
The differences in the distribution characteristics and prognostic value of tumor-infiltrating T lymphocyte subsets between lung adenocarcinoma and lung squamous cell carcinoma
Highlight box
Key findings
• Compared with lung adenocarcinoma (LUAD), the proportion of CD8+ tumor-infiltrating lymphocytes (TILs) was higher and CD4+/CD8+ ratio was lower in lung squamous cell carcinoma (LUSC); higher proportion of CD4+, CD8+ TILs and lower CD4+/CD8+ ratio predicted longer survival for LUAD patients, but the prognostic significance of these indicators in patients with LUSC is very limited.
What is known and what is new?
• The distribution of TIL subsets was different in different pathological types of cancer.
• This research compared the distribution and prognostic significance of CD4+ and CD8+ T cells in TILs between LUAD and LUSC.
What is the implication, and what should change now?
• The proportions of tumor-infiltrating CD4+ and CD8+ lymphocyte subsets and their clinical significance are very different between LUAD and LUSC. Clinical immunomodulatory therapy should be treated differently according to the pathological type of patients.
Introduction
In recent years, the application of immunotherapy in non-small cell lung cancer (NSCLC) has made significant progress, in which immune checkpoint inhibitors represented by anti-programmed death protein 1 (PD-1)/PD-ligand 1 (PD-L1) monoclonal antibodies have become mainstream drugs, including Atezolizumab and Pembrolizumab (1,2). By blocking the PD-1 receptor on the surface of tumor cells and binding to the PD-L1 ligand on immune cells, these drugs enhance the anti-tumor activity of T cells and promote their recognition and killing of tumors. The reactivity of immunotherapy is affected by many factors, including the characteristics of tumor microenvironment (TME) (3) and the expression level of specific genes (4,5), so individualized precision therapy has become the future development direction of immunotherapy. For example, Kuncman et al. (6) found that high expression of FMS-related tyrosine kinase 3 in TME is associated with increased infiltration of immune cells in NSCLC, and can improve susceptibility to immunotherapy and radiotherapy and prolong disease-free survival (DFS) in patients; An IMpower010 study showed that adjunct immunotherapy with Atezolizumab significantly extended DFS and overall survival (OS) in patients with stage Ib-IIIa NSCLC who expressed positive PD-L1 (7). The TME is mainly composed of tumor cells, tumor-infiltrating lymphocytes (TILs), extracellular matrix (ECM), tumor-associated stromal cells, etc. (8), and TILs are mainly composed of T lymphocytes. Studies have shown that there is a close correlation between tumor immunotherapy and the distribution of tumor infiltrating T lymphocyte subsets. For example, Xu et al. (9) found that in patients with advanced gastric cancer and esophageal cancer, the CD4+/CD8+ ratio of circulating T cells is closely related to the efficacy of PD-1 inhibitors, which can be used to predict patients’ OS. However, there are relatively few studies on the distribution characteristics of CD4+, CD8+ in TILs of NSCLC patients.
Lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are the most common pathological types of NSCLC. Studies have shown that there are significant differences in the distribution of negative, positive-low expression and positive-high expression of PD-L1 between LUAD and LUSC (10), and checkpoint inhibitors, such as nivolumab, have also shown some differences in curative effect in the treatment of LUAD and LUSC (11). Therefore, analysis of the differences in the tumor immune microenvironment between LUAD and LUSC patients is highly important for the customization of personalized immunotherapy programs for lung cancer patients. In our clinical experience, we found that there were differences in the distribution of CD4+ and CD8+ T cells in TILs between LUAD and LUSC, so we speculated that whether the difference in TILs distribution characteristics was the cause of different immunotherapy outcomes between LUAD and LUSC. This study aims to observe and compare the distribution characteristics of CD4+ and CD8+ infiltrating T lymphocyte subsets in the stroma of LUAD and LUSC, and analyze the prognostic value of CD4+ and CD8+ distribution characteristics, respectively, so as to provide theoretical reference for the personalized treatment of these two types of lung cancer. We present this article in accordance with the REMARK reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-62/rc).
Methods
Patients
Patients with LUAD or LUSC who underwent surgery at The Second Affiliated Hospital of Zhengzhou University between October 2020 and October 2022 were enrolled. Inclusion criteria: (I) patients with complete clinical data, aged 18–80 years; (II) patients were diagnosed with primary LUAD or LUSC by imaging, laboratory and pathological examinations, and the TNM stage was stage I, II or IIIa and IIIb; (III) no anticancer treatment was given before surgery; (IV) patients who provided informed consent to participate in this study and who were willing to authorize the surgically removed tumor specimens to the hospital for scientific research use. The exclusion criteria for patients were severe infectious diseases; immune system diseases; blood system diseases; heart, liver and kidney diseases; and other organic disorders. All patients underwent thoracoscopic radical resection of lung cancer. This study was a prospective observational study. The survival time of all patients was followed up by regular review or telephone until February 29, 2024, with progression-free survival (PFS) defined as the time from the surgery to recurrence or distant metastases, and OS defined as the time from the surgery to death. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the medical ethics committee of The Second Affiliated Hospital of Zhengzhou University (No. 2020243). Informed consent was taken from all the patients.
Pathological observation
Fresh lung cancer specimens were obtained surgically, part of those were paraffin embedded after rinsing, sliced (5 µm) and stored after paraffin embedding. After the paraffin sections were dewaxed and hydrated with xylene and gradient alcohol, they were placed in hematoxylin solution for 5 minutes, rinsed and placed in eosin solution for 3 minutes, and then the sections were placed in 95% alcohol I for 15 minutes, 95% alcohol II for 5 minutes, anhydrous ethanol I for 5 minutes, anhydrous ethanol II for 5 minutes. Then the slides were sealed with neutral gum. The stained sections were observed under a microscope. According to the guideline by the International TILs Working Group (12), several suitable views for observing TILs were selected under a low-power lens (100×), and the proportion of TILs area was assessed (defined as a). Then, the proportion of lymphocytes in each field of view was calculated under a high-power lens (defined as b), and the proportion of TILs was calculated by a×b. Five fields of view were taken for each sample.
Flow cytometry
Three slices of fresh lung cancer tissue (approximately 0.5×0.5×0.5 cm3, avoiding the junction) were cut and placed on sterile phosphate buffer saline (PBS). After being cut into pieces with sterile scissors, trypsin was added and digested in water bath at 37 ℃ for 30 min. Normal saline was added to terminate digestion. Single-cell suspension was obtained through 300-mesh nylon mesh filtration after centrifugation and rinsing. The cell suspension was rinsed with PBS buffer, and centrifuged at 1,500 rpm (600 g) for 5 min. Then, the supernatant was discarded, and the samples were added with red blood cell lysis buffer (6 mL) for 5 min. The samples were then rinsed with PBS (5 min × 2 times) and centrifuged at 1,500 rpm (600 g) for 5 min, after which the supernatant was discarded. PBS buffer and 12 mL of human lymphocyte isolation solution were added, the mixture was centrifuged at 2,000 rpm (800 g) for 20 min, and the middle mononuclear cell layer was obtained. After washing with PBS for 5 min × 2 times, the mixture was centrifuged at 800 rpm for 10 min at 4 ℃, and mononuclear cells were obtained from the precipitate. 1×106 cells were removed, and 1 µL of the corresponding CD3, CD4, and CD8 antibodies and fluorescent secondary antibodies were added. At the same time, a blank tube and a control tube were set up and allowed to react at room temperature in the dark for 20 min. Then, 1 mL of flow buffer was added, the mixture was centrifuged at 1,500 rpm for 5 min, the supernatant was removed, and 200 µL of flow buffer was added, and took to a flow cytometer. forward scatter (FSC)/side scatter (SSC) gates and CD3 were used to detect the proportion of CD3+ T cells proportion. The proportion of CD3+CD4+ and CD3+CD8+ cells in CD3+ was detected respectively, and then the overall proportion of CD4+ and CD8+ cells and the ratio of CD4+/CD8+ were calculated.
Statistical analysis
All the data were summarized by Microsoft Excel, and imported into IBM SPSS 19.0 for analysis. The TILs-related indicators between LUAD and LUSC groups were compared by independent sample t-tests. The correlation between CD4+, CD8+ T cell distribution characteristics in TIL and clinicopathological features was analyzed by comparing the differences in TIL indicators among different tumor size groups (maximum tumor diameter ≥3 and <3 cm), degree of differentiation groups (low and moderate/high groups), lymphatic metastasis groups (metastasis and non-metastasis groups) and TNM stage groups, and the correlation with prognosis were analyzed by Kaplan-Meier (K-M) survival analysis and Cox regression analysis. The standard of statistical significance was 0.05.
Results
General data
A total of 78 LUAD patients and 56 LUSC patients were enrolled. There were some cases in this study that were lost to follow-up, and the outcome was defined as “censored”. At the end of follow-up, the median follow-up time of all patients was 35 months and the median survival time was 34 months. In 78 LUAD patients, 57 recurred or metastasized, and 45 died; in 56 LUSC patients, 32 recurred or metastasized, and 19 died. The comparison of general data between LUAD and LUSC are shown in Table 1. It can be seen that there is difference in the proportion of smoke between the LUAD and LUSC groups (P=0.004), but no statistical differences in other data.
Table 1
| Data | LUAD (n=78) | LUSC (n=56) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 63.37±10.12 | 64.41±8.68 | 0.621 | 0.54 |
| Sex (male/female) | 42/36 | 35/21 | 0.999 | 0.32 |
| Smoke | 58 (74.4) | 28 (50.0) | 8.413 | 0.004 |
| Tumor size (≥3 cm) | 40 (51.3) | 38 (67.9) | 3.681 | 0.06 |
| Differentiated degree | 0.48 | |||
| Low | 41 (52.6) | 26 (46.4) | 0.491 | |
| Moderate | 37 (47.4) | 30 (53.6) | ||
| Lymph node metastasis | 25 (32.1) | 25 (44.6) | 2.210 | 0.14 |
| TNM | 4.347 | 0.11 | ||
| I | 44 (56.4) | 25 (44.6) | ||
| II | 11 (14.1) | 16 (28.6) | ||
| III | 23 (29.5) | 15 (26.8) | ||
| Neoadjuvant chemotherapy | 41 (52.6) | 35 (62.5) | 1.311 | 0.25 |
| CD4+ (%) | 17.24±0.75 | 16.15±0.91 | 0.925 | 0.36 |
| CD8+ (%) | 17.09±0.57 | 19.60±1.10 | 2.196 | 0.03 |
| CD3+CD4+ (%) | 51.43±5.00 | 45.76±2.57 | 8.565 | <0.001 |
| CD3+CD8+ (%) | 50.04±5.06 | 55.76±2.65 | 8.497 | <0.001 |
| CD4+/CD8+ | 1.05±0.023 | 0.82±0.011 | 8.562 | <0.001 |
Data are presented as n (%) or mean ± standard deviation. TIL, tumor-infiltrating lymphocyte; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; TNM, tumor-node-metastasis.
Comparison of tumor-infiltrating CD4+ or CD8+ T lymphocyte subsets between LUAD and LUSC
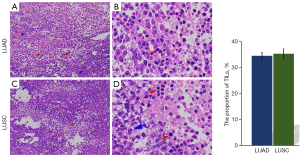
Pathological observation show that compared with tumor cells, TILs are relatively small, round in shape, and have darker staining. There is no significant difference in TILs density between LUAD and LUSC groups (P=0.71, Figure 1). Flow cytometry was used to detect the proportions of CD4+ and CD8+ T cells in TILs, the results show that the CD8+ proportion is higher in LUSC and CD4+/CD8+ ratio is lower than those in LUAD tissues (P=0.03, P<0.001, respectively, Table 1).
Correlations between the distributions of TIL subsets and clinicopathological features in LUAD and LUSC
In the LUAD group, the CD4+/CD8+ ratio was correlated with tumor size, and the CD4+ proportion, CD8+ proportion and CD4+/CD8+ ratio were correlated with TNM stage: the CD4+/CD8+ ratio in the ≥3 cm group was significantly higher than that in the <3 cm group (P=0.04); The CD4+ proportion and CD8+ proportion in the stage III group were significantly lower (P=0.02, P=0.04), and the CD4+/CD8+ ratio was significantly higher than those in the stage I group (P=0.04, Figure 2).

In the LUSC group, the CD4+ proportion was correlated with tumor size, and the CD4+ proportion and CD8+ proportion were correlated with degree of differentiation and TNM stage: the CD4+ proportion in the ≥3 cm group was lower than that in <3 cm group (P=0.04), both the CD4+ proportion and CD8+ proportion in the low-differentiation-degree group and stage I group were significantly higher than those in the high-differentiation-degree group (P<0.001, P=0.002, respectively) and stage III group (P=0.006, P=0.006, respectively, Figure 3).

Correlations between the distribution of TIL subsets and survival of LUAD patients and LUSC patients
K-M analysis was used to analyze the correlation between TIL subsets and the median survival of LUAD and LUSC patients. The results show that in LUAD group, the CD4+ proportion, CD8+ proportion and CD4+/CD8+ ratio were closely related to patients’ median survival: the PFS and OS of patients in the high CD4+ proportion group, high CD8+ proportion group and low CD4+/CD8+ group were significantly longer than those in the low CD4+ proportion group, low CD8+ proportion group and high CD4+/CD8+ group, respectively (PFS: P<0.001, P=0.007, P<0.001, respectively; OS: P=0.009, P=0.057, P=0.004, respectively; Figure 4, Table 2). However, in the LUSC group, no correlation relationship was found between the CD4+ proportion, CD8+ proportion, CD4+/CD8+ ratio and patients’ survival (PFS: P=0.12, P=0.09, P=0.22, respectively; OS: P=0.14, P=0.19, P=0.22, respectively; Figure 5, Table 2).

Table 2
| Groups | CD4+ (%) | CD8+ (%) | CD4+/CD8+ |
|---|---|---|---|
| LUAD | |||
| PFS (months) | |||
| Low group | 22 | 24 | 30 |
| High group | 30 | 29 | 21 |
| χ2 | 14.714 | 7.310 | 16.665 |
| P | <0.001 | 0.007 | <0.001 |
| OS (months) | |||
| Low group | 29 | 30 | 37 |
| High group | 39 | 38 | 28 |
| χ2 | 6.844 | 3.628 | 8.421 |
| P | 0.009 | 0.057 | 0.004 |
| LUSC | |||
| PFS (months) | |||
| Low group | 23 | 22 | 22 |
| High group | 32 | 30 | 28 |
| χ2 | 2.423 | 2.822 | 1.481 |
| P | 0.12 | 0.10 | 0.22 |
| OS (months) | |||
| Low group | 33 | 38 | 33 |
| High group | 39 | 38 | 38 |
| χ2 | 2.205 | 1.695 | 1.517 |
| P | 0.14 | 0.19 | 0.22 |
TIL, tumor-infiltrating lymphocyte; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PFS, progression free survival; OS, overall survival.

CD4+/CD8+ ratio is an independent risk factor for survival in LUAD patients but not in LUSC patients
Cox multivariate regression analysis of PFS and OS was performed for LUAD and LUSC patients, respectively, and age (converted into the bitaxonomic variable at 65 years), sex, smoking status, tumor size (converted into the bitaxonomic variable at 3 cm), differentiation degree, lymphatic metastasis status, TNM stage, neoadjuvant chemotherapy, TILs density, CD3+CD4+ proportion, CD3+CD8+ proportion and CD4+/CD8+ (transformed into binary variables according to the median value) were included as variables. The results showed that in LUAD patients, age, smoke, tumor size and higher CD4+/CD8+ (>1.04) were independent risk factors for PFS (P=0.01, P=0.01, P=0.001, P<0.001, respectively), and differentiation degree (moderate/high) and higher CD8+ proportion (≥17.4%) were independent protective factors (P=0.04, P=0.004, P<0.001, respectively); Tumor size and CD4+/CD8+ (>1.04) were independent risk factors for OS (P=0.006, P=0.01, respectively), the differentiation degree (moderate/high) was an independent protective factor (P=0.01, Table 3). In LUSC, TNM stage was an independent risk factor for PFS (P<0.001), and differentiation degree (moderate/high), neoadjuvant chemotherapy and higher TILs level were independent protective factors (P<0.001, P=0.004, P<0.001, respectively); The age, tumor size and TNM stage were independent risk factors for OS (P=0.005, P=0.07, P=0.005, respectively, Table 3). There was no independent correlations between CD4+ proportion, CD8+ proportion, or CD4+/CD8+ ratio and PFS or OS.
Table 3
| Variables | B | SE | Wald | Sig. | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| LUAD | |||||||
| PFS | |||||||
| Age (≥65 years) | 0.693 | 0.278 | 6.188 | 0.01 | 1.999 | 1.158 | 3.450 |
| Smoke | 0.791 | 0.323 | 5.978 | 0.01 | 2.205 | 1.170 | 4.155 |
| Tumor size (≥3 cm) | 0.956 | 0.295 | 10.515 | 0.001 | 2.601 | 1.460 | 4.635 |
| Differentiation degree (moderate/high) | −0.595 | 0.291 | 4.188 | 0.04 | 0.552 | 0.312 | 0.975 |
| CD8+ (≥17.4%) | −0.872 | 0.301 | 8.403 | 0.004 | 0.418 | 0.232 | 0.754 |
| CD4+/CD8+ (>1.04) | 1.107 | 0.296 | 14.038 | 0.000 | 3.027 | 1.696 | 5.402 |
| OS | |||||||
| Tumor size (≥3 cm) | 0.853 | 0.311 | 7.541 | 0.006 | 2.347 | 1.277 | 4.316 |
| Differentiation degree (moderate/high) | −0.816 | 0.324 | 6.320 | 0.01 | 0.422 | 0.234 | 0.835 |
| CD4+/CD8+ (>1.04) | 0.794 | 0.316 | 6.334 | 0.01 | 2.213 | 1.192 | 4.108 |
| LUSC | |||||||
| PFS | |||||||
| Differentiation degree (moderate/high) | −1.860 | 0.513 | 13.127 | <0.001 | 0.156 | 0.057 | 0.426 |
| Tumor-node-metastasis (TNM) | 17.566 | <0.001 | |||||
| TNM (stage II) | 4.074 | 1.035 | 15.497 | <0.001 | 58.787 | 7.734 | 446.866 |
| TNM (stage III) | 4.443 | 1.083 | 16.829 | <0.001 | 85.025 | 10.178 | 710.269 |
| Neoadjuvant chemotherapy | −2.791 | 0.962 | 8.416 | 0.004 | 0.061 | 0.009 | 0.404 |
| Tumor-infiltrating lymphocytes (>50/high power field) | −2.230 | 0.540 | 17.044 | <0.001 | 0.108 | 0.037 | 0.310 |
| OS | |||||||
| Age (≥65 years) | 1.872 | 0.672 | 7.757 | 0.005 | 6.498 | 1.741 | 24.254 |
| Tumor size (≥3 cm) | 1.840 | 0.998 | 3.398 | 0.07 | 6.295 | 0.890 | 44.509 |
| TNM | 10.409 | 0.005 | |||||
| TNM (stage II) | 2.140 | 0.861 | 6.180 | 0.01 | 8.502 | 1.573 | 45.963 |
| TNM (stage III) | 3.778 | 1.177 | 10.310 | 0.001 | 43.716 | 4.357 | 438.645 |
PFS, progression free survival; OS, overall survival; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; SE, standard error; CI, confidence interval; TNM, tumor-node-metastasis.
Discussion
Key findings
The results in the present study reveal no significant difference in the level of TILs between LUAD and LUSC tissues, but the proportion of CD8+ T cells in LUSC tissues is relatively higher and the CD4+/CD8+ ratio is relatively lower than those in LUAD tissues. In LUAD, the CD4+/CD8+ ratio in TILs is closely related to tumor size and TNM stage, and higher proportion of CD4+ T cells, CD8+ T cells and lower CD4+/CD8+ ratio are correlated with longer PFS and OS; however, in LUSC, the proportions of CD4+ T cells and CD8+ T cells are correlated with tumor size, or degree of differentiation and TNM stage, but there is no correlation between the proportion of CD4+ T cells, CD8+ T cells, CD4+/CD8+ ratio and prognosis, none of them are independent factors of PFS or OS. The results confirm that the proportions of CD4+ and CD8+ T cell subsets and the CD4+/CD8+ ratio in TILs between LUSC and LUAD have different prognostic value.
Strengths and limitations
The presented study confirms that the proportions of tumor-infiltrating CD4 and CD8 lymphocyte subsets and their clinical significance are very different between LUAD and LUSC, suggesting that clinical immunotherapy should be treated differently according to the pathological type of patients. Limitations of this study include small sample size, short follow-up time and simple classification of TIL lymphocyte subsets, in follow-up studies, it is necessary to expand the sample size, extend the follow-up time and increase the classification of T cells subsets.
Comparison with similar research
Current studies mostly focus on the correlation between the level of TILs or one of the subsets and the prognosis of lung cancer (13,14), but there is no clear conclusion on the correlation between the imbalance of CD4+ and CD8+ subsets in infiltrating T lymphocytes and prognosis, and the difference in the characteristics of TILs among different pathological types of lung cancer are also less reported. A few studies have reported differences in the distribution of TILs in different pathological types of tumors. Dong et al. found that there were differences in TILs levels between esophageal squamous cell carcinoma and gastric adenocarcinoma, with the median cell proportion being 1.92% and 0.12% respectively (15), which was different from the results of this study, which showed no difference in TILs levels between LUAD and LUSC. In the existing studies on the correlation between CD4+ and CD8+ TILs and tumor prognosis, it is basically believed that the greater the number of CD8+ T lymphocytes, the better the prognosis. For example, Mlika et al. showed that the CD8/CD4 ratio was a prognostic factor for the OS of NSCLC patients, and the CD8+ TIL proportion was a prognostic factor for relapse-free survival, with an optimal cut-off value of 67.8/ high-power field (16); a study from Japan found that the level of CD8+ TILs in cervical squamous cell carcinoma was a protective factor for good prognosis after radiotherapy (17); a study from Greece suggested that for patients with surgically treatable NSCLC, the higher the proportion of CD4+ TILs cells and the CD4+/CD8+ ratio, the lower the proportion of CD8+, and the poorer the prognosis (18). Chinese scholar Qin M’s study confirmed that both CD4+ TILs and CD8+ TILs have certain application value in the prognosis of colorectal cancer (19); Similarly, Tao et al. also showed that colorectal cancer patients with high levels of CD8+ and CD4+ CD8+ in TILs had higher DFS and OS than patients with low levels, and CD8+ and CD4+ CD8+ were independent influencing factors for DFS and OS (20). The above study is consistent with the results of this study on LUAD patients. However, at present, there are few studies on the difference in prognostic value of CD4+ or CD8+ TILs in different pathological types of lung cancer. Only Chen et al. showed that the level of CD8+ TILs in LUSC was relatively higher than LUAD, and higher TIL density was a poor prognostic factor in LUAD, but a favorable prognostic factor in LUSC (21), which is consistent with the results of this study. Huai et al. analyzed the data from 182 patients with esophageal cancer in the TCGA database and found that high levels of CD8+ TIL cells predict shorter survival in esophageal squamous cell carcinoma, and high levels of resting memory CD4+ T cells predict shorter survival in esophageal adenocarcinoma (22), which is different from the results of this study. The different conclusions may be related to factors such as sample size, TNM stage of patients, race and region, the patients in this study were all those who received surgical treatment and had early TNM stage, the proportion of patients in stage I and stage II exceeded 70%.
Explanations of findings
After the occurrence of a tumor, tumor cells act as foreign bodies to activate the immune system. In general, the level of TILs will increase (8) and play a role of tumor-killing, among which CD8+ T cells and natural killer (NK) cells are direct effector cells that mediate the killing of tumors (23), and CD4+ T cells generally help to make the immune response as durable as possible (24). Generally, the antitumor effect of TILs is relatively strong in the early stage of tumor progression, with the development of disease, especially for recurrent tumors after treatment (25), tumor cells gradually adapt to the immune microenvironment, promote the deterioration of immune microenvironment and may lead to the imbalance in the subsets of infiltrating T cells, and further cause the gradual weakening or even failure of the immune system, forming a vicious cycle.
Activation of both CD4+ T cells and CD8+ T cells is necessary to inhibit tumor cells and play an effective antitumor role. Although CD8+ cells play a killing role in TILs, they usually require the assistance of CD4+ T cells to perform the best function. Therefore, the CD4+/CD8+ ratio is considered to be an important parameter for evaluating T-cell function and subpopulation balance and has different characteristics in different types of tumors (26). The heterogeneity of lung cancer is based on the abundance of cell types in the lung. The cells of origin of LUSC are proximal airway cells, including ciliary cells, basal cells and neuroendocrine cells, especially pseudostratified epithelial cells (27). The distal airway and small airway are composed of alveolar epithelial cells, rod cells and bronchoalveolar stem cells, which are the cells of origin of LUAD (28).
Therefore, it is speculated that the cell types of origin of LUSC and LUAD may be responsible for the differences in the tumor immune microenvironment, which in turn leads to differences in the tumor location, histologic grade, pleura invasion, distribution of infiltrating T-cell subsets (29) LUSC may cause more obvious inversion of CD4+/CD8+ cells in TILs for various reasons, such as insufficient oxygen supply. In this case, the greater the proportion of CD8+ cells in LUAD was, the stronger the tumor-killing effect, indicating better survival, so the prognostic significances of the CD8+ T cells proportion and CD4+/CD8+ ratio are obvious. However, in LUSC, the CD4+/CD8+ ratio is unbalanced or even inverted, even if the proportion of CD8+ is high, the tumor killing effect cannot be well performed in the absence of the corresponding proportion of CD4+ T cells that play an auxiliary role. At this point, the proportion of CD8+ T cells or CD4+/CD8+ ratio is weakly correlated with prognosis, and it is difficult to become an independent influencing factor for patient survival.
Implications and actions needed
Tumor infiltrating lymphocyte therapy is one of the most important anticancer therapies. The presented study reveal that the distribution characteristics and clinical significance of tumor-infiltrating CD4 and CD8 subsets in LUAD and LUSC are very different, therefore, personalized treatment should be carried out according to the pathological types during the treatment process, and reasonable decisions should be made in combination with the prognosis assessment of lung cancer patients.
Conclusions
In conclusion, this study suggests that there are significant differences in the distribution characteristics and prognostic values of CD4+ and CD8+ TIL subsets between LUAD and LUSC. There is no significant difference in the overall level of TILs between the two types of lung cancer, but the CD4+/CD8+ ratio in TILs of LUAD is higher, and the proportion of CD8+ T cells CD4+/CD8+is lower than that in LUSC. In LUAD, the higher proportion of CD8+ or CD4+ T cells and lower CD4+/CD8+ ratio in TILs are associated with better prognosis and CD4+/CD8+ independently influences patients’ survival. However, in LUSC, there are obvious inversion of CD4+/CD8+ and lower proportion of CD4+ T cells, resulting in a very weak correlation between the proportions of CD4+ and CD8+ T cells, CD4+/CD8+ and patient prognosis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-62/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-62/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-62/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-62/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the medical ethics committee of The Second Affiliated Hospital of Zhengzhou University (No. 2020243). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Felip E, Altorki N, Zhou C, et al. Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol 2023;34:907-19. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Genova C, Dellepiane C, Carrega P, et al. Therapeutic Implications of Tumor Microenvironment in Lung Cancer: Focus on Immune Checkpoint Blockade. Front Immunol 2022;12:799455. [Crossref] [PubMed]
- van Elsas MJ, Labrie C, Etzerodt A, et al. Invasive margin tissue-resident macrophages of high CD163 expression impede responses to T cell-based immunotherapy. J Immunother Cancer 2023;11:e006433. [Crossref] [PubMed]
- Zheng H, Wang M, Zhang S, et al. Comprehensive pan-cancer analysis reveals NUSAP1 is a novel predictive biomarker for prognosis and immunotherapy response. Int J Biol Sci 2023;19:4689-708. [Crossref] [PubMed]
- Kuncman Ł, Orzechowska M, Milecki T, et al. High FLT3 expression increases immune-cell infiltration in the tumor microenvironment and correlates with prolonged disease-free survival in patients with non-small cell lung cancer. Mol Oncol 2024;18:1316-26. [Crossref] [PubMed]
- Kenmotsu H, Sugawara S, Watanabe Y, et al. Adjuvant atezolizumab in Japanese patients with resected stage IB-IIIA non-small cell lung cancer (IMpower010). Cancer Sci 2022;113:4327-38. [Crossref] [PubMed]
- Qayoom H, Sofi S, Mir MA. Targeting tumor microenvironment using tumor-infiltrating lymphocytes as therapeutics against tumorigenesis. Immunol Res 2023;71:588-99. [Crossref] [PubMed]
- Xu S, Zhu Q, Wu L, et al. Association of the CD4(+)/CD8(+) ratio with response to PD-1 inhibitor-based combination therapy and dermatological toxicities in patients with advanced gastric and esophageal cancer. Int Immunopharmacol 2023;123:110642. [Crossref] [PubMed]
- Ito A, Tarukawa T, Suzuki Y, et al. Clinicopathological and Molecular Characteristics Promoting PD-L1 Expression in Early-stage Lung Adenocarcinoma and Squamous Cell Carcinoma. Anticancer Res 2023;43:5197-204. [Crossref] [PubMed]
- Kaneda T, Kurata T, Yoshida T, et al. Massive digital gene expression analysis reveals different predictive profiles for immune checkpoint inhibitor therapy between adenocarcinoma and squamous cell carcinoma of advanced lung cancer. BMC Cancer 2022;22:154. [Crossref] [PubMed]
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Rakaee M, Adib E, Ricciuti B, et al. Association of Machine Learning-Based Assessment of Tumor-Infiltrating Lymphocytes on Standard Histologic Images With Outcomes of Immunotherapy in Patients With NSCLC. JAMA Oncol 2023;9:51-60. [Crossref] [PubMed]
- Na K, Lee S, Kim DK, et al. CD81 and CD82 expressing tumor-infiltrating lymphocytes in the NSCLC tumor microenvironment play a crucial role in T-cell activation and cytokine production. Front Immunol 2024;15:1336246. [Crossref] [PubMed]
- Dong H, Yao L, Fan J, et al. Characteristics of auto-quantified tumor-infiltrating lymphocytes and the prognostic value in adenocarcinoma of the esophagogastric junction, gastric adenocarcinoma, and esophageal squamous cell carcinoma. Aging (Albany NY) 2024;16:11027-61. [Crossref] [PubMed]
- Mlika M, Saidi A, Mejri N, et al. Prognostic impact of tumor-infiltrating lymphocytes in non-small cell lung carcinomas. Asian Cardiovasc Thorac Ann 2022;30:177-84. [Crossref] [PubMed]
- Miyasaka Y, Yoshimoto Y, Ando K, et al. CD8-positive Tumor-infiltrating Lymphocytes and Prognosis in Radiotherapy for Uterine Cervical Squamous Cell Carcinoma. Anticancer Res 2023;43:2077-84. [Crossref] [PubMed]
- Giatromanolaki A, Anestopoulos I, Panayiotidis MI, et al. Prognostic Relevance of the Relative Presence of CD4, CD8 and CD20 Expressing Tumor Infiltrating Lymphocytes in Operable Non-small Cell Lung Cancer Patients. Anticancer Res 2021;41:3989-95. [Crossref] [PubMed]
- Qin M, Chen G, Hou J, et al. Tumor-infiltrating lymphocyte: features and prognosis of lymphocytes infiltration on colorectal cancer. Bioengineered 2022;13:14872-88. [Crossref] [PubMed]
- Tao Y, Xie Y. Prognostic impact of CD4+ and CD8+ tumor-infiltrating lymphocytes in patients with colorectal cancer. Acta Chir Belg 2024;124:35-40. [Crossref] [PubMed]
- Chen L, Cao MF, Zhang X, et al. The landscape of immune microenvironment in lung adenocarcinoma and squamous cell carcinoma based on PD-L1 expression and tumor-infiltrating lymphocytes. Cancer Med 2019;8:7207-18. [Crossref] [PubMed]
- Huai Q, Guo W, Han L, et al. Identification of prognostic genes and tumor-infiltrating immune cells in the tumor microenvironment of esophageal squamous cell carcinoma and esophageal adenocarcinoma. Transl Cancer Res 2021;10:1787-803. [Crossref] [PubMed]
- Park J, Hsueh PC, Li Z, et al. Microenvironment-driven metabolic adaptations guiding CD8(+) T cell anti-tumor immunity. Immunity 2023;56:32-42. [Crossref] [PubMed]
- Zander R, Schauder D, Xin G, et al. CD4(+) T Cell Help Is Required for the Formation of a Cytolytic CD8(+) T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 2019;51:1028-1042.e4. [Crossref] [PubMed]
- Watermann C, Pasternack H, Idel C, et al. Recurrent HNSCC Harbor an Immunosuppressive Tumor Immune Microenvironment Suggesting Successful Tumor Immune Evasion. Clin Cancer Res 2021;27:632-44. [Crossref] [PubMed]
- Wang K, Shen T, Siegal GP, et al. The CD4/CD8 ratio of tumor-infiltrating lymphocytes at the tumor-host interface has prognostic value in triple-negative breast cancer. Hum Pathol 2017;69:110-7. [Crossref] [PubMed]
- Sarode P, Mansouri S, Karger A, et al. Epithelial cell plasticity defines heterogeneity in lung cancer. Cell Signal 2020;65:109463. [Crossref] [PubMed]
- Marjanovic ND, Hofree M, Chan JE, et al. Emergence of a High-Plasticity Cell State during Lung Cancer Evolution. Cancer Cell 2020;38:229-246.e13. [Crossref] [PubMed]
- Wang BY, Huang JY, Chen HC, et al. The comparison between adenocarcinoma and squamous cell carcinoma in lung cancer patients. J Cancer Res Clin Oncol 2020;146:43-52. [Crossref] [PubMed]


