (20-31) and adrenal surgery (32-36) is frequently under discussion. According to some studies, obesity is suggested to play a detrimental role on patient outcomes following abdominal surgery, as obese (Ob) patients usually record higher postoperative morbidity rates, if compared to non-obese (NOb) ones (20,23,24,30,32,35). However, other studies underlined lack of association between obesity and onset of postoperative complications (21,26-28,31,33,36). They even acknowledged a preservative impact of obesity on postoperative mortality after digestive surgery, the so called “obesity paradox” (21,25,26). Unfortunately, most studies focused on surgery of intraperitoneal organs or peculiar types of surgery (i.e., bariatric surgery), ruling out surgical procedures of retroperitoneal structures such as adrenal glands (34).
Therefore, we intended to offer updated evidence thanks to a comparison between intraoperative and perioperative outcomes in non-obese and obese patients, who underwent TLA for benign or malignant adrenal diseases. We present this article in accordance with the PRISMA reporting checklist (37) (available at https://cco.amegroups.com/article/view/10.21037/cco-24-55/rc).
Methods
Our meta-analysis was based on previously published studies with no additional data other than those related to original patient population. Thus, approval by Ethics committee and informed patient consent were not required.
Search strategy
PubMed/MEDLINE, Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials-CENTRAL), Web of Science (Science and Social Science Citation Index), and Scopus databases were used to identify articles of interest.
Combination of non-MeSH/MeSH terms was as follows:
- PubMed/MEDLINE
((obese[Title/abstract]) AND (adrenalectomy[Title/abstract])) OR ((obesity[Title/abstract]) AND (adrenalectomy[Title/abstract])). Filters applied: English
- Cochrane Library
obese in Title Abstract Keyword AND adrenalectomy in Title Abstract Keyword OR obesity in Title Abstract Keyword AND adrenalectomy in Title Abstract Keyword—(word variations have been searched). Language: English
- Web of Science
Obese (Topic) AND adrenalectomy (Topic) OR obesity (Topic) AND adrenalectomy (Topic) and English (Languages)
- Scopus
(TITLE-ABS-KEY (obese) AND TITLE-ABS-KEY (adrenalectomy) OR TITLE-ABS-KEY (obesity) AND TITLE-ABS-KEY (adrenalectomy)) AND (LIMIT-TO (LANGUAGE, “English”))
Final analysis was carried out on 21st of March 2024.
Additionally, the reference lists of relevant studies were manually reviewed to identify any articles that may have been missed during the electronic search.
Inclusion and exclusion criteria
We enclosed comparative population studies (case series, case-control studies, cohort studies, controlled clinical trials and randomized clinical trials) concerning non-obese and obese adult patient populations (over 18 years of age) undergoing TLA for benign or malignant adrenal diseases.
Furthermore, studies comparing patient populations having mixed transperitoneal robotic/open adrenalectomy + TLA or TLA + retroperitoneal laparoscopic adrenalectomy (RLA) data were ruled out, as well as studies analyzing fewer than 3 outcomes of interest (see “Outcomes” section).
We determined to rule out abstracts, posters, letters to the Editor, editorials, case reports and previously published systematic reviews and/or meta-analyses, although previously published systematic reviews or meta-analyses were taken into account in order to detect comparative studies left out through our systematic search.
Due to paucity of data retrieved in the course of first unsystematic search, we ruled out limitations connected to date of issue.
Outcomes
We evaluated two groups of outcomes: intraoperative and postoperative ones.
Intraoperative outcomes included operative time, intraoperative complications rate, estimated blood loss (EBL), transfusion rate, and conversion to open surgery rate.
Postoperative outcomes included overall postoperative complications rate, major (Clavien-Dindo or CD ≥ III) postoperative complications rate, and length of hospital stay.
Data extraction
Two independent reviewers (M.Zi. and A.M.) selected papers based on title, abstracts, keywords, and full-texts. All collected results were then reviewed by a third independent reviewer (C.G.).
Following data were collected from included papers:
- Demographic data [author’s surname and year of publication, study period, study country, study type, population size, gender and age, American Society of Anaesthesiologists (ASA) score, body mass index (BMI), adrenal side, adrenal size, adrenal disease, follow-up duration];
- Intraoperative outcomes data (operative time, intraoperative complications rate, EBL, transfusion rate, conversion to open surgery rate);
- Postoperative outcomes data [overall postoperative complications rate, major (CD ≥ III) postoperative complications rate, length of hospital stay].
Quality assessment
Two independent reviewers made use of RoB 2 and ROBINS-I tools for a proper quality assessment of the different included studies (38,39).
Version 2 Cochrane Risk-of-Bias tool for randomized trials (RoB 2) helped in assessing the risk of bias in randomized trials (38). It included a fixed set of bias domains that were focused on different aspects of study design, conduct and reporting (38). Each set included a series of questions (“reporting questions”) aimed at collecting data on study characteristics, that contributed to the risk of bias (38). An algorithm suggested bias risk from each domain, based on answers to reporting questions (38). Risk of bias was classified as “Low”, “High”, or “Some Concerns” (38).
ROBINS-I tool assessed the risk of bias in non-randomized studies comparing health outcomes in two or more interventions (39). In risk assessment, reporting questions having a substantial factual nature aimed at easing judgment on the risk of bias (39). Answers to reporting questions gave a framework for domain-level judgments on the risk of bias, which then served as a basis for an overall judgment in a particular outcome (39). Ratings were “Low Risk”, “Moderate Risk”, “Severe Risk” and “Critical Risk”, where “Low risk” meant the risk of bias in a high-quality randomized study (39). Only in outstanding cases, a non-randomized study may be given rating of low risk, due to confounding variables (39).
Statistical analysis
We used “Review Manager (RevMan) [Computer program] Version 5.4. The Cochrane Collaboration, 2020” to perform our meta-analysis (40). In case of dichotomous outcomes, odds ratios (ORs) and corresponding 95% confidence intervals (CIs) followed Mantel-Haenszel (MH) method. In case of continuous outcomes, weighted mean differences (WMDs) and corresponding 95% CIs followed inverse variance method. In the lack of mean and standard deviation (SD) for an end-point, we used reported median range and interquartile range (IQR), if provided, according to Hozo formulas (41). Moreover, if a study had sample sizes, means, and SDs separately for two or more subgroups in each of the intervention groups, the Cochrane’s formula was used to combine the numbers into a single sample size, mean, and SD for each group of intervention (42).
I2 statistics were used to assess statistical heterogeneity. <25%, 25–50% and >50% I2 values were classified as follows: low, moderate, and high. Due to heterogeneous discrepancies in general population characteristics, in addition to discrepancies in minimally invasive surgical approaches, a random-effects model served as default in all statistical analyses with P<0.05 statistical significance.
Results
Search results
According to 21st of March 2024 final literature search, 1,265 potentially interesting studies were found (Figure 1). After removing duplicate publications and excluding those irrelevant for title and abstract, 62 full-texts were considered eligible. Finally, just 8 comparative studies underwent qualitative and quantitative synthesis, as they complied with inclusion criteria (43-50). No additional records were found through other sources (references list).
Quality of studies
According to ROBINS-I, all non-randomized studies recorded moderate overall bias (43-50) (see Table S1). Due to lack of identification of randomized trials, RoB 2 tool was not used.
Study and population features
Table 1 shows study and population features. The eight studies retrieved through the systematic search had all observational nature. In particular, 7 studies had a retrospective design and 1 had a prospective one. They came from Western and Eastern countries and recorded an observational period of a nearly 30 years (1994–2020).
Table 1
| Authors/year | Study type | Study country | Study period | Group | Patient population | Gender, n | Age (years), mean ± SD | BMI (kg/m2), mean ± SD | Obesity criteria | ASA score, n | FU, mean ± SD | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | I–II | III–IV | ||||||||||
| Kazaryan et al., 2011 (43) | Prospective | Norway | 1997–2010 | Obese | 39 | 14 | 25 | 54.75±14.76 | 35.68±4.56 | ≥30 kg/m2 | n/a | n/a | n/a |
| Non-obese | 133 | 44 | 89 | 49.67±16.55 | 24.45±3.11 | n/a | n/a | ||||||
| Dancea et al., 2012 (44) | Retrospective | USA | 2000–2010 | Obese | 49 | 18 | 31 | 50.1±13.0 | n/a | ≥30 kg/m2 | n/a | n/a | n/a |
| Non-obese | 31 | 17 | 14 | 56.1±14.0 | n/a | n/a | n/a | ||||||
| Economopoulos et al., 2016 (45) | Retrospective | USA | 2002–2014 | Obese | 157 | 69 | 88 | 53.95±5.09 | n/a | ≥30 kg/m2 | n/a | n/a | n/a |
| Non-obese | 166 | 55 | 111 | 52.95±6.58 | n/a | n/a | n/a | ||||||
| Pędziwiatr et al., 2017 (46) | Retrospective | Poland | 2006–2015 | Obese | 174 | 51 | 123 | 58.15±11.12 | n/a | ≥30 kg/m2 | 97 | 77 | n/a |
| Non-obese | 346 | 129 | 217 | 54.25±14.99 | n/a | 237 | 109 | ||||||
| Inaishi et al., 2018 (47) | Retrospective | Japan | 2011–2016 | Obese | 28 | 14 | 14 | 37.0±7.47 | 28.08±2.76 | ≥25 kg/m2 | n/a | n/a | n/a |
| Non-obese | 70 | 27 | 43 | 53.75±15.29 | 20.65±2.94 | n/a | n/a | ||||||
| Ortenzi et al., 2019 (48) | Retrospective | Italy | 1994–2017 | Obese | 79 | 26 | 53 | 53.4±13.8* | n/a | ≥30 kg/m2 | n/a | n/a | 6.3±4.2 years |
| Non-obese | 149 | 43 | 106 | n/a | n/a | n/a | |||||||
| Altın et al., 2021 (49) | Retrospective | Turkey | 2008–2018 | Obese | 35 | 6 | 29 | 53.0±9.9 | n/a | ≥30 kg/m2 | n/a | n/a | 37.5±20.45 months |
| Non-obese | 30 | 15 | 15 | 47.6±14.7 | n/a | n/a | n/a | ||||||
| Rodríguez-Hermosa et al., 2021 (50) | Retrospective | Spain | 2003–2020 | Obese | 90 | 47 | 43 | 57.0±11.6 | 32.2±2.8 | ≥30 kg/m2 | 26 | 64 | n/a |
| Non-obese | 70 | 31 | 39 | 48.5±13.9 | 24.2±2.6 | 36 | 34 | ||||||
*, pooled population: non-obese + obese. SD, standard deviation; BMI, body mass index; ASA, American Society of Anaesthesiologists; FU, follow-up; n/a, not available.
Pooled population included 1,646 patients with samples size ranged between 65 and 520. Most patients had female sex (1,040; 63.2%), while the mean age and the mean BMI of the individual populations analyzed ranged between 37 and 59.2 years old and between 20.65 and 35.68 kg/m2, respectively.
Non-obese population included 995 patients (60.5%) with samples size ranged between 30 and 346. Most patients had female sex (634; 63.7%), while the mean age and the mean BMI of the individual populations analyzed ranged between 47.6 and 56.1 years old and between 20.65 and 27.28 kg/m2, respectively.
Obese population included 651 patients (39.5%) with samples size ranged between 28 and 174. Most patients had female sex (406; 62.4%), while the mean age and the mean BMI of the individual populations analyzed ranged between 37 and 59.2 years old and between 28.08 and 35.68 kg/m2, respectively.
Table 2 shows the surgical and histopathological characteristics of adrenal pathology. The lesions involved the left adrenal gland in a slightly larger portion than the right one with a mean diameter between 2.63 and 5 cm. Non-functioning/hormonally inactive tumors and Cushing’s syndrome/glucocorticosteroid-secreting tumors were the most frequently treated adrenal diseases.
Table 2
| Authors/year | Group | Adrenal side (n) | Adrenal size (cm), mean ± SD | Adrenal disease (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Bilateral | Primary aldosteronism | Pheochromocytoma | Cushing | Nonfunctional lesion | Metastasis | Other | |||
| Kazaryan et al., 2011 (43) | Obese | 13 | 26 | 0 | 2.65±1.29 | 15 | 3 | 8 | 11 | n/a | 2 |
| Non-obese | 66 | 67 | 0 | 4.84±2.90 | 32 | 35a | 17 | 37a | n/a | 13 | |
| Dancea et al., 2012 (44) | Obese | 19b | 28b | 0 | 4.5±3.1 | n/a | n/a | n/a | n/a | n/a | n/a |
| Non-obese | 13 | 18 | 0 | 4.3±2.0 | n/a | n/a | n/a | n/a | n/a | n/a | |
| Economopoulos et al., 2016 (45) | Obese | n/a | n/a | n/a | 3.48±0.86 | 27 | 34 | 38 | 48 | 9 | 1 |
| Non-obese | n/a | n/a | n/a | 3.85±0.95 | 30 | 51 | 15 | 56 | 9 | 5 | |
| Pędziwiatr et al., 2017 (46) | Obese | 79 | 95 | 0 | 4.23±2.70 | 25 | 32 | 30 | 84 | 0 | 3 |
| Non-obese | 179 | 167 | 0 | 4.23±2.11 | 34 | 87 | 29 | 191 | 0 | 5 | |
| Inaishi et al., 2018 (47) | Obese | 12 | 16 | 0 | 2.63±1.66 | 11 | 9 | 4 | 2 | 2 | 0 |
| Non-obese | 26 | 44 | 0 | 3.3±1.41 | 15 | 20 | 22 | 7 | 6 | 0 | |
| Ortenzi et al., 2019 (48) | Obese | 41 | 35 | 3 | 4.3±1.8 | 0 | 0 | 79 | 0 | 0 | 0 |
| Non-obese | 80 | 61 | 8 | 4.26±1.67 | 0 | 0 | 149 | 0 | 0 | 0 | |
| Altın et al., 2021 (49) | Obese | 14 | 21 | 0 | 4.7±1.8 | 9 | 2 | 19 | 5 | 0 | 0 |
| Non-obese | 17 | 13 | 0 | 4.7±2.8 | 8 | 5 | 5 | 8 | 3 | 1 | |
| Rodríguez-Hermosa et al., 2021 (50) | Obese | 43 | 47 | 0 | 5.0±2.9 | 13 | 17 | 27 | 19 | 14 | |
| Non-obese | 34 | 36 | 0 | 5.0±2.4 | 10 | 22 | 11 | 15 | 12 | ||
a, one patient in group I had both pheochromocytoma and adenoma in her left adrenal gland; b, BMIs of two Ob patients were not reported. SD, standard deviation; n/a, not available; BMI, body mass index; Ob, obese.
Meta-analyses results
Operative time
All 8 included studies (1,646 patients: NOb 995, Ob 651) recorded operative time (Figure 2) (43-50). Meta-analysis of pooled results showed that operative time [mean difference (MD): −4.15, 95% confidence interval (CI): −10.68, 2.38, P=0.21] did not have statistically significant discrepancies between the two groups. The recorded heterogeneity was high and significant from a statistical point of view (I2=76%, P<0.001).

Intraoperative complications
Three out of 8 included studies (317 patients: NOb 194, Ob 123) reported intraoperative complications rate (Figure 3) (43,44,49). Meta-analysis of pooled results showed that intraoperative complications rate [odds ratio (OR): 0.86, 95% CI: 0.29, 2.59, P=0.79] recorded statistically non-significant discrepancies between the two groups. The recorded heterogeneity was low, although negligible from a statistical perspective (I2=0%, P=0.42).

EBL
Five out of 8 included studies (1,086 patients: NOb 666, Ob 420) recorded EBL (Figure 4) (44,46-48,50). Meta-analysis of pooled results showed that EBL (MD: −16.35, 95% CI: −42.48, 9.79, P=0.22) recorded statistically non-significant discrepancies between the two groups. The recorded heterogeneity was high and significant from a statistical point of view (I2=91%, P<0.001).

Transfusion
Four out of 8 included studies (625 patients: NOb 382, Ob 243) reported transfusion rate (Figure 5) (43,48-50). Meta-analysis of pooled results showed that transfusion rate (OR: 0.56, 95% CI: 0.11, 2.78, P=0.48) recorded statistically non-significant discrepancies between the two groups. The recorded heterogeneity was low, although negligible from a statistical perspective (I2=0%, P=0.76).
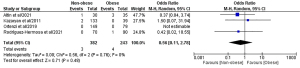
Conversion to open surgery
All 8 included studies (1,646 patients: NOb 995, Ob 651) reported conversion to open surgery rate (Figure 6) (43-50). Meta-analysis of pooled results showed that conversion to open surgery rate (OR: 0.74, 95% CI: 0.34, 1.60, P=0.44) recorded statistically non-significant discrepancies between the two groups. The recorded heterogeneity was low, although negligible from a statistical point of view (I2=0%, P=0.83).

Overall postoperative complications
All 8 included studies (1,646 patients: NOb 995, Ob 651) recorded overall postoperative complications rate (Figure 7) (43-50). Meta-analysis of pooled results showed that overall postoperative complications rate (OR: 0.72, 95% CI: 0.44, 1.17, P=0.18) recorded statistically non-significant discrepancies between the two groups. The recorded heterogeneity was low, although negligible from a statistical perspective (I2=17%, P=0.30).

Major (Clavien-Dindo or CD ≥ III) postoperative complications
Six out of 8 included studies (1,246 patients: NOb 713, Ob 533) recorded major (CD ≥ III) postoperative complications rate (Figure 8) (44-47,49,50). Meta-analysis of pooled results showed that major (CD ≥ III) postoperative complications rate (OR: 0.79, 95% CI: 0.23, 2.77, P=0.72) recorded statistically non-significant discrepancies between the two groups. The recorded heterogeneity was moderate, although negligible from a statistical point of view (I2=27%, P=0.23).

Length of hospital stay
All 8 included studies (1,646 patients: NOb 995, Ob 651) recorded length of hospital stay (Figure 9) (43-50). Meta-analysis of pooled results showed that length of hospital stay (OR: 0.06, 95% CI: −0.29, 0.41, P=0.75) recorded statistically non-significant discrepancies between the two groups. The recorded heterogeneity was high and significant from a statistical perspective (I2=88%, P<0.001).

Subgroup analysis
Subgroup analysis was carried out as a consequence of discrepancies in study designs. We investigated different outcomes, just taking into account studies with ≥30 kg/m2 obesity criteria. Our subgroup analysis confirmed 7 out of 8 outcomes of pooled analysis (see Figures S1-S8). Just the operative time, which in the pooled analysis was close to statistical significance, was statistically significantly lower in the NOb group (MD: −6.18, 95% CI: −12.15, −0.20, P=0.04) (I2=72%, P=0.001).
Publication bias
As we included 8 studies, we did not carry out an analysis of publication bias. Indeed, in compliance with Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0), tests for funnel plot asymmetry should be carried out just in meta-analyses including at least 10 studies, as fewer studies prevent tests from identifying the case from real asymmetry (51).
Discussion
Our meta-analysis examined several short-term intraoperative and postoperative outcomes of comparative studies in non-obese and obese patient populations undergoing TLA for benign or malignant adrenal disorders. We found just 2 meta-analyses dealing with the same topic, one including 5 comparative studies and the other 8 comparative ones (34,52). However, they both comprised comparative studies of patients undergoing TLA and the less common RLA (34,52). No distinction was made between the two approaches, although such distinction is mandatory, given significant anatomical and surgical discrepancies that many Authors pointed out in recent years. Furthermore, significantly fewer outcomes were analyzed in both meta-analyses (34,52).
Our study on pooled population of 1,646 patients (995 non-obese patients and 651 obese ones), who underwent TLA for benign or malignant adrenal diseases, recorded absence of statistically significant discrepancies in all short-term outcomes taken into account. Therefore, lack of a significant impact of obesity on intraoperative and postoperative TLA-related outcomes emerged from our study. Although the subgroup analysis detected a statistically shorter operative time in the NOb group compared to the Ob group, none of the intraoperative and postoperative morbidity outcomes as well as the length of hospital stay showed differences between the two groups.
Many factors could more or less significantly have affected aforementioned meta-analyzed results, among them: (I) laterality and size of adrenal lesion; (II) surgically treated adrenal pathology; (III) learning curve (LC) of surgeons, thus making a discussion about this topic mandatory.
Adrenal glands are retroperitoneally located bilateral organs (19). They are marked by anatomical relationship with prominent structures that could complicate surgical dissection (19,53). Connections between right adrenal gland and inferior vena cava and associations between left adrenal gland and spleen, pancreatic tail, splenic vessels and left renal vein play a paramount role (19,53). As right adrenal gland is a partly retrocaval one and it drains directly into inferior vena cava through a short central vein, right adrenalectomy is supposed to be more challenging than left adrenalectomy (19,53). Recently, few studies have compared right and left adrenalectomies, all laparoscopic ones and almost all TLAs (5/6) (53). Resulting data were integrated into a recent meta-analysis by Wang et al., where Authors found that right adrenalectomy group (361 patients) underwent higher EBL and higher conversion rate to open surgery, if compared to left adrenalectomy group (419 patients), with no significant discrepancies in terms of operative time, overall complications, major (CD ≥ III) complications, length of hospitalization (53). Authors concluded that, despite significant limitations in meta-analyzed studies, greater attention should be given to laparoscopic right adrenalectomy, due to its greater risk of bleeding (53).
Size of adrenal lesion represents a further important variable. In case of large lesions (e.g., >6 cm) many guidelines choose open adrenalectomy, rather than laparoscopic adrenalectomy, given the greater risk of capsule rupture through minimally invasive surgery. It should be noted that 6 cm cut-off was assumed by panel members on a mainly discretionary basis and not on good evidence from clinical studies. However, same guidelines report that this cut-off does not prove every <6 cm tumor should undergo laparoscopic adrenalectomy and every >6 cm tumor should undergo open adrenalectomy. Of the two Gan et al.’s recently published meta-analyses, the 2022 one analyzed both safety and effectiveness of minimally invasive adrenalectomy (laparoscopic and robotic ones) versus open adrenalectomy in patients with ≥5 cm adrenal lesions (10 observational studies; 898 patients) (54). Authors concluded that minimally invasive adrenalectomy gave advantages over open adrenalectomy, in terms of treating large adrenal lesions (including shorter length of hospitalization, drainage time, fasting time, less EBL and transfusions), whereas operative time and complications were similar (54). The 2023 published meta-analysis investigated the role of laparoscopic adrenalectomy in patients with ≥6 cm pheochromocytomas (55). Studying a 600-patient total population from 8 observational studies, Authors identified statistically significant discrepancies in the Large group (longer operative time and length of hospitalization, greater EBL, episodes of hypertension and/or hypotension and conversion to open surgery) compared to the Small group, in the absence of significant differences in overall complication rates (55).
Unfortunately, studies included in our meta-analysis did not investigate the impact of laterality and lesion size on surgical outcomes.
In addition to the two above-described factors (laterality and size of adrenal lesion), the role of adrenal pathology to be surgically treated cannot be overlooked. In the presence of prospective malignant lesions (e.g., adrenocortical carcinoma or adrenal gland metastases) risk of lesion rupture should be avoided and/or a simultaneous lymphadenectomy (in selected cases) performed in compliance with oncological radicality. Careful handling of lesion must also be carried out in case of pheochromocytoma, whose management can trigger the release of catecholamines and subsequent risk of intraoperative hemodynamic instability.
Comparative studies included in our meta-analysis considered multiple types of adrenal disorders, although no one was singly analyzed, in order to assess its impact on possible outcomes.
Many studies analyzed LC in adrenal surgery, dealing with both laparoscopic adrenal surgery per se and, more significantly, individual different laparoscopic approaches such as TLA. Reports regarding TLA LC examined different parameters. This variable is related to lack of standard definition of appropriate evaluation parameters.
Many studies suggested stabilization of operative time as a measure of LC. In 2002, both Valeri et al. and Pillinger et al. first described how stabilization of operative time can be considered a fundamental step in achieving LC (56,57). Valeri et al. came to the conclusion that 25 interventions were necessary to complete LC, while Pillinger et al. stated that 40 interventions were needed (56,57). Subsequently, some Authors included other evaluation parameters to operative time. Goitein et al. highlighted a reduction in operative time and rate of intraoperative complications by gaining experience, leading to localization of flattening LC after performing approximately 30 cases (58). Thirty surgical interventions were suggested as LC point leading to TLA (58). Ali et al. and Frizer et al. demonstrated a significant reduction in conversion to open surgery rate, as well as operative time during LC after the first 40 and 40–50 procedures, respectively (59,60). Finally, Maccabee et al. established flatten LC in 20 performed interventions (61). However, Authors did not find a significant impact of operative time on LC, in contrast to complication rate and blood losses (61).
As far as laparoscopic adrenalectomy is concerned, LC is estimated to be between 20–40 cases for experienced laparoscopic surgeons. LC impact on laparoscopic adrenal surgery outcomes seems to be highly important, although no included study analyzed this issue.
As already stated, TLA represents one of two laparoscopic approaches to adrenalectomy (13-18). Retroperitoneal approach bears different advantages over TLA (62). Among them, we underline possible avoidance of intraabdominal cavity and an easier approach to adrenal gland, thus avoiding both manipulation of intraabdominal structures and patient repositioning during bilateral procedures (62). Different studies underline how RLA means shorter operative time, lower EBL and a shorter length of hospital stay, with no significant discrepancies in terms of conversion to open surgery and postoperative complications (13-18). However, in a surgical perspective, RLA is a more challenging method, due to its narrower working space and less familiar anatomical view available to surgeons (13-18).
At present, scientific literature shows how RLA and TLA are similar, as far as histology of treated lesion is concerned (13-18,63). As a matter of fact, non-functioning and functioning benign lesions as well as metastases are effectively treated in both procedures (13-18,63-65). Although open approach is highly advisable, disagreement arouses in terms of clear or suspected primary adrenal malignancies (10,11,66-69). In such cases, some (few) Authors suggest both laparoscopic methods, although in very selected cases and in high-volume centers (66-69).
On the other hand, neither lesion size (>6 cm) nor patient obesity seem to restrict safety and effectiveness related to both procedures (13,34,52,54,55,63,70). Some Authors treated even 12 cm diameter benign lesions (63,64) or severely obese patients (43-46,48-50,71-73).
Eventually, TLA shows a significant advantage in allowing concurring surgery on other organs, while RLA shows its advantage in the treatment of patients with a significant history of previous abdominal surgery.
From a surgical point of view, obesity has long been considered as detrimental factor in postoperative outcomes (22). Furthermore, obesity is associated with deep metabolic disorders (74). At present, adipose tissue is acknowledged not only as a reserve of lipids but as a deeply active metabolic organ showing endocrine, paracrine and immunological features (74). Metabolic syndrome is an additional consequence of exceeding adipose tissue, in particular of intraabdominal or visceral ones (74). In this occurrence, prothrombotic and proinflammatory states are associated with insulin resistance (74).
We might question whether obesity is harmful or helpful in patients undergoing elective or emergency surgery (22). Obesity paradox shows that moderate obesity provides metabolic reserve and an altered immune state, that may be beneficial (21).
Although our meta-analysis underlines the lack of significant discrepancies in terms of overall and major postoperative complications between the two groups in both pooled and subgroup analyses, studies on postoperative morbidity and mortality in obese patients led to different outcomes. In particular, obese patient undergoing bariatric and non-bariatric surgery seemed to be more prone to develop pulmonary disorders (hypoventilation syndrome, pneumonia, atelectasis, pulmonary embolism), cardiovascular disorders (atrial arrhythmias, thromboembolic accidents), surgical site infections, wound healing complications, systemic infections (urinary tract infections, in particular), renal failure (74). Risk of cholelithiasis is also peculiar to patients who undergo bariatric surgery (75).
Present meta-analysis showed several non-negligible limitations: (I) we did not detect any randomized controlled study, except for observational studies, all lacking in propensity-score matching analysis; (II) numbers of included studies and of enrolled patients were small; (III) the study time frame witnessed variation of diagnostic methods, surgical techniques and skills; (IV) general population and surgically treated adrenal disease (adrenal histology, adrenal side, adrenal size) characteristics had heterogeneous nature.
Despite drawbacks, our study has significant strong points, which previous meta-analyses missed. In the lack of randomized controlled trials or propensity score matching observational studies, our meta-analysis offered the highest level of evidence, as we included higher number of comparative (double-arm) studies on TLA in both non-obese and obese populations than previous studies. Eventually, our meta-analysis included a significantly higher number of outcomes, if compared to those that others discussed.
Conclusions
By including comparative studies of non-obese and obese adult patients undergoing TLA for benign or malignant adrenal disorders, our meta-analysis showed that TLA did not record any statistically different short-term outcomes between the two populations. Therefore, we can say that obesity does not impact TLA safety and effectiveness.
Our results need deep analysis because of significant biases among meta-analyzed studies, slight overall sample size and paucity of analyzed events. Thus, well-designed randomized controlled trials, possibly multicentre ones, are of paramount importance, if we want to endorse meta-analysis’s outcomes and structure an appropriate and uniform patient selection.
Acknowledgments
We thank Daniela Masi (Azienda USL-IRCCS di Reggio Emilia) for support in English editing.
Funding: This study was partially supported by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-55/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-55/coif). M.Z. serves as an unpaid editorial board member of Chinese Clinical Oncology from April 2024 to March 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The local ethics committee (Comitato Etico dell’Area Vasta Emilia Nord, Italy) ruled that no formal ethics approval was required in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hupe MC, Imkamp F, Merseburger AS. Minimally invasive approaches to adrenal tumors: an up-to-date summary including patient position and port placement of laparoscopic, retroperitoneoscopic, robot-assisted, and single-site adrenalectomy. Curr Opin Urol 2017;27:56-61. [Crossref] [PubMed]
- Uludağ M, Aygün N, İşgör A. Surgical Indications and Techniques for Adrenalectomy. Sisli Etfal Hastan Tip Bul 2020;54:8-22. [PubMed]
- Taffurelli G, Ricci C, Casadei R, et al. Open adrenalectomy in the era of laparoscopic surgery: a review. Updates Surg 2017;69:135-43. [Crossref] [PubMed]
- Mihai R. Open adrenalectomy. Gland Surg 2019;8:S28-35. [Crossref] [PubMed]
- Carr AA, Wang TS. Minimally Invasive Adrenalectomy. Surg Oncol Clin N Am 2016;25:139-52. [Crossref] [PubMed]
- Kwak J, Lee KE. Minimally Invasive Adrenal Surgery. Endocrinol Metab (Seoul) 2020;35:774-83. [Crossref] [PubMed]
- Raffaelli M, De Crea C, Bellantone R. Laparoscopic adrenalectomy. Gland Surg 2019;8:S41-52. [Crossref] [PubMed]
- Makay O, Erol V, Ozdemir M. Robotic adrenalectomy. Gland Surg 2019;8:S10-6. [Crossref] [PubMed]
- Fassnacht M, Tsagarakis S, Terzolo M, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2023;189:G1-G42. [Crossref] [PubMed]
- Mihai R, De Crea C, Guerin C, et al. Surgery for advanced adrenal malignant disease: recommendations based on European Society of Endocrine Surgeons consensus meeting. Br J Surg 2024;111:znad266.
- Yip L, Duh QY, Wachtel H, et al. American Association of Endocrine Surgeons Guidelines for Adrenalectomy: Executive Summary. JAMA Surg 2022;157:870-7. [Crossref] [PubMed]
- Gagner M, Lacroix A, Bolté E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med 1992;327:1033. [Crossref] [PubMed]
- Conzo G, Tartaglia E, Gambardella C, et al. Minimally invasive approach for adrenal lesions: Systematic review of laparoscopic versus retroperitoneoscopic adrenalectomy and assessment of risk factors for complications. Int J Surg 2016;28:S118-23. [Crossref] [PubMed]
- Arezzo A, Bullano A, Cochetti G, et al. Transperitoneal versus retroperitoneal laparoscopic adrenalectomy for adrenal tumours in adults. Cochrane Database Syst Rev 2018;12:CD011668. [Crossref] [PubMed]
- Jiang YL, Qian LJ, Li Z, et al. Comparison of the retroperitoneal versus Transperitoneal laparoscopic Adrenalectomy perioperative outcomes and safety for Pheochromocytoma: a meta-analysis. BMC Surg 2020;20:12. [Crossref] [PubMed]
- Meng C, Du C, Peng L, et al. Comparison of Posterior Retroperitoneoscopic Adrenalectomy Versus Lateral Transperitoneal Laparoscopic Adrenalectomy for Adrenal Tumors: A Systematic Review and Meta-Analysis. Front Oncol 2021;11:667985. [Crossref] [PubMed]
- Gavriilidis P, Camenzuli C, Paspala A, et al. Posterior Retroperitoneoscopic Versus Laparoscopic Transperitoneal Adrenalectomy: A Systematic Review by an Updated Meta-Analysis. World J Surg 2021;45:168-79. [Crossref] [PubMed]
- Zhang M, Wang H, Guo F, et al. Retroperitoneal laparoscopic adrenalectomy versus transperitoneal laparoscopic adrenalectomy for pheochromocytoma: a systematic review and meta-analysis. Wideochir Inne Tech Maloinwazyjne 2023;18:11-9. [PubMed]
- Madani A, Lee JA. Surgical Approaches to the Adrenal Gland. Surg Clin North Am 2019;99:773-91. [Crossref] [PubMed]
- Mullen JT, Davenport DL, Hutter MM, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol 2008;15:2164-72. [Crossref] [PubMed]
- Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg 2009;250:166-72. [Crossref] [PubMed]
- Doyle SL, Lysaght J, Reynolds JV. Obesity and post-operative complications in patients undergoing non-bariatric surgery. Obes Rev 2010;11:875-86. [Crossref] [PubMed]
- Gurunathan U, Rapchuk IL, Dickfos M, et al. Association of Obesity With Septic Complications After Major Abdominal Surgery: A Secondary Analysis of the RELIEF Randomized Clinical Trial. JAMA Netw Open 2019;2:e1916345. [Crossref] [PubMed]
- Khorgami Z, Sclabas GM, Aminian A, et al. Mortality in open abdominal aortic surgery in patients with morbid obesity. Surg Obes Relat Dis 2019;15:958-63. [Crossref] [PubMed]
- Maloney SR, Reinke CE, Nimeri AA, et al. The Obesity Paradox in Emergency General Surgery Patients. Am Surg 2022;88:852-8. [Crossref] [PubMed]
- El Moheb M, Jia Z, Qin H, et al. The Obesity Paradox in Elderly Patients Undergoing Emergency Surgery: A Nationwide Analysis. J Surg Res 2021;265:195-203. [Crossref] [PubMed]
- Chisholm JA, Jamieson GG, Lally CJ, et al. The effect of obesity on the outcome of laparoscopic antireflux surgery. J Gastrointest Surg 2009;13:1064-70. [Crossref] [PubMed]
- Cullinane C, Fullard A, Croghan SM, et al. Effect of obesity on perioperative outcomes following gastrointestinal surgery: meta-analysis. BJS Open 2023;7:zrad026. [Crossref] [PubMed]
- Pan W, Sun Z, Xiang Y, et al. The correlation between high body mass index and survival in patients with esophageal cancer after curative esophagectomy: evidence from retrospective studies. Asia Pac J Clin Nutr 2015;24:480-8. [PubMed]
- Ramadan B, Dahboul H, Mouawad C, et al. Obesity: A risk factor for postoperative complications in laparoscopic surgery for colorectal cancer. J Minim Access Surg 2024;20:12-8. [Crossref] [PubMed]
- Balentine CJ, Enriquez J, Cruz G, et al. Obesity does not increase complications following pancreatic surgery. J Surg Res 2011;170:220-5. [Crossref] [PubMed]
- Kazaure HS, Roman SA, Sosa JA. Obesity is a predictor of morbidity in 1,629 patients who underwent adrenalectomy. World J Surg 2011;35:1287-95. [Crossref] [PubMed]
- Paun D, Petris R, Ganescu R, et al. Outcome of Laparoscopic Adrenalectomy in Obese Patients. Maedica (Bucur) 2015;10:231-6. [PubMed]
- Danwang C, Agbor VN, Bigna JJ. Obesity and postoperative outcomes of the patients with laparoscopic adrenalectomy: a systematic review and meta-analysis. BMC Surg 2020;20:194. [Crossref] [PubMed]
- Mínguez Ojeda C, Gómez Dos Santos V, Lorca JÁ, et al. Influence of obesity and overweight in surgical outcomes of adrenalectomy for primary adrenal disease: A cohort study of 146 cases. Endocrinol Diabetes Nutr (Engl Ed) 2023;70:564-71. [Crossref] [PubMed]
- Knewitz DK, Castillo-Larios R, Evans LA, et al. Impact of Body Mass Index ≥35 kg/m(2) on Minimally Invasive Adrenalectomy. J Laparoendosc Adv Surg Tech A 2024;34:359-64. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [Crossref] [PubMed]
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Cochrane Review Manager (RevMan). Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 22 March 2024).
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Higgins JPT, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Cochrane Handbook for Systematic Reviews of Interventions [Version 5.1.0]. Available online: https://handbook-5-1.cochrane.org/chapter_7/table_7_7_a_formulae_for_combining_groups.htm (accessed on 22 March 2024).
- Kazaryan AM, Marangos IP, Røsok BI, et al. Impact of body mass index on outcomes of laparoscopic adrenal surgery. Surg Innov 2011;18:358-67. [Crossref] [PubMed]
- Dancea HC, Obradovic V, Sartorius J, et al. Increased complication rate in obese patients undergoing laparoscopic adrenalectomy. JSLS 2012;16:45-9. [Crossref] [PubMed]
- Economopoulos KP, Phitayakorn R, Lubitz CC, et al. Should specific patient clinical characteristics discourage adrenal surgeons from performing laparoscopic transperitoneal adrenalectomy? Surgery 2016;159:240-8. [Crossref] [PubMed]
- Pędziwiatr M, Major P, Pisarska M, et al. Laparoscopic transperitoneal adrenalectomy in morbidly obese patients is not associated with worse short-term outcomes. Int J Urol 2017;24:59-63. [Crossref] [PubMed]
- Inaishi T, Kikumori T, Takeuchi D, et al. Obesity does not affect peri- and postoperative outcomes of transabdominal laparoscopic adrenalectomy. Nagoya J Med Sci 2018;80:21-8. [PubMed]
- Ortenzi M, Balla A, Ghiselli R, et al. Minimally invasive approach to the adrenal gland in obese patients with Cushing's syndrome. Minim Invasive Ther Allied Technol 2019;28:285-91. [Crossref] [PubMed]
- Altın Ö, Sarı R. The effect of obesity in laparoscopic transperitoneal adrenalectomy. Turk J Surg 2021;37:126-32. [Crossref] [PubMed]
- Rodríguez-Hermosa JI, Planellas-Giné P, Cornejo L, et al. Comparison of Outcomes between Obese and Nonobese Patients in Laparoscopic Adrenalectomy: A Cohort Study. Dig Surg 2021;38:237-46. [Crossref] [PubMed]
- Sterne JAC, Egger M, Moher D, et al. Chapter 10: Addressing reporting biases. In: Cochrane Handbook for Systematic Reviews of Interventions [Version 5.1.0]. Available online: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm (accessed on 22 March 2024).
- Xia Z, Liu H, Gu P, et al. Peri- and postoperative outcomes of laparoscopic adrenalectomy in nonobese versus obese patients: a systematic review and meta-analysis. Wideochir Inne Tech Maloinwazyjne 2022;17:430-40. [Crossref] [PubMed]
- Wang Y, Yang Z, Chang X, et al. Right laparoscopic adrenalectomy vs. left laparoscopic adrenalectomy: a systematic review and meta-analysis. Wideochir Inne Tech Maloinwazyjne 2022;17:9-19. [PubMed]
- Gan L, Meng C, Li K, et al. Safety and effectiveness of minimally invasive adrenalectomy versus open adrenalectomy in patients with large adrenal tumors (≥5 cm): A meta-analysis and systematic review. Int J Surg 2022;104:106779. [Crossref] [PubMed]
- Gan L, Peng L, Meng C, et al. The role of laparoscopic adrenalectomy in the treatment of large pheochromocytomas (>6 cm): a meta-analysis and systematic review. Int J Surg 2023;109:1459-69. [Crossref] [PubMed]
- Valeri A, Borrelli A, Presenti L, et al. The influence of new technologies on laparoscopic adrenalectomy: our personal experience with 91 patients. Surg Endosc 2002;16:1274-9. [Crossref] [PubMed]
- Pillinger SH, Bambach CP, Sidhu S. Laparoscopic adrenalectomy: a 6-year experience of 59 cases. ANZ J Surg 2002;72:467-70. [Crossref] [PubMed]
- Goitein D, Mintz Y, Gross D, et al. Laparoscopic adrenalectomy: ascending the learning curve. Surg Endosc 2004;18:771-3. [Crossref] [PubMed]
- Ali JM, Liau SS, Gunning K, et al. Laparoscopic adrenalectomy: auditing the 10 year experience of a single centre. Surgeon 2012;10:267-72. [Crossref] [PubMed]
- Fiszer P, Toutounchi S, Pogorzelski R, et al. Laparoscopic adrenalectomy--assessing the learning curve. Pol Przegl Chir 2012;84:293-7. [Crossref] [PubMed]
- Maccabee DL, Jones A, Domreis J, et al. Transition from open to laparoscopic adrenalectomy: the need for advanced training. Surg Endosc 2003;17:1566-9. [Crossref] [PubMed]
- Callender GG, Kennamer DL, Grubbs EG, et al. Posterior retroperitoneoscopic adrenalectomy. Adv Surg 2009;43:147-57. [Crossref] [PubMed]
- Van Den Heede K, Vatansever S, Girgin T, et al. Posterior retroperitoneal versus transperitoneal laparoscopic adrenalectomy in adults: results from the EUROCRINE® surgical registry. Langenbecks Arch Surg 2023;408:241. [Crossref] [PubMed]
- Conzo G, Pasquali D, Gambardella C, et al. Long-term outcomes of laparoscopic adrenalectomy for Cushing disease. Int J Surg 2014;12:S107-11. [Crossref] [PubMed]
- Conzo G, Gambardella C, Candela G, et al. Single center experience with laparoscopic adrenalectomy on a large clinical series. BMC Surg 2018;18:2. [Crossref] [PubMed]
- Creamer J, Matthews BD. Laparoscopic adrenalectomy for cancer. Surg Oncol Clin N Am 2013;22:111-24. vi-vii. [Crossref] [PubMed]
- Giordano A, Feroci F, Podda M, et al. Minimally invasive versus open adrenalectomy for adrenocortical carcinoma: the keys surgical factors influencing the outcomes-a collective overview. Langenbecks Arch Surg 2023;408:256. [Crossref] [PubMed]
- Nakanishi H, Miangul S, Wang R, et al. Open Versus Laparoscopic Surgery in the Management of Adrenocortical Carcinoma: A Systematic Review and Meta-analysis. Ann Surg Oncol 2023;30:994-1005. [Crossref] [PubMed]
- Carlisle K, Blackburn KW, Japp EA, et al. Laparoscopic surgery for adrenocortical carcinoma: Estimating the risk of margin-positive resection. J Surg Oncol 2024;129:691-9. [Crossref] [PubMed]
- Tsai IC, Hsieh YC, Tseng WH, et al. Retroperitoneal laparoscopic adrenalectomy for large adrenal tumors-analysis of tumor size and adverse events: a retrospective single-center study. Front Surg 2024;10:1284093. [Crossref] [PubMed]
- Hu Q, Hang Z, Ho Y, et al. Impact of Obesity on Perioperative Outcomes of Retroperitoneal Laparoscopic Adrenalectomy. Urol Int 2015;95:361-6. [Crossref] [PubMed]
- Zonča P, Bužga M, Ihnát P, et al. Retroperitoneoscopic Adrenalectomy in Obese Patients: Is It Suitable? Obes Surg 2015;25:1203-8. [Crossref] [PubMed]
- Seow YT, Nyandoro MG, Poh S, et al. The Impact of Obesity on Mortality and Complications in Posterior Retroperitoneoscopic Adrenalectomy. Cureus 2023;15:e42421. [Crossref] [PubMed]
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881-7. [Crossref] [PubMed]
- Pizza F, D'Antonio D, Lucido FS, et al. The Role of Ursodeoxycholic Acid (UDCA) in Cholelithiasis Management After One Anastomosis Gastric Bypass (OAGB) for Morbid Obesity: Results of a Monocentric Randomized Controlled Trial. Obes Surg 2020;30:4315-24. [Crossref] [PubMed]



