Transdermal granisetron for the prevention of nausea and vomiting following moderately or highly emetogenic chemotherapy in Chinese patients: a randomized, double-blind, phase III study
Introduction
Chemotherapy-induced nausea and vomiting (CINV) are commonly occurring, which have a negative impact on patient’s quality of life and lead to poor compliance with further chemotherapy. In addition, nausea and vomiting can result in dehydration, electrolyte imbalances, anorexia and malnutrition, further withdrawal from treatment (1-3). Thus, the prevention of CINV could be one of the most challenging supportive care issues in oncology.
The development of the 5-HT3-receptor antagonists (5-HT3RAS, i.e., granisetron, ondansetron) represents a significant advance in antiemetic therapy. All of these agents show considerable efficacy in preventing CINV, with acute responses for single agents ranging from 40% to 86% (1). The granisetron transdermal delivery system (GTDS;Sancuso®, ProStrakan, Inc., USA) has been recently developed and approved by the US Food and Drug Administration (FDA) for prevention and controlling of CINV (4). Sancuso® is the first GTDS for prevention and control of CINV, which contain 34.3 mg of granisetron and can provide continuous delivery of granisetron through the skin, with releasing 3.3 mg of granisetron per 24 h for up to 7 days (4-7). Meanwhile, it maintained the plasma concentration (Cavg) of 2.2 ng/mL over 6 days, similar to that obtained with 2 mg of oral granisetron administered every day during the same period of time (8). Compared with other 5-HT3RAS, GTDS carries important advantages over repeated injections or oral dosing in terms of patient convenience and compliance. It may be especially valuable in patients for whom swallowing is difficult or absorption of oral medication is uncertain, such as cancer patients with gastrointestinal surgery (7,9-12).
A published phase III trial in 641 patients receiving multiday moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC) regimen demonstrated that the GTDS was not inferior to oral granisetron in complete control (CC) of CINV (13). Though 71% of patients included in the GTDS group received cisplatin-based HEC and without the use of neurokinin-1 (NK-1) RA, the efficacy was satisfying and promising. Only 11.9% of Asian patients were included in the study and 43 Asian patients was administrated GTDS (13). Until now, not enough data was reported about GTDS in Asian population.
This is the first phase III study to evaluate the efficacy and tolerability of GTDS in patients receiving MEC or HEC in China.
Methods
Patients and treatment
Cancer patients who were administrated to multiday (≥2 days) MEC or HEC [according to NCCN Clinical Practice Guidelines in Oncology: Antiemesis (1)] were eligible for enrollment in the study. Inclusion criteria included Eastern Cooperative Oncology Group (ECOG) Status ≤2 and a life expectancy of ≥3 months. The primary exclusion criteria included contraindications to 5-HT3RAs; uncontrolled nausea (≥ grade 2) and vomiting within 72 h before chemotherapy initiation and/or baseline QTc prolongation.
Study design
This was a randomized, active control, double-blind, parallel-group study, conducted at 15 centers in China. The primary objective was to demonstrate GTDS efficacy compared to oral granisetron in Chinese patients. Secondary objectives included the assessment of the safety, tolerability and adhesive properties of the GTDS.
Patients were randomized in a 1:1 ratio using a central randomization system to receive either a GTDS patch and placebo capsules or a placebo patch and active capsules (2 mg granisetron). Stratification was based on gender, the severity of emetogenic chemotherapy (moderate or high risk) and chemotherapy duration (2 or ≥3d).
Patches were applied to the upper arm for 24–48 h before the initiation of chemotherapy and left in place for 7 days. Patients received 1 mg tablet 1–2 h before administration of chemotherapy on day 1 firstly, then 1mg tablet every 12 h during the chemotherapy period. Corticosteroid was not used as prophylactic medicine in the study. Chemotherapy regimen was required to include moderate to high risk emetogenic agents and the duration for 2 days or more. The chemotherapy regimens containing paclitaxel or pemetrexed were excluded due to corticosteroid as pretreatment. Corticosteroid can be used as rescue medicine and was recorded in the study.
All patients provided written informed consent before enrollment into the study. The study protocol was approved by the Ethics Committee Review Board at each participating center and was registered with ClinicalTrials.gov (Identifier, NCT01937156).
Efficacy parameters
The primary efficacy endpoint was the percentage of patients achieving complete control of CINV (CC; no vomiting and/or retching, no more than mild nausea, and no need for rescue medication) from the first administration until 24 h after the last administration of chemotherapeutic agents (PEEP). The CC of per day (day 1, 2, 3, 4, 5) during PEEP was observed.
Secondary efficacy endpoints were the following: the time to failure of CC during the efficacy observation period (the chemotherapy initiation until 24 h after patch removal), the percentage of patients failing CC due to nausea, vomiting, or receipt of rescue medication (total days and per day), the percentage of patients achieving complete response (CR; no vomiting and/or retching, no use of rescue medication) during PEEP, patients’ global satisfaction with antiemetic therapy (assessed using a 10-cm visual analog scale (VAS) at the time of patch removal), the frequency of vomiting per day and the severity of nausea during the efficacy observation period, and the percentage adhesion of the patch over the application period.
Study visits and evaluation
Assessments of efficacy, tolerability and safety variables were performed throughout the study period, including a 14-day follow-up after patch removal. Global satisfaction in the control of nausea and vomiting was evaluated by patients themselves.
Statistical analysis
Statistical analyses were performed in the safety set (SS; all patients who received at least one dose of study treatment), the full analysis set (FAS; all SS patients who had ≥1 efficacy assessment), and the per protocol set (PPS; all FAS patients who had no protocol violations that directly affected the primary endpoint). The primary and secondary efficacy endpoints were evaluated with the FAS and the PPS.
Chi-square test, Fisher’s exact test and Kaplan-Meier were used for statistical analysis. A two-sided P<0.05 was considered statistical significance. All analyses were performed using the SAS ver. 9.3.
Results
Between August 2013 and January 2014, 313 patients were randomized to the GTDS group (n=157) or the oral granisetron group (n=156). All patients received one cycle chemotherapy. Of these patients, 313 were in the SS, 310 were in the FAS, and 281 were in the PPS. Of 9 patients who dropped out of the study, 3 patients withdrew due to serious adverse events (SAE) and 6 patients withdrew from the study by themselves.
The baseline demographic, medical characteristics and chemotherapy regimens were comparable between the groups (Table 1). In the FAS, baseline characteristics were similar. The chemotherapy regimens including chemotherapy duration, emetogenic degree and cisplatin-contained or not were well balanced between the groups.
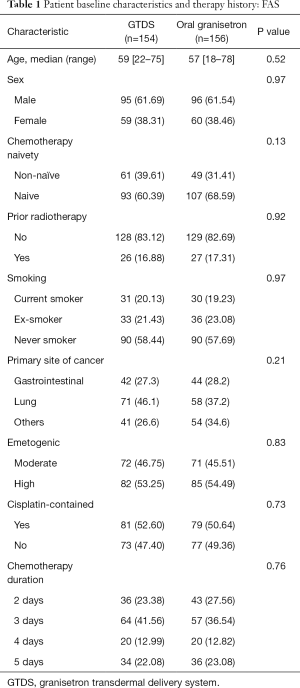
Full table
Primary efficacy analysis
In the FAS, the number of patients who achieved CC during the PEEP was 72 (46.75%) in the GTDS group and 92 (58.97%) in the oral granisetron group. There was statistical significance between the groups (P=0.0404), indicating that the oral granisetron was superior relative to the GTDS. In the PPS, CC was achieved by 67 (47.52%) patients in the GTDS group and 83 (59.29%) patients in the oral granisetron group during the PEEP. There was no statistical significance between the groups (P=0.0559).
Further analyses showed that the difference of CC percentage occurred on the first day of chemotherapy between the groups. In the FAS, the CC percentage of CINV was 70.13% in the GTDS group and 91.03% in the oral granisetron group on day 1 of chemotherapy. Statistical significance was observed (P<0.0001). But in the following day 2 to day 5, the CC remained stable lever in the GTDS group (69.48–79.63%). While in the oral granisetron group, the CC decreased to 76.3% on day 2 and then remained the similar level from day 3 to day 5 (67.26–76.28%). Overall, the percentage CC of CINV was similar and there was no statistical significance in both groups (P>0.05). The same conclusion was also drawn from the PPS analyses.
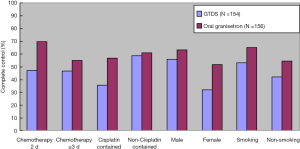
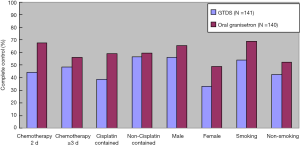
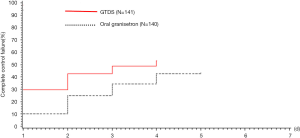
The predefined subgroups analyses included chemotherapy duration, sex, cisplatin-contained in the chemotherapy regimen and smoking history. In the FAS and PPS population receiving 2 days or ≥3 days chemotherapy, male and smoking history, the differences in CC percentage had no statistical significance between the groups during PEEP (P>0.05). There was statistical significance in terms of cisplatin-contained regimen and female (P=0.0089 and 0.0268) (Figures 1,2).
Secondary efficacy analysis
During the efficacy observation phase, FAS analyses showed that the percentage of patients who achieved CC was 46.1% in the GTDS group while 56.4% in the oral granisetron group. There was no statistical significance between the groups (P=0.0621). The percentage of patients who achieved CC of delay vomiting (the second day of chemotherapy to 24 h after patch removal) wasn’t also statistically significance between the GTDS groups and the Oral granisetron group (53.25% vs. 56.41%, P=0.5448). The difference of the percentage CC mainly occurred on day 1. In the following day 2 to day 5, no statistical significance was observed (P>0.05).
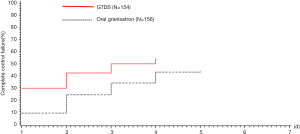
To analyze the failure time and the ratio to CC, Kaplan-Meier survival graft showed that the failure of CC mainly occurred on day 1 in the GTDS group, which had more negative influence on the study results. However, the failure percentage of CC per day was similar in both groups during the following period. In the FAS and PPS, there was no statistical significance between the groups (P<0.05) (Figures 3,4).
In FAS, complete response (CR) of vomiting was statistically different between the GTDS group and the oral granisetron group during PEEP (51.30% vs. 64.10%). The difference also occurred on day 1. In the following day 2 to day 5, no statistical significance was observed between the groups (P>0.05). During the efficacy observation phase, the frequency of vomiting (episodes per day) and severity of nausea had no statistical difference from day 2 to day 7. Patients’ satisfaction with antiemetic therapy was high in both groups. The mean VAS score for patients was 80.50 in the GTDS group and 88.0 in the oral granisetron group (P=0.7442). There was also no statistical significance between the groups for ECOG change. The adhesion of active and placebo patches were similar (P>0.05). The PPS results were in accordance with the FAS results.
Safety analysis
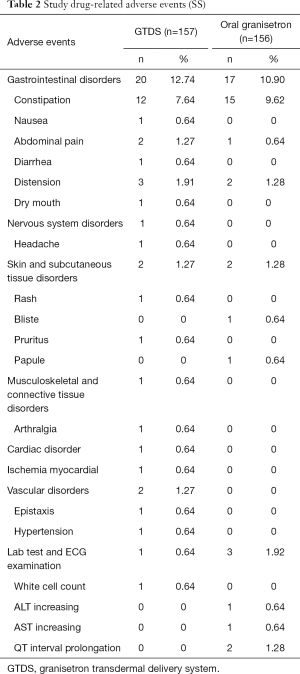
A total of 313 patients were included in the SS, of whom 212 experienced adverse events (AE). The main adverse events included constipation, anorexia, cough and fatigue. The study drug-related adverse events were summarized in Table 2.The most common study drug-related AE in both groups was constipation, which was reported more frequently in patients receiving oral granisetron. ECG data showed that QTC prolongation of 2 patients was identified in the oral granisetron group, which was considered granisetron related. All adverse events were of mild to moderate severity and tolerable. The incidence of serious adverse event (SAE) was lower, which only one occurred upper digestive tract hemorrhage in the GTDS group and 4 patients occurred platelet decreasing, white cell decreasing, acute appendicitis and diarrhea in the oral granisetron group, respectively. All SAE were not related to study treatments.
Full table
Discussion
The current randomized study indicated that GTDS had stable efficacy in the control of CINV whether per day or total day of chemotherapy. In the GTDS group, the CC was 70.13% on day 1 and fluctuated from 69.48% to 79.63% from day 2 to day 5. The CC in the oral granisetron group was 91.03% on day 1 which was higher than that in the GTDS group. In the following days, the CC were almost equivalent in both groups. Compared with the oral granisetron group, the difference of the CC in the GTDS group mainly occurred on the first day, which was the important factor to affect the overall efficacy. On the whole, the percentage of patients who achieved CC with the GTDS treatment seemed to be higher on later days of chemotherapy. The results suggested that the GTDS had better antiemetic efficacy in delayed emesis which was possibly related to its continuous delivery of granisetron.
Chinese PK study reported that key pharmacokinetic parameters (Cavg) of GTDS were in coincidence with oral granisetron. In addition, the plasma concentration of GTDS reached median peak time (Tmax) at 72 h in Chinese patients (SOLASIA PHARMA K.K. 2013, The pharmacokinetics study report of Comparing 52 cm transdermal granisetron patch for 6 days with oral 1mg/per day BID granisetron in Chinese healthy volunteers: an open, randomized, single center and double cross-over study SP-0102.unpublished data), while the Tmax was 48 h that was observed in the first phase I study of GTDS conducted in Germany (Clinical Study Report 392MD/4/C.2003.Strakan Pharmacuetical Ltd. unpublished data). Tmax delay in Chinese people also indicated that the antiemetic efficacy in the GTDS group was worse than that in the oral granisetron group due to the lower plasma concentration of granisetron on the first day after patch application. Therefore, further investigation is need to explore whether add oral or intravenous granisetron to the first day of GTDS therapy or not, so as to achieve therapeutic levels of granisetron more rapidly.
In the study, the CC during PEEP was lower than that the previous study (13,14). In the Boccia study, CC was achieved by 60% of patients in the GTDS group, and 65% in the oral granisetron group during PEEP. It’s worth to highlight that dexamethasone was administrated in the vast majority of patients as the part of the antiemetic regimen in the Boccia study, while corticosteroid was prohibited from using as prophylactic medicine in our study. Thus, it was possible that whether corticosteroids as prophylactic antiemetic treatment or not leaded to the differences of CC between the studies. In addition, only 11.9% of Asian patients were included in the aforementioned study (13). Therefore, there was the trend that whether the efficacy of GTDS or oral granisetron seemed to be lower in Chinese patients receiving MEC or HEC. Those results also suggested that differences were present between Asian patients and non-Asian patients in the control of CINV, which was possibly related to heterogeneous populations.
Another randomized study was conducted in Korea, which the GTDS showed non-inferior efficacy to IV and oral granisetron in the control of CINV in patients receiving MEC (15). In comparison to our study, the CC were higher in the GTDS group and the oral granisetron group (69.9% vs. 72.5%). It was possible due to the difference of emetogenic chemotherapy degree and corticosteroids usage. In the study, the chemotherapy regimen was restricted to the MEC degree and 10 mg of dexamethasone intravenously was administrated to all patients. While in our study, the HEC regimens accounted for 53.25% and no patient used corticosteroid as prophylactic medicine. Thus, if the patients received MEC, GTDS may be more alternative option in the control of CINV.
In the subgroup analysis of our study, in terms of cisplatin-contained regimen and female, the oral granisetron group was superior to the GTDS group in the control of CINV. Thus, if the patients was female or received the cisplatin-contained regimen in clinical practice, GTDS as monotherapy may be suggested to be reconsidered in China.
The GTDS was well tolerated, with acceptable adverse events. The incidence of adverse events related to study drug was very low, 16.6% in the GTDS group and 12.2% in the oral granisetron group. Constipation was the most common adverse event. The study showed similar safety profile compared to previous GTDS studies.
In summary, GTDS is effective and safe in the control of CINV in Chinese patients, especially associated with MEC. It offers a more convenient alternative route in the control of CINV.
Acknowledgements
This work was sponsored by the Strakan International Limited Company.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All patients provided written informed consent before enrollment into the study. The study protocol was approved by the Ethics Committee Review Board at each participating center and was registered with ClinicalTrials.gov (Identifier, NCT01937156).
References
- NCCN. NCCN Clinical practice guidelines in oncology: antiemesis Version 1. 2014.
- Keating GM, Duggan ST, Curran MP. Transdermal granisetron: a guide to its use in preventing nausea and vomiting induced by chemotherapy. CNS Drugs 2012;26:787-90. [Crossref] [PubMed]
- Boccia RV, Clark G, Howell JD. Use of transdermal and intravenous granisetron and the ability of the Hesketh score to assess nausea and vomiting induced by multiday chemotherapy. Cancer Manag Res 2012;4:171-6. [Crossref] [PubMed]
- Sancuso (granisetron transdermal system) product information. ProStrakan Inc, Bedminster, NJ. 2008.
- Tuca A. Use of granisetron transdermal system in the prevention of chemotherapy-induced nausea and vomiting: a review. Cancer Manag Res 2009;2:1-12. [Crossref] [PubMed]
- Patel D, Chaudhary SA, Parmar B, et al. Transdermal drug delivery system: a review. The Pharma Innovation 2012;1:66-75. Available online: http://www.thepharmajournal.com/archives/2012/vol1issue4/PartA/14.pdf
- Simmons K, Parkman HP. Granisetron transdermal system improves refractory nausea and vomiting in gastroparesis. Dig Dis Sci 2014;59:1231-4. [Crossref] [PubMed]
- Howell J, Smeets J, Drenth HJ, et al. Pharmacokinetics of a granisetron transdermal system for the treatment of chemotherapy-induced nausea and vomiting. J Oncol Pharm Pract 2009;15:223-31. [Crossref] [PubMed]
- Kraut L, Fauser AA. Anti-emetics for cancer chemotherapy-induced emesis: Potential of alternative delivery systems. Drugs 2001;61:1553-62. [Crossref] [PubMed]
- Seol YM, Kim HJ, Choi YJ, et al. Transdermal granisetron versus palonosetron for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: a multicenter, randomized, open-label, cross-over, active-controlled, and phase IV study. Support Care Cancer 2016;24:945-52. [Crossref] [PubMed]
- Grossman J, Caspi A. Sancuso® Granisetron transdermal delivery system: a formulation for chemotherapy-induced nausea and vomiting. P&T Product Profiler 2011;2:1-30. Available online: http://www.doc88.com/p-6039952960563.html
- Schulmeister L. Granisetron transdermal system: a new option to help prevent chemotherapy-induced nausea and vomiting. Clin J Oncol Nurs 2009;13:711-4. [Crossref] [PubMed]
- Boccia RV, Gordan LN, Clark G, et al. Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer 2011;19:1609-17. [Crossref] [PubMed]
- Howell J, Clark G, Yellowlees A, et al. Efficacy, safety and tolerability of a transdermal granisetron patch for prevention of single-dose chemotherapy-induced nausea and vomiting: phase II trial results. J Oncol Pharm Pract 2009;15:20.
- Kim JE, Hong YS, Lee JL, et al. A randomized study of the efficacy and safety of transdermal granisetron in the control of nausea and vomiting induced by moderately emetogenic chemotherapy in Korean patients. Support Care Cancer 2015;23:1769-77. [Crossref] [PubMed]