
E203K mutation in MAP2K1 (MEK1) causes acquired resistance to PD-1 blockade but responds to trametinib: a case report
Highlight box
Key findings
• Mitogen-activated protein kinase kinase 1 (MEK1 E203K) mutation is responsible for the resistance to immune checkpoint inhibitors but responds well to trametinib.
What is known and what is new?
• It’s reported that the aberrant activation of the mitogen-activated protein kinase (MAPK) pathway may be an important mechanism of resistance to immunotherapy. This clinical case presents compelling evidence of the involvement of the MEK1 E203K mutation in the resistance to immune checkpoint inhibitors.
• It’s reported that activation mutations of MEK1 showed robust resistance to allosteric MEK inhibitors. However, the case is the first reported instance of a patient with an MEK1 E203K mutation responding well to trametinib.
What is the implication, and what should change now?
• The MEK1 E203K mutation is associated with resistance to immune checkpoint inhibitors. However, the MEK inhibitor Trametinib has shown promising results in treating tumors with this mutation.
Introduction
Recently, blockade of immune checkpoint molecules with monoclonal antibodies has emerged as a promising strategy in gastric cancer (1,2). However, mechanisms of its acquired resistance are poorly understood. Patients with Epstein-Barr virus-associated gastric cancer (EBVaGC), characterized by the infiltration of lymphocytes and programmed death ligand 1 (PD-L1) overexpression, are reported to benefit from immunotherapy (3,4). This unique tumor microenvironment of EBVaGC belongs to type II according to the tumor immunity in the microenvironment (TIME) classification (5), highlight the potential for using Epstein-Barr virus (EBV) as a biomarker for response to immunotherapy. These distinctive clinicopathologic characteristics and immunotherapy response of EBVaGC are receiving increasing clinical attention (6-9). However, no studies have focused on the change of molecular characteristics and immune microenvironment after immunotherapy to explore the mechanisms of immune resistance. Here, we present a clinical case with EBVaGC to reveal that mitogen-activated protein kinase (MAPK) pathway activation, due to mitogen-activated protein kinase kinase 1 (MAP2K1/MEK1) mutation, might be responsible for the resistance to programmed cell death protein 1 (PD-1) blockade, but showed a rapid and durable response to MEK inhibitor trametinib. Trametinib is an extremely potent allosteric inhibitor of MEK 1/2, which was the first MEK inhibitor approved for use in treatment of advanced BRAF V600E/K mutant melanoma as a single agent and in combination with BRAF inhibitor, dabrafenib (10). We present this case in accordance with the CARE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-61/rc).
Case presentation
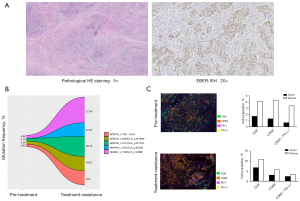
A 45-year-old male patient was admitted to the hospital due to intermittent upper abdominal pain that had persisted for 6 months. On his admission, there were no symptoms or signs. The patient was diagnosed with gastric cancer following a gastroscopy. He has no family genetic history or other special medical history. On July 19, 2015, he underwent D2 radical gastrectomy followed by Roux-en-Y anastomosis for gastric cancer, with a postoperative pathological staging of pT2N0M0 (stage IB, with no vascular invasion and perineural invasion). The tumor presented the morphology of lymphoepithelioma-like carcinomas, and was positive for EBV encoded small RNA in situ hybridization (EBER-ISH) (Figure 1A). HER2 immunostaining was negative, and four mismatch repair proteins (MLH1, MSH2, MSH6 and PSM2) were proficient. The patient did not receive adjuvant chemotherapy after surgery, but was regularly followed up. Four years later (31-Oct-2019), contrast-enhanced computed tomography (CT) revealed relapse in the liver and para-aortic lymph nodes. Biopsy was performed on 02-Dec-2019 and the combined positive score (CPS) of PD-L1 score by immunohistochemistry (IHC) was 50.
For the first line treatment, from 09-Dec-2019 to March 2020, he was treated with chemotherapy regimen of SOX (oxaliplatin + S-1) plus sintilimab (a fully humanized anti-PD-1 monoclonal antibody) for three cycles. Contrast-enhanced CT showed the live and partial lymph node lesions were complete remission on 12-Mar-2020. Next, consolidating radiotherapy (56 Gy/2 Gy/28 f) was administered to the residual lesions from 11-Apr-2020 to 20-May-2020, and another two cycles of SOX plus Sintilimab were continued. After these treatments, contrast-enhanced CT revealed these residual para-aortic lesions achieved complete remission on August 03, 2020. However, the lymph node along the distal splenic artery (station No. 11d, according to the Japanese Gastric Cancer Classification), which was achieved complete response before the radiotherapy and not included in the radiation field, relapsed again.
Re-biopsy of this lesion was performed on Aug-06-2020. Molecular characteristics and tumor immune microenvironment of the pre-treatment and treatment-resistant tumors were examined by next-generation sequencing (NGS) test and multiplex IHC (mIHC), respectively. The CPS of PD-L1 score was 50 before treatment, while re-biopsy of the treatment-resistant lesion showed CPS of 100, indicating the express of PD-L1 was increased after treatment. Both pre-treatment and treatment-resistant tumors had low tumor mutational burden (TMB) (2.89 and 4.82 mutations/mb, respectively). No mutations conferring microsatellite instability (MSI) or DNA-repair defects were detected. Most mutations were shared in both tumors, such as ARID1A, ARID1B and SMAD2 mutation. Moreover, no loss-of-function mutation events were observed in JAK1 and JAK2, PTEN, or B2M. However, 10 nonsynonymous mutations were lost from the pre-treatment tumor (not seen in the treatment-resistant tumor), and 1 missense mutation (MAP2K1, c.607G>A, p.E203K) was exclusive to the immunotherapy-resistant lesion (Figure 1B). The raw data of NGS have been submitted to NCBI Sequence Read Archive (PRJNA766700). Moreover, the results of mIHC showed the treatment-resistant lesion had more infiltrated immunocytes in tumor (Figure 1C). After normalization by immunocytes infiltrated in stroma, the treatment-resistant tumor had more increase of CD68+PD-L1+ macrophages (0.71% and 0.20%, respectively) compared with CD8+ T cells (0.62% and 0.41%, respectively) (Table 1).
Table 1
| Tissue category | %CD8 | %CD68 | %CD68+&PD-L1+ |
|---|---|---|---|
| Pre-treatment tumor | 1.722/4.159 (0.41) | 1.325/4.339 (0.31) | 0.689/3.423 (0.20) |
| Treatment-resistant tumor | 6.8/10.971 (0.62) | 3.078/5.755 (0.53) | 2.434/3.417 (0.71) |
PD-L1, programmed death ligand 1.
For the second-line therapy, he was treated with weekly albumin paclitaxel plus sintilimab from 11-Sep-2020 to 28-Oct-2020. However, after two cycles, CT showed the progressive of disease again, indicating a true acquired resistance to PD-1 blockade, on 20-Nov-2020.
For the second-line therapy, due to the secondary MEK1 E203K mutation, he pursued an off-label use of an allosteric MEK inhibitor (trametinib, 2 mg, qd) after informed consent on 27-Nov-2020, and tolerated this treatment well after 1 week of fatigue and loss of appetite. Fortunately, he rapidly achieved partial response after 1 month (07-Jan-2021), and the tumor volume was significantly reduced and the enhancement was weakened. This response sustained for eight months until the development of multiple metastases in the liver on Aug-26-2021 (Figure 2).
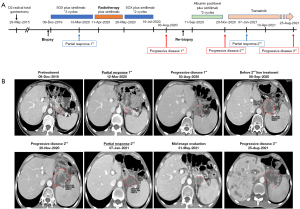
All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Sichuan University West China Hospital (No. 2021-196) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
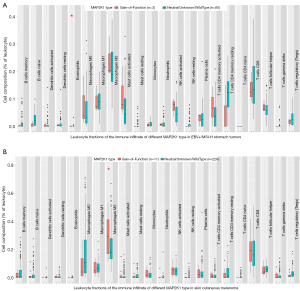
Although patients with EBVaGC greatly benefit from immune checkpoint inhibitors (ICIs) (3,4,11), the underlying mechanisms of acquired resistance have not fully been elucidated. In this case, although the express of PD-L1 was increased after first-line treatment, re-introduction with PD-1 antibody in second-line treatment was ineffective, confirming MAP2K1 E203K mutation couldn’t benefit from PD-1 antibody. None of the known mechanisms, such as mutation of B2M, JAK1, or JAK2, could explain the acquired resistance (12). Oncogenic signaling pathways, such as MAPK, PI3K and WNT/β-catenin, were also considered as important intrinsic mechanisms of resistance to immunotherapy (13,14). In this patient, a gain-of-function mutation of MAP2K1 (E203K) was exclusive to the immunotherapy-resistant lesion. However, the specific mechanism of MAP2K1 activating mutation involved in immune resistance has not been clarified. We try to explain the mechanism by means of data mining based on The Cancer Genome Atlas (TCGA). The mutation frequency of MAP2K1 was highest in skin cutaneous melanoma (6.4%), but 1.6% in stomach adenocarcinoma. The high frequency mutational sites included P124S/L and E203K/V (Figure 3A). Consistent with previous reports (11,15,16), EBV+ and MSI-high (MSI-H) stomach tumors exhibited immune activation compared with MSS tumors (Figure 3B). For the ‘Estimation of STromal and Immune cells in MAlignant Tumours using Expression data’ (ESTIMATE) analysis (17), the overall immune infiltration (“ImmuneScore”) didn’t show statistical differences between the two MAP2K1 groups (Figure 3C). For the cell-type identification by estimating relative subsets of RNA transcripts (CIBERSORT) analysis (18), in immunotherapy-sensitive stomach tumors (EBV+ and MSI-H), MAP2K1 gain-of-function mutation tumors had a higher proportion of eosinophils (Figure 4A). Correspondingly, in skin cutaneous melanoma, with the highest MAP2K1 mutation frequency, gain-of-function tumors had evidence of higher proportion of M2 polarized macrophages (Figure 4B). In this case, mIHC revealed CD68+PD-L1+ macrophages were higher proportion in treatment-resistant lesion, which might partially contribute to the immunotherapy resistance (Table 1).
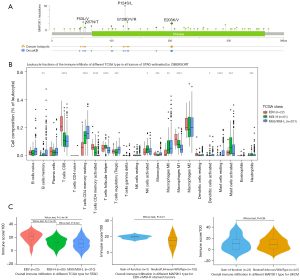
The clinical trials of trametinib on patients harboring MEK1 mutations are very rare. It’s reported that activation mutations of MEK1 showed robust resistance to allosteric MEK inhibitors, which didn’t compete with ATP for binding and preferentially inhibit the inactive conformation of the MEK enzyme (19). Molecular dynamic simulations also demonstrated that trametinib had stronger interactions with the non-active MAP2K1 (wild-type or A52V mutant) than E203K active mutant (20). Another activation mutation, MAP2K1 V211D, was also reported to be resistant to allosteric MEK inhibitors in a patient with colon cancer treated with binimetinib plus panitumumab (21). However, a patient with histiocytic sarcoma harboring an activating MEK1 F53L mutation showed a rapid and durable complete response to trametinib (22). These results suggest that different MEK1 mutations may respond differently to trametinib (23). As an important MEK high-frequency mutation, E203K mutation has not been reported to be sensitive to MEK inhibitors. However, in this case, the patient with an MEK1 E203K mutation received a rapid and durable response by the treatment of trametinib.
Conclusions
In conclusion, we reported a new potential mechanism of resistance to ICIs, mediated by the gain-of-function mutation of diver gene MAP2K1, at least in this EBVaGC patient. these results are consistent with preclinical findings of aberrant activation of the MAPK pathway as an alternative important mechanism of resistance to ICIs. Moreover, this is the first reported case of tumor patient harboring MEK1 E203K mutation which responded well to trametinib. If independently validated, MEK1 E203K mutation might be a potential indicator of trametinib therapy in a variety of malignancies. The analysis from TCGA showed MAP2K1 gain-of-function mutation could influence the tumor immune microenvironment by augmenting the expression of immunosuppression cytokines and the infiltration of immunosuppression lymphocytes, indicating clinical studies of the new immunotherapy regimen combining inhibitors of MAPK signaling and immune checkpoint blockade may be warranted.
Acknowledgments
Next generation sequencing (NGS) and associated bioinformatic analyses were performed at a CAP (College of American Pathologists)-accredited laboratory (GeneCast Biotechnology Co., Ltd.).
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-61/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-61/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-61/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Sichuan University West China Hospital (No. 2021-196) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27-40. [Crossref] [PubMed]
- Lin F, Chen Y, Huang B, et al. Application of immune checkpoint inhibitors for resectable gastric/gastroesophageal cancer. Front Pharmacol 2024;15:1391562. [Crossref] [PubMed]
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449-58. [Crossref] [PubMed]
- Bos J, Groen-van Schooten TS, Brugman CP, et al. The tumor immune composition of mismatch repair deficient and Epstein-Barr virus-positive gastric cancer: A systematic review. Cancer Treat Rev 2024;127:102737. [Crossref] [PubMed]
- Kim TK, Herbst RS, Chen L. Defining and Understanding Adaptive Resistance in Cancer Immunotherapy. Trends Immunol 2018;39:624-31. [Crossref] [PubMed]
- Xie T, Liu Y, Zhang Z, et al. Positive Status of Epstein-Barr Virus as a Biomarker for Gastric Cancer Immunotherapy: A Prospective Observational Study. J Immunother 2020;43:139-44. [Crossref] [PubMed]
- Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019;30:1479-86. [Crossref] [PubMed]
- Qiu MZ, He CY, Yang DJ, et al. Observational cohort study of clinical outcome in Epstein-Barr virus associated gastric cancer patients. Ther Adv Med Oncol 2020;12:1758835920937434. [Crossref] [PubMed]
- Salnikov MY, MacNeil KM, Mymryk JS. The viral etiology of EBV-associated gastric cancers contributes to their unique pathology, clinical outcomes, treatment responses and immune landscape. Front Immunol 2024;15:1358511. [Crossref] [PubMed]
- Dayimu A, Gupta A, Matin RN, et al. A randomised phase 2 study of intermittent versus continuous dosing of dabrafenib plus trametinib in patients with BRAF(V600) mutant advanced melanoma (INTERIM). Eur J Cancer 2024;196:113455. [Crossref] [PubMed]
- Panda A, Mehnert JM, Hirshfield KM, et al. Immune Activation and Benefit From Avelumab in EBV-Positive Gastric Cancer. J Natl Cancer Inst 2018;110:316-20. [Crossref] [PubMed]
- Ricciuti B, Lamberti G, Puchala SR, et al. Genomic and Immunophenotypic Landscape of Acquired Resistance to PD-(L)1 Blockade in Non-Small-Cell Lung Cancer. J Clin Oncol 2024;42:1311-21. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol 2020;20:25-39. [Crossref] [PubMed]
- Grogg KL, Lohse CM, Pankratz VS, et al. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol 2003;16:641-51. [Crossref] [PubMed]
- Chiaravalli AM, Feltri M, Bertolini V, et al. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Arch 2006;448:344-53. [Crossref] [PubMed]
- Yoshihara K, Shahmoradgoli M, Martínez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 2013;4:2612. [Crossref] [PubMed]
- Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12:453-7. [Crossref] [PubMed]
- Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A 2009;106:20411-6. [Crossref] [PubMed]
- Zhu J, Li C, Yang H, et al. Computational Study on the Effect of Inactivating/Activating Mutations on the Inhibition of MEK1 by Trametinib. Int J Mol Sci 2020;21:2167. [Crossref] [PubMed]
- Gao Y, Maria A, Na N, et al. V211D Mutation in MEK1 Causes Resistance to MEK Inhibitors in Colon Cancer. Cancer Discov 2019;9:1182-91. [Crossref] [PubMed]
- Gounder MM, Solit DB, Tap WD. Trametinib in Histiocytic Sarcoma with an Activating MAP2K1 (MEK1) Mutation. N Engl J Med 2018;378:1945-7. [Crossref] [PubMed]
- Lian T, Li C, Wang H. Trametinib in the treatment of multiple malignancies harboring MEK1 mutations. Cancer Treat Rev 2019;81:101907. [Crossref] [PubMed]


