
Oncoplastic surgery in the treatment of breast cancer: a review of evolution and surgical training
Introduction
The treatment of breast cancer (BC) has evolved substantially over the last century. The combination of multimodal treatment, surgery, and regional and systemic adjuvant therapies has allowed the evolution of breast-conserving surgery (BCS) and sentinel lymph node biopsy (SLNB), minimizing the impact of radical surgery (1-3). Breast reconstruction, whether with autologous flaps or more recently with implants and expanders, has also had a positive impact on women’s quality of life (QOL) (4). The breast surgeon, whose role in the past was restricted to oncological surgery, is currently responsible, in most cases, for the initial decisions involved in the interface between surgical treatment and adjuvant therapies, and their decisions impact oncological outcomes. Furthermore, breast surgeons have gradually taken on a leading role in the repair of oncological treatment, in a set of techniques currently known as oncoplastic breast surgery (OBS) (5,6).
OBS arose from the interest of breast surgeons in facilitating the resection of breast tumors associated with an improvement in the aesthetic result, as well as from a growing demand from patients for the availability of this service (7). Initially related to therapeutic mammoplasty, OBS currently has a broader concept, also encompassing breast volume replacement with autologous flaps, implants or even fat grafting (8). The classifications of these procedures in the universe of oncoplasty have evolved over the years (9), but there are still many barriers to the dissemination of these techniques among surgeons (10).
The aim of this study is to describe the evolution of OBS, the most used techniques, their oncological outcomes compared to traditional surgeries, classifications and training models described through a non-systematic review.
Evolution of breast surgery
The surgical treatment of BC has evolved substantially since the radical mastectomy recommended by Halsted (1). Back in the 19th century, Halsted developed the concept of “radical surgery”, which consisted of the complete removal of the breast, including the skin, nipple-areola complex (NAC), pectoral muscles, and drainage chains, regardless of the extent of the disease.
Little changed in the surgical treatment of BC until the 1970s, despite the emergence of “less” radical modalities, the so-called “modified radical mastectomies”. In fact, less radical mastectomies, sparing the pectoral muscles or even without associated axillary dissection (AD) (simple mastectomy) have been shown to be comparable to the Halstedian modality (11). The emergence of BCS has brought great advantages in QOL with similar OS when compared to the radical modality, as evidenced in several randomized studies (3,11-17). Surgical advances were undeniable, but BCS still often had a “radical” appearance. Axillary surgery was still radical, since AD was the standard surgical routine, regardless of initial axillary status. This began to change with SLNB in the 1990s: randomized studies demonstrated that SLNB was effective in defining axillary status compared to AD, with no impact on oncological outcome, but with lower morbidity (18). Currently, SLNB can be used alone in cases of limited lymph node disease in upfront surgery or even after NCT, including when the axilla was initially positive (19-23). The type of axillary surgery would also influence the complications of future OBS, especially immediate breast reconstructions (IBRs) (24).
Breast reconstruction
Total breast reconstruction, initially delayed and traditionally performed using myocutaneous flaps, such as the latissimus dorsi (LD) flap or the transverse rectus abdominis myocutaneous (TRAM) flap, begins a new chapter in the surgical treatment of BC (25). Patients who have previously undergone mastectomy would have the possibility of replacing breast volume, with a great impact on QOL (26). The advent of implants and expanders, moreover, was responsible for the dissemination of IBR: the possibility of performing breast reconstruction without operating on another site besides the breast brought great advantages, including reduced morbidity, time and surgical risk (4).
Breast implants have come a long way to the present day, including discussions about oncological safety: the risk of developing new neoplasms, such as lymphoma, or even LR after BC treatment and even immunological reaction are recurring themes (27-29). Another issue related to implants is their positioning in relation to skin flaps and the pectoralis muscle. Initially, implants were used in a prepectoral position, but high rates of complications associated with skin flap necrosis, infection, exposure, and loss of implants changed this understanding, with submuscular positioning gaining popularity (30). The use of temporary submuscular expanders in the so-called 2-stage IBR, with the use of an expander initially followed by exchange for a definitive implant in a second surgery, was also a milestone in IBR, as it enabled breast reconstruction with implants even in cases where there was insufficient skin (4). Furthermore, the use of an expander in a scenario in which chest wall radiotherapy was recommended would be an opportunity to minimize capsular contracture in the second stage of reconstruction, through corrections in the implant pocket, such as capsulotomy and repositioning of the inframammary fold (31). More recently, immediate reconstruction with definitive implants has been frequently used, even in the prepectoral position, facilitated by a better understanding of the infectious processes (biofilm) related to surgery, as well as by the greater preservation of the skin resulting from preserving mastectomies, allowing in many cases a mastectomy that not only spares the skin, but also preserves the muscles (32,33). However, as with BCS, the cosmetic results of total reconstructive surgery were often unsatisfactory, related to the oncological surgical volume used, inadequate flaps and the need for adjuvant radiotherapy (34).
Interface between the mastologist and multimodal therapy
Undoubtedly, the cosmetic outcome of BC surgery would depend on the volume of resection in relation to the size of the breast. Over the years, however, with the advent of multimodal systemic treatment, oncological surgeries could be, paradoxically, smaller and without impacting local regional recurrence (LRR). Systemic treatment impacted the control of distant disease, but it also reduced LR. There are several examples of this impact: the addition of adjuvant hormone therapy in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 study significantly reduced LR. The addition of chemotherapy evidenced in the NSABP B-13 study also reduced LR (35), as did the addition of trastuzumab in BC that overexpresses the human epidermal growth factor receptor 2 (HER-2) receptor. In a study of HER-2-overexpressing BC, with tumors ≤5 cm and negative axilla undergoing BCS, the use of adjuvant trastuzumab (n=102) was compared to the absence of this therapy (36). The estimated 3-year LRR-free survival was 90% in the group that did not use trastuzumab compared to 99% among patients who received the treatment. Another example was the use of NCT, increasing the BCS rate: a meta-analysis of 10 non-contemporaneous randomized studies with patients (n=4,756) undergoing NAC, demonstrated that cases that received NAC had a higher BCS rate (65% versus 49%) (37). It was also observed that LR was higher in the neoadjuvant group compared to upfront surgery followed by adjuvant chemotherapy. On the other hand, it is assumed that, at the time these women were treated, many current technologies were not available. Anti-HER-2 therapy, for example, would only be available in early BC a few years later. Marking lesions before chemotherapy, especially with clips, was not routine. Imaging exams, as well as pathology, have evolved substantially over the years. More contemporary studies demonstrate, even more, the impact of these technologies, without important differences in locoregional control (38-40). Axillary surgery has also had a major impact on multimodal therapy, migrating from radical AD recommended over a century ago to SLNB, with no impact on LRR, but improved QOL, whether in upfront surgery or after neoadjuvant treatment, even in cases with an initially positive axilla (22,38,40).
Total mastectomies have come to be associated with skin and/or nipple preservation, facilitating IBR with implants and obtaining better cosmetic results, often through a reduced scar (41-45). There has been much discussion about the oncological safety of preserving mastectomies, especially those sparing the NAC. Inadequate flap thickness could theoretically be a risk factor for LR. The concept of a thin flap, with a specific previously stipulated thickness, was a dogma that emerged with radical surgery. Currently, there is an understanding that flap thickness should not be a fixed value, but rather variable among patients, depending on body mass (46). Furthermore, “thin” flaps, with a thickness <5 mm, do not guarantee the absence of breast parenchyma, even in skin-sparing mastectomies (47). These surgeries may, in fact, have similar oncological outcomes. A study conducted in Italy with 1,989 patients who underwent nipple-sparing mastectomy (NSM) between 2003 and 2011, including ductal carcinoma in situ (DCIS) (n=278) and invasive carcinoma (n=1,711), evaluated LR, NAC recurrence, NAC necrosis, and overall survival (OS) (44). After a mean follow-up of 97 months, 91/1,711 (5.3%) cases with invasive disease presented LR and 11/278 (4.0%) cases with initial in situ disease recurred in the breast. Only 36 patients (1.8%) presented recurrence in the NAC, with 66 (3.3%) of total necrosis. The estimated 5-year OS for invasive and in situ carcinomas, respectively, were 96.1% and 99.2%. Another related discussion would be the appropriate recommendation for these mastectomies, including distance from the tumor to the NAC, traditionally stipulated as at least ≥2 cm, tumor size and the need for intraoperative freezing of the NAC base. A study conducted in a Brazilian public hospital evaluated unselected cases of BC undergoing NSM, demonstrating excellent locoregional and distant control, regardless of the distance from the tumor to the NAC or the use of intraoperative analysis of the NAC base (41). After a mean follow-up of 34 months, approximately 5% of cases presented LR, with estimated 5-year OS and DFS of 98% and 83%, respectively.
Skin-sparing mastectomy associated with skin reduction is a more complex example of skin-sparing mastectomy, which involves the combination of techniques (sparing mastectomy and Wise skin resection) for breasts that are candidates for skin-sparing mastectomy but have excess skin and/or excess breast volume. This technique was initially described by Nava et al.: the authors described a combined technique to reconstruct medium-sized ptotic breasts in a single stage, with a distance from the areola to the inframammary fold greater than 8 cm, in a single stage, using anatomical implants. The mastectomy was performed after deepithelialization of the lower half of the breast following the conventional Wise pattern. In the reconstruction, the implant was positioned in a pocket of the pectoralis major muscle previously sutured to the upper edge of the lower dermal flap. The overall complication rate was approximately 20% (48). Although feasible, the applicability of skin-reducing mastectomy is controversial, especially due to high complication rates. A systematic review of the risks and benefits of pattern-wise skin-sparing mastectomy, specifically in relation to complications, patient-reported outcomes, and aesthetic results, involving 24 studies and 879 patients (1,184 reconstructed breasts) showed an overall complication rate of 21% (49).
Oncoplasty
The main focus in BC surgery should be effective treatment of the disease, however, the priority of oncological treatment should not leave the importance of the cosmetic result in second place. The advent of more conservative techniques was an advance in improving the cosmetic result, however, it was not enough to guarantee an adequate aesthetic result: the volume of resection in BCS, for example, or even the location of the tumor in the breast, could lead to unsatisfactory cosmetic results, in addition to making resection of the lesions difficult (50). This concept still needed to be expanded and partial or total reconstructive surgery tactics needed to be added to the surgical treatment of BC. In fact, these techniques were gradually being incorporated into oncological breast surgery.
Historically, breast reconstructive procedures were mostly performed by plastic surgeons referred by a breast surgeon, where oncological surgery was performed and secondarily reconstructive surgery. However, these techniques began to be applied in the treatment of BC, giving rise to the concept of OBS (5). One of the first problems faced by oncoplastic surgeons is the division of the surgical procedure into two fronts (oncological and reconstructive) and the difficulty in combining techniques from different specialties. Some articles have cited collaborations between breast surgeons and plastic surgeons when performing OBS, while others have described the procedure being performed by breast surgeons trained in plastic surgery (7). There are currently no clear guidelines available on the requirements for performing OBS by breast surgeons, especially with regard to IBR. However, there may be advantages in performing the oncological and reconstructive procedures by the same surgeon. One study evaluated mastectomy and IBR cases between 2015 and 2019, dividing them into single-surgeon (SS) (breast surgeon with reconstruction fellowship) or dual-surgeon (DS) (breast surgeon and a surgeon with reconstruction fellowship) cohorts (51). The authors included 158 patients in their analysis [SS (n=45); DS (n=113)]. SS patients underwent surgery 13.2 days earlier than DS patients (P<0.01) and required significantly fewer preoperative (1.9 versus 3.4; P<0.01) and postoperative visits (6.8 versus 10.7; P<0.01). Operative duration was comparable in both groups (SS: 245 minutes; DS: 245 minutes; P=0.99). The authors found no significant differences in surgical site infection, seroma, hematoma, abdominal donor site healing, or flap and prosthesis loss between the groups. The authors also found that patients with two surgeons had a significantly higher rate of mastectomy flap necrosis (20% versus 4% P=0.01), which held true in logistic regression when controlling for other variables. BREAST-Q data demonstrated that patients with a single surgeon had significantly higher overall scores (P=0.04) and were significantly more satisfied with their results, surgeon, and the information provided (P=0.03, P=0.03, and P=0.01, respectively). Another retrospective study was conducted on OBS performed over a 6-year period by a single surgeon approach (oncologic and reconstructive surgery) or a team with two surgeons, oncologic and reconstructive surgery separately (52). Primary outcomes were positive margin rates and overall complication rates; secondary outcomes were LRR, DFS, and OS. A total of 217 patients were identified; 117 were SS cases and 100 were DS cases. Positive margin rates were not significantly different (SS: 10.9% versus DS: 9%; P=0.81), nor were complication rates (SS: 11.1% versus DS: 15%; P=0.42). LRR rates were also not significantly different (SS: 1.7% versus DS: 0%; P=0.5). DFS and OS were not significantly different at the 1-, 3-, and 5-year time points (P=0.20 and P=0.23, respectively) (52,53).
Oncoplasty safety
Traditionally, the term oncoplasty was initially used in BCS, associated with the displacement of breast volume, often through a “therapeutic mammoplasty” (5). This tactic could have the impact of facilitating resections, expanding the possibility of BCS. A study conducted in France with 101 patients treated at the Curie Institute between 1985 and 1999, whose cosmetic result was theoretically possibly adverse in conventional BCS, demonstrated that the integration of mammoplasty with oncological surgery allowed a wide excision (average breast parenchyma resection of 222 grams), with a favorable cosmetic result in 82% of cases and a LR and OS rate at 5 years of 9.4% and 95.7% respectively (54). The oncologic safety of OBS compared with conventional BCS was an initial concern but has been shown to be similar in several studies (55-66) (Table 1). A more recent study by Clough et al. evaluated 350 patients who received therapeutic mammoplasty between 2004 and 2016 (65). The 5-year cumulative incidences for LR, regional, and distant were 2.2%, 1.1%, and 12.4%, respectively. Another retrospective cohort study evaluated prospectively maintained databases from one institution to identify patients who underwent surgery for BCS between 2007 and 2014 (66). Surgeries were categorized as BCS, OBS, total mastectomy (TM), or TM with IBR (TM + R). A total of 10,607 operations were performed for 9,861 patients. Median follow-up was 3.4 years. Use of OBS resulted in a nearly fourfold increase in the percentage of all BC surgeries during the study period. OBS had a lower rate of seroma formation and lower rates of positive or close margins compared with BCS. There was no difference in OS or recurrence-free survival when comparing BCS and OBS. A meta-analysis of 11 studies including 3,789 patients comparing these techniques showed that recurrence rates were not different, but reoperation rates were lower with OBS (67).
Table 1
| Study | Period | Follow-up | Oncoplasty, n | Control, n | Recurrence oncoplasty (%) | Recurrence control (%) |
|---|---|---|---|---|---|---|
| Clough (65) | 2004–2016 | 55 months | 350 | – | LR: 2.2 | – |
| Mansell (64) | 2009–2012 | 56 months | 104 | 558 (BCS)/318 (TM) | LR: 2.0 | LR: 3.4 (BCS), 2.6 (TM) |
| Fitoussi (59) | 1986–2007 | 49 months | 540 | – | LR: 6.8 | – |
| Rose (63) | 2008–2013 | 4.1 years | 197 | 1,399 (BCS) | Recurrence: 6.0 | Recurrence: 3.6 (BCS) |
| André (56) | 2010–2016 | 64 months | 243 simple, 215 complex | 3,720 (BCS) | LR: 1.4 (complex) | LR: 1.0 (simple), 1.5 (BCS) |
| Hing (61) | 2009–2013 | 82 months | 174 | 365 (BCS) | LR: 1.7 | LR: 2.2 (BCS) |
| De Lorenzi (58) | 2000–2008 | 7.2 years | 454 | 908 (BCS) | LR: 6.7 | LR: 4.2 (BCS) |
| Fitzal (60) | 2010–2013 | 74.5 months | 297 (level II) | 2,217 (BCS) | LR: 3.6 | LR: 2.7 (BCS) |
| Kelemen (62) | 2010–2017 | 51 months | 350 | 350 (BCS) | LR: 1.1 | LR: 3.1 (BCS) |
| Almeida (55) | 2011–2015 | 50.4 months | 98 | 768 (BCS) | LR: 6.1 | LR: 3.9 (BCS) |
| Chakravorty (57) | 2003–2010 | 28 months | 150 | 440 (BCS) | LR: 2.7 | LR: 2.2 (BCS) |
| Carter (66) | 2007–2014 | 3.4 years | 1,177 | 3,559 | RFS: 94.6 | RFS: 96.1 (BCS) |
BCS, breast-conserving surgery; LR, local recurrence; RFS, recurrence-free survival; TM, mastectomy.
Theoretically, OBS would be a compromise between conventional BCS and mastectomy. Therefore, oncological safety studies comparing OBS not only with conventional BCS but also with mastectomy would be appropriate, since in many cases OBS would be performed in cases of patients who are candidates for mastectomy. One study compared the oncological safety of OBS versus conventional alternatives, including mastectomy, to assess the risk of recurrence (68). In this meta-analysis, 18 studies met the inclusion criteria, including over 18,103 patients. The primary outcome (recurrence) was not significantly different between the techniques, OBS or mastectomy. This study also evaluated the reoperation rate which, after adjustment for biases, was statistically non-significant between the groups.
The addition of greater complexity to BCS was also associated with a higher rate of surgical complications. A study using the American College of Surgeons database evaluated all post-surgical complications in traditional BCS and OBS in more than 100,000 cases with BC, of which 9,126 were OBS (69). OBS was more associated with overall surgical complications compared to BCS (3.8% versus 2.6%; P<0.001). These complication rates, however, may not be related to the delay in the initiation of adjuvant oncological treatment. A prospective multicenter British cohort study (TeaM study) evaluated almost 900 patients undergoing therapeutic mammoplasty, of which approximately 47% of cases were surgeries with a Wise-type mammoplasty resection pattern, and contralateral mammoplasty was performed in one third of cases (32%) (70,71). The overall complication rate was 23% and the reoperation rates due to complications were approximately 2.8%. However, when cases with complications (n=168) were compared to those patients who did not have postoperative complications (n=527), no significant difference was found in the time, in days, to start adjuvant treatment. In a Cochrane review, several effects of OBS were reviewed in comparison to conventional BCS or even in relation to mastectomy (71,72). Oncological outcomes, QOL and cosmetic results in women with BC were evaluated. A total of 178,813 women were included in 78 cohort (non-randomized) studies. In the first comparison, OBS versus standard BCS, no difference was observed in LR, either in the assessment of LR-free survival or LR rate, as was the case for DFS, but OBS could reduce the rate of reexcisions. On the other hand, OBS could increase the number of women who have at least one complication. Regarding cosmesis, in general, OBS had a trend towards a more favorable outcome. In the next comparison, OBS versus mastectomy alone without reconstruction, OBS could increase LR-free survival compared with mastectomy. Furthermore, OBS could reduce complications compared with mastectomy. In the last comparison, OBS versus mastectomy with IBR, OBS had no difference in LR-free survival or in DFS when compared with mastectomy with IBR. IBR, on the other hand, could reduce the rate of complications compared with mastectomy with reconstruction.
Oncoplasty costs
Another factor that should be analyzed in relation to OBS is the cost of the procedure. A single-center retrospective study calculated the costs of all patients undergoing BC surgery between January 2014 and December 2016 (72). Conventional BCS, OBS, and IBR mastectomy were evaluated. Treatment costs were calculated using hospital financial data. A total of 220 patients were included: 74 patients in the conventional BCS group, 78 with OBS, and 68 in the IBR mastectomy group. Conventional BCS had lower costs compared to OBS and IBR mastectomy. The costs of OBS and IBR mastectomy were comparable. Complication rates were 5.5% for conventional BCS, followed by 17% for OBS and 34% for IBR mastectomy. It is possible to assume, however, that in countries with limited access to resources, OBS is likely to have a financial advantage over IBR mastectomy, due to the use of implants or expanders, which may often not be available.
Classification of oncoplasty
It was necessary to standardize techniques to minimize complications and establish objective criteria for selection. Clough et al., for example, presented a classification of surgery, by topographies in the breast, “quadrant by quadrant”, which was widely accepted and adopted among surgeons: OBS was stratified by levels, based on the volume of breast parenchyma resected: type I (which involved resection of up to 20% of the breast volume) and II with a larger volume resection (20% to 50%), where mammoplasty techniques are normally necessary in order to avoid an inadequate aesthetic result (Table 2) (9).
Table 2
| Study | Level | Reference |
|---|---|---|
| Clough (volume) (9) | I | Up to 20% volume |
| II | 20–50% volume | |
| de Andrade Urban (surgery type) (73) | I | Simple displacement |
| II | Mammoplasty; fat grafting; implants†; bilateral | |
| III | Autologous flaps | |
| Canada (volume) (74) | I | Up to 15% volume |
| II | 15–25% volume | |
| III | >25% volume | |
| American Society of Breast Surgeons (type/volume) (75) | I (volume displacement) | Up to 20% volume |
| II (volume displacement or replacement) | 20–50% volume |
†, involves reconstruction with definitive implants, expanders and breast augmentation.
Urban proposed three levels of competence for OBS, according to the complexity of the surgery: level I for simple procedures that do not require specific training in plastic/reconstructive surgery; level II for mammoplasty/therapeutic mastopexy, breast augmentation, fat grafting, as well as reconstruction with implants and bilateral procedures; and level III for procedures using autologous flaps (Table 2) (73).
A similar classification system has been proposed in Canada, which introduced a third level of volume replacement (74). Level I is the least complex, with less than 15% of the breast volume resected. Level II techniques involve resection of between 15% and 25% of the breast parenchyma volume, or when the tumors are in adverse surgical locations, while level III involves resections greater than 25% of the volume and requires more complex reconstructive techniques (Table 2).
In 2019, the American Society of Breast Surgeons (ASBrS) published definitions and a classification system in OBS to facilitate discussion and training. The ASBrS definition defined principles of volume displacement or replacement. These concepts have gained considerable popularity in the pragmatic definition of different oncoplastic techniques. Volume replacement would include those situations in which volume is added, or replaced, using flaps or implants to correct the defect (75). Volume displacement is defined as the closure of the oncologic resection defect (lumpectomy) and the redistribution of residual volume after resection (breast preservation) and is divided into two levels: level I (< 20%) and level II (20–50%) (Table 2).
Hoffmann proposed a complexity-based classification encompassing both ablative and conservative surgeries (76). Procedures would be classified by levels of complexity (5), ranging from simple procedures to flap reconstruction with microsurgery in both categories. In general, ablative surgeries would involve simple (level 1) or complex (level 2) mastectomies, without reconstruction; implant reconstruction procedures would be level 3; reconstruction with local flaps (level 4); reconstruction with pedicled distant flaps (LD or TRAM) (level 5); free flaps with microsurgery (level 6). A similar classification for BCS: level 1 (simple excision without parenchymal mobilization); level 2 (more complex surgery, inframammary, with mobilization of up to 25% of the parenchyma); level 3 (more complex surgery, inframammary, with mobilization >25% of the parenchyma); level 4 (complex procedures, adaptive mastopexy, local flaps); level 5 (reduction mammoplasty); level 6 (pedicled or free flaps). This complexity-based system could facilitate operational reporting, billing procedures, and research (Table 3).
Table 3
| Level | Ablative surgery | BCS |
|---|---|---|
| Level 1 | Simple mastectomy without reconstruction | Simple excision |
| Level 2 | Complex mastectomy without reconstruction | Mobilization ≤25%† |
| Level 3 | Implant reconstruction | Mobilization >25% |
| Level 4 | Local flap reconstruction | Mastopexy/flap‡ |
| Level 5 | Distant autologous flap reconstruction§ | Mammoplasty |
| Level 6 | Free flap reconstruction (microsurgery) | Flaps (autologous) |
†, mobilization of the parenchyma, usually inframammary incisions; ‡, local flaps; §, pedicled latissimus dorsi or rectus abdominis muscle flap. BCS, breast-conserving surgery.
In 2019, the ASBrS published definitions and a classification system in OBS to facilitate discussion and training. The ASBrS definition defined principles of volume displacement or replacement. These concepts have gained considerable popularity in the pragmatic definition of different oncoplastic techniques. Volume replacement would include those situations in which volume is added, or replaced, using flaps or implants to correct the defect (76). Volume displacement is defined as the closure of the oncologic resection defect (lumpectomy) and the redistribution of residual volume after resection (breast preservation) and is divided into two levels: level I (<20%) and level II (20–50%) (Table 2).
Oncoplastic techniques
Volume displacement is generally used to correct small or moderate defects after BCS. A wide variety of dermoglandular volume displacement techniques have been described: (I) glandular rotation, a method of redistribution of the breast parenchyma, can be used in almost all breast topographies, without skin resection. The skin is dissected from the parenchyma/subcutaneous tissue and mobilized in order to correct the defect; (II) elevation, displacement or centralization of the NAC can be used to correct nipple asymmetry, when the breast volume needs to be mobilized. The NAC can be dissected from the underlying breast tissue to compensate for any deviations; (III) the round block technique is often used in breasts with moderate ptosis or hypertrophy, with tumor location in the periareolar region. This technique involves drawing circles of different diameters around the NAC, resecting the intermediate skin, facilitating access to the tumor and resection of the lesion with margins. (IV) The radial technique can be used when the tumor is located in the medial or lateral quadrants, especially in cases of larger tumors and need for skin removal. The skin incision is performed radially followed by tumor resection with margins up to the deep pectoral plane. Partial skin undermining allows glandular rotation into the defect. Mobilization of the NAC can prevent displacement; (V) mammoplasty/mastopexy can be used in patients with larger breasts and ptosis, allowing wide excisions with a superior or inferior pedicled flap depending on the location of the tumor, and the same technique can be used in the contralateral breast.
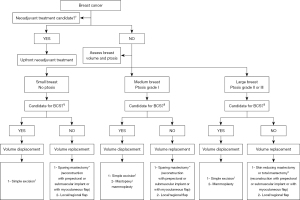
Volume replacement corrects a defect by replacing an implant or autologous tissue, and is mainly used when large volumes of resection are necessary in relatively small breasts, in which the defect cannot be corrected by parenchymal displacement. Several approaches to volume replacement with autologous tissue have been described. For lateral defects, the lateral intercostal artery perforator (LICAP) and lateral thoracic artery perforator (LTAP) flaps can be used. Defects in the lower poles of the breast can be filled using the anterior intercostal artery perforator (AICAP) flap, while medial and even medial upper quadrant defects can be filled using the medial intercostal artery perforator (MiCAP) flap. In patients with larger defects, other options may be considered, such as the thoracodorsal artery perforator (TDAP) flap. Autologous reconstruction with myocutaneous flaps, either pedicled or free, are also options in this setting. The most commonly used pedicled flap is the LD flap, with the muscle and overlying skin replacing the excised volume. The myosubcutaneous LD flap or mini LD (mLD) flap can be used when the skin overlying the tumor can be preserved. The mLD technique is similar to the LD technique except that the skin overlying the LD does not need to be used. The deep inferior epigastric artery perforator (DIEP) flap is the most commonly used free flap. Other autologous options for women who are not suitable for DIEP flaps include the TRAM, vertical rectus abdominis myocutaneous (VRAM), transverse upper gracilis (TUG), profunda artery perforator (PAP), lumbar artery perforator (LAP), superior gluteal artery perforator (SGAP), and inferior gluteal artery perforator (IGAP) flaps. Many surgeons now consider the DIEP flap to be the gold standard in autologous total free breast reconstruction, with low flap failure rates (77), although these techniques involve greater complexity and a trained multidisciplinary team that is not available in many settings. Autologous techniques can also be combined with fat grafting or implants, which can be used to increase volume. All these techniques must be selected in a context that involves the needs of systemic treatment (neoadjuvant or adjuvant), disease subtype, patient’s desire and breast type (Figure 1).
New technologies and fat grafting
A topic related to volume replacement with implants or expanders is the use of acellular dermal matrix (ADM). ADMs have become a component of implant-based IBR, with their use continuing to increase (78,79). In general, ADMs consist of biomaterials that allow vascular ingrowth and integration with tissues, with the aim of reinforcing them, and are frequently used in prepectoral and dual-plane IBR associated with the pectoralis major muscle. ADM emerged in IBR to reduce implant exposure and minimize capsular contracture (80). However, its use has been controversial, with conflicting results regarding complications (81). The use of ADM also represents a significant cost for IBR. The use of implants may also influence the technique, results and cost. Polyurethane implants, for example, have been frequently used in breast reconstruction, including as an alternative to the use of ADMs in prepectoral reconstruction, with lower cost and possibly lower complication rates, including seroma and infection (82-84). In a study of 784 nipple-sparing mastectomies (453 patients) and immediate reconstruction with prepectoral polyurethane implants, no major complications were reported: the authors concluded that the use of these implants may be an alternative in this scenario (85).
Fat grafting is a technique that aids in cosmetic refinement and has become very popular in OBS and cosmetic surgery (76,86). It consists of harvesting adipose tissue from a donor site through liposuction, such as the abdomen, knee or flanks, using a syringe with appropriate pressure and needle, and transferring it to the recipient area. This technique was not widely accepted until the 1980s, after all, reports demonstrated variable degrees of absorption and consequent asymmetry, possibly due to the technique used at the time (87). In oncology, initial preclinical studies in patients with BC suggested that fat grafting could modify microenvironment, increasing LR (88-90). More recent studies, on the other hand, have demonstrated the safety of fat grafting. A study with 60 months of follow-up in 719 breasts treated with BC and 305 cases of prophylactic surgeries or benign alterations that used fat grafting compared to controls did not demonstrate significant differences: the cumulative rates of LRR at 5 years were 1.6% and 4.1% for cases and controls, respectively, while systemic recurrence occurred in 2.4% of cases and 3.6% of controls (P=0.514). There was no primary BC in healthy breasts reconstructed with the aid of fat grafting (89). A meta-analysis of 9 studies (4,247 patients) demonstrated no significant differences in the primary objective, LRR, between the group undergoing fat grafting compared to the control group (hazard ratio: 0.92) (90).
QOL
Overall, BCS improves QOL. Based on systematic reviews, we can infer that patients undergoing BCS or mastectomy with IBR have better QOL compared to those undergoing mastectomy without reconstruction (91). Oncoplastic surgery may also impact QOL when compared to conventional BCS. A recent meta-analysis evaluated, using the BREAST-Q questionnaire, the QOL of 11,186 BC patients in 55 studies who underwent conventional BCS compared to BCS with oncoplastic surgery (level I or II). Oncoplastic surgery was associated with greater satisfaction with the breast (72% vs. 62.9%; P=0.02) and psychosocial well-being (78.9% vs. 73.3%; P=0.0001), based on the patient-reported outcomes (92).
Oncoplastic in special situations
Locally advanced breast cancer (LABC) is very common in developing countries or countries with limited access to resources. OBS has increased the possibility of BCS in more advanced tumors, especially in T3, multifocal and multicentric tumors, which are natural candidates for mastectomy, giving rise to the concept of extreme oncoplasty, representing an advance in BCS, mainly using modifications of the Wise pattern and geometric compensation (93). Recent studies have shown oncological safety of BCS and acceptable complication rates, especially compared to mastectomies with immediate reconstruction (94-96). In the literature, others different techniques are described for “wider” breast resections, ranging from the use of thoracobdominal flaps (97-101) to the use of myocutaneous flaps (102-105).
A related topic is the possibility of performing oncoplastic surgery in large DCIS. In general, these cases are traditionally managed with mastectomy, due to the risk of greater LR in conservative surgery, but recently the possibility of performing BCS with oncoplastic techniques has gained popularity, with studies demonstrating acceptable complication rates, margin involvement and local control compared to radical surgery (106-109).
NAC, historically used in LABC, has been increasingly used in early BC. A current discussion has been the possibility of a higher rate of complications in breast surgery in cases undergoing NAC, including oncoplastic surgery. A study with 429 patients and 713 breasts in a single center evaluated the impact of NAC in OBS, without changing complication rates or even delaying adjuvant radiotherapy (110). These data are like those related to total breast reconstruction after NAC, demonstrating that this procedure is also safe after neoadjuvant systemic treatment (111). “No ink on margins” has been the BCS cutoff point in upfront surgery but has been controversial after NAC. Oncoplastic surgery could provide an advantage in facilitating the achievement of clear margins after NAC. A recent meta-analysis of 11 studies and 4,594 patients compared oncoplastic BCS to conventional BCS or even mastectomy: positive margin rate and OS were not statistically different between the groups. However, the margin re-excision rate was significantly lower in oncoplastic surgery than in conventional BCS (2.9% vs. 6.1%). Cosmetic outcomes and patient-reported outcome measures were also marginally in favor of oncoplastic surgery (112).
Oncoplasty training
In recent years, interest in OBS has grown among mastologists in several countries. In the United States, most breast surgeons show interest in oncoplasty, as does Canada (113-119). The evolution of the breast surgeon in OBS seemed inevitable in many places. The general surgeon, or even the mastologist with prior training in obstetrics/gynecology, began to specialize exclusively in breast surgery, being one of the great drivers of the development of oncoplasty, as well as the interest of patients in breast repair. In countries with limited access to economic resources, training of the mastologist in oncoplasty would be a great advantage. However, these techniques have not been fully implemented for several reasons, including the education of surgeons (120). Specific training is needed for senior physicians and inclusion of these techniques for younger surgeons (121-123).
There is multiple training models described (69,124-127), but there is no universal training model. In the United Kingdom (UK), as recently as the 1990s, few general surgeons in training chose breast surgery as a specialty, at a time when the demand for breast surgery, especially reconstructive surgery, was growing, driven by patient interest. OBS attracted new surgeons to the breast field, especially due to the greater complexity of the techniques, with many training activities carried out, many of which continue to this day, such as cadaver training courses. OBS was included in the curriculum of breast specialists, training in multidisciplinary teams and the creation of fellowships in oncoplasty, with close collaboration between breast surgeons and plastic surgeons having an impact on the reconstruction of thousands of women (120).
Training in other countries, however, varies substantially, even within Europe. There is still a great lack of homogeneity in most European countries or even between different centers in the same country. The European Society of Mastology (EUSOMA) has continued efforts to standardize the training of mastologists. In 2000, guidelines for specialist breast units were published and in 2007 training standards for surgeons were defined (124). A more detailed recommendation was produced by EUSOMA in 2013 with the aim of ensuring quality and accreditation of breast centers, with appropriate proportions of IBR and BCS being part of this process (127). Regular examinations are now carried out to assess training, knowledge and skills in OBS in Europe, with requirements for specialist certification in breast surgery.
In Brazil, there has been a greater focus on OBS training in recent years. The Brazilian Society of Mastology (SBM) is the official society of mastologists. Since 2008, the SBM has supported several measures to improve and train mastologists: (I) continuing education in oncoplasty, through hands-on courses, in which senior or younger surgeons can develop expertise in these techniques; (II) dedicated fellowships in oncoplasty; (III) inclusion of oncoplasty in mastology residency programs (128). The SBM also participates directly in the administration of these hands-on courses, as well as in the selection of professors and students. In 16 years, this course model has allowed the proportion of mastologists qualified for OBS to increase from 30% to 50%, a fact not demonstrated in other countries (129). Furthermore, it has encouraged the creation of similar courses in other countries, such as Argentina and Peru, with surgeries performed in both in-person and virtual formats (128).
Social impact of oncoplasty
In countries with limited access to resources, the availability of the full range of oncological treatments is not homogeneous, including a surgical team qualified in breast repair, which has an impact on the IBR rate (130). Adequate training of breast surgeons, with greater availability of reconstructive surgery, could ultimately impact the QOL of thousands of women. Furthermore, a considerable percentage of women have LABC. Educational strategies for chest wall reconstruction are essential in this patient profile. Producing more surgeons qualified in OBS has the potential to reduce mastectomy rates. A recent study assessed whether the adoption of OBS helped reduce mastectomies performed at a UK referral center (120). All BC procedures between April 2016 and July 2023 were evaluated, categorizing the procedures into: BCS, mastectomy, OBS, and total reconstructions. During the period, 3,875 surgeries were recorded (3,638 patients). The BCS rate increased from 66.2% in 2016 to 80.7% in 2023. Using a linear regression model, BCS increased by 2.1% annually (coefficient =2.12; P=0.0069) and OBS increased from 10.5% to 22.9% (coefficient =2.14; P=0.00017), with a positive correlation between these two variables observed (coefficient =0.86; P=0.0056), suggesting that OBS helped to reduce mastectomy rates.
Conclusions
OBS is a major chapter in the treatment of BC, combined with the advent of multimodal treatment, with the breast surgeon taking on a major role in bringing this knowledge together. On the other hand, educational models need to evolve and be optimized to improve the education of surgeons. Medical societies can play a major educational and driving role. Many surgeons with specialized oncoplastic training, for example, often encounter an additional obstacle during the credentialing process (incorporation of specific billing codes), preventing the integration of these advanced techniques into their practice or workplace. Ultimately, resolving these bottlenecks could impact the lives of thousands of women.
Acknowledgments
None.
Footnote
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-140/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-140/coif). F.P.C. received consulting fees from AstraZeneca; received payment or honoraria from Roche, Pfizer, Libbs, MSD, AstraZeneca and Daiichi Sankyo; received support for attending meetings from MSD; and served as board member for MSD and AstraZeneca. F.P.Z. received payment for lectures as speaker for the following companies: Astra Zeneca, Merck, Novartis, Daiichi-Sankyo and Roche; received support for attending San Antonio Breast Cancer Symposium for AstraZeneca; and served as board member for Merck. E.C.M. received payment or honoraria from Lilly. A.M. received consulting fees from Roche, Merck, Novartis and Eli Lilly; received payment or honoraria from Roche, Merck, Novartis, Eli Lilly, Daiichi Sankyo and Exact Science; received support for attending meetings from Roche and Merck; served as board member for Roche; and has stock or stock options from Eli Lilly and Novo Nordisk. M.A. received payment or honoraria from Gilead, AstraZeneca and Daiichi Sankyo. F.P.B. received payment from MSD, Lilly, Roche, Novo Nordisk and GCA aesthetics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halsted WS. I. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann Surg 1907;46:1-19. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- Cordeiro PG. Breast reconstruction after surgery for breast cancer. N Engl J Med 2008;359:1590-601. [Crossref] [PubMed]
- Audretsch WP, Rezai M, Kolotas C, et al. Tumor-Specific Immediate Reconstruction in Breast Cancer Patients. Semin Plast Surg 1998;11:71-100. [Crossref]
- Weber WP, Morrow M, Boniface J, et al. Knowledge gaps in oncoplastic breast surgery. Lancet Oncol 2020;21:e375-85. [Crossref] [PubMed]
- Rainsbury R. Oncoplastic training in the UK and perspectives for the future. Mastology 2017;27:265-70. [Crossref]
- Heeling E, van Hemert AKE, Vrancken Peeters MTFD. A clinical perspective on oncoplastic breast conserving surgery. Transl Breast Cancer Res 2023;4:29. [Crossref] [PubMed]
- Clough KB, Kaufman GJ, Nos C, et al. Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 2010;17:1375-91. [Crossref] [PubMed]
- Bitoiu B, Grigor E, Zeitouni C, et al. Current Practices and Trends of Plastic and Oncoplastic Breast Surgeons in Canada. Plast Surg (Oakv) 2025;33:35-41. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Arriagada R, Lê MG, Rochard F, et al. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol 1996;14:1558-64. [Crossref] [PubMed]
- Blichert-Toft M, Rose C, Andersen JA, et al. Danish randomized trial comparing breast conservation therapy with mastectomy: six years of life-table analysis. Danish Breast Cancer Cooperative Group. J Natl Cancer Inst Monogr 1992;19-25. [PubMed]
- De la Cruz Ku G, Karamchandani M, Chambergo-Michilot D, et al. Does Breast-Conserving Surgery with Radiotherapy have a Better Survival than Mastectomy? A Meta-Analysis of More than 1,500,000 Patients. Ann Surg Oncol 2022;29:6163-88. [Crossref] [PubMed]
- Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 2002;347:567-75. [Crossref] [PubMed]
- Poggi MM, Danforth DN, Sciuto LC, et al. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy: the National Cancer Institute Randomized Trial. Cancer 2003;98:697-702. [Crossref] [PubMed]
- van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143-50. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Barrio AV, Montagna G, Mamtani A, et al. Nodal Recurrence in Patients With Node-Positive Breast Cancer Treated With Sentinel Node Biopsy Alone After Neoadjuvant Chemotherapy-A Rare Event. JAMA Oncol 2021;7:1851-5. [Crossref] [PubMed]
- Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455-61. [Crossref] [PubMed]
- Cavalcante FP, Millen EC, Zerwes FP, et al. Role of Axillary Surgery After Neoadjuvant Chemotherapy. JCO Glob Oncol 2020;6:238-41. [Crossref] [PubMed]
- Cavalcante FP, Zerwes FP, Souza ABA, et al. The use of blue dye alone for sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initially node-positive breast cancer. Eur J Surg Oncol 2024;50:107967. [Crossref] [PubMed]
- Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. [Crossref] [PubMed]
- Madsen RJ, Esmonde NO, Ramsey KL, et al. Axillary Lymph Node Dissection Is a Risk Factor for Major Complications After Immediate Breast Reconstruction. Ann Plast Surg 2016;77:513-6. [Crossref] [PubMed]
- Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg 1971;47:565-7. [Crossref] [PubMed]
- van Bommel ACM, de Ligt KM, Schreuder K, et al. The added value of immediate breast reconstruction to health-related quality of life of breast cancer patients. Eur J Surg Oncol 2020;46:1848-53. [Crossref] [PubMed]
- Cohen Tervaert JW, Mohazab N, Redmond D, et al. Breast implant illness: scientific evidence of its existence. Expert Rev Clin Immunol 2022;18:15-29. [Crossref] [PubMed]
- Cordeiro PG, Ghione P, Ni A, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg 2020;73:841-6. [Crossref] [PubMed]
- Maarse W, Teunis T. Implant Surface Texture and Breast Cancer Recurrence. JAMA Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 1981;67:312-7. [Crossref] [PubMed]
- Cordeiro PG, Jazayeri L. Two-Stage Implant-Based Breast Reconstruction: An Evolution of the Conceptual and Technical Approach over a Two-Decade Period. Plast Reconstr Surg 2016;138:1-11. [Crossref] [PubMed]
- Cavalcante FP, Lima TO, Alcantara R, et al. Immediate prepectoral versus submuscular breast reconstruction in nipple-sparing mastectomy: a retrospective cohort analysis. Rev Bras Ginecol Obstet 2024;46:e-rbgo76.
- Urban C, González E, Fornazari A, et al. Prepectoral Direct-to-Implant Breast Reconstruction without Placement of Acellular Dermal Matrix or Mesh after Nipple-Sparing Mastectomy. Plast Reconstr Surg 2022;150:973-83. [Crossref] [PubMed]
- Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation: a multicenter analysis of long-term health-related quality of life and satisfaction. Ann Surg Oncol 2014;21:2159-64. [Crossref] [PubMed]
- Fisher B, Redmond C. Systemic therapy in node-negative patients: updated findings from NSABP clinical trials. National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst Monogr 1992;105-16. [PubMed]
- Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer 2012;118:1982-8. [Crossref] [PubMed]
- Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018;19:27-39. [Crossref] [PubMed]
- Cavalcante FP, Millen EC, Zerwes FP, et al. Progress in Local Treatment of Breast Cancer: A Narrative Review. Rev Bras Ginecol Obstet 2020;42:356-64. [Crossref] [PubMed]
- Mamtani A, Sevilimedu V, Le T, et al. Is local recurrence higher among patients who downstage to breast conservation after neoadjuvant chemotherapy? Cancer 2022;128:471-8. [Crossref] [PubMed]
- Millen EC, Cavalcante FP, Zerwes F, et al. The Attitudes of Brazilian Breast Surgeons on Axillary Management in Early Breast Cancer-10 Years after the ACOSOG Z0011 Trial First Publication. Ann Surg Oncol 2022;29:1087-95. [Crossref] [PubMed]
- Cavalcante FP, Araújo MMP, Veras IM, et al. Oncological Outcomes of Nipple-Sparing Mastectomy in an Unselected Population Evaluated in a Single Center. Rev Bras Ginecol Obstet 2022;44:1052-8. [Crossref] [PubMed]
- Cavalcante FP, Lima MVA. Nipple-sparing mastectomy with periareolar incision and two-stage reconstruction: Initial analysis of 31 cases. Breast J 2018;24:940-3. [Crossref] [PubMed]
- Cavalcante FP, Lima TO, Alcantara R, et al. Inframammary versus Periareolar Incision: A Comparison of Early Complications in Nipple-sparing Mastectomy. Plast Reconstr Surg Glob Open 2023;11:e5367. [Crossref] [PubMed]
- Galimberti V, Morigi C, Bagnardi V, et al. Oncological Outcomes of Nipple-Sparing Mastectomy: A Single-Center Experience of 1989 Patients. Ann Surg Oncol 2018;25:3849-57. [Crossref] [PubMed]
- Lanitis S, Tekkis PP, Sgourakis G, et al. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg 2010;251:632-9. [Crossref] [PubMed]
- Frey JD, Salibian AA, Choi M, et al. Optimizing Outcomes in Nipple-sparing Mastectomy: Mastectomy Flap Thickness Is Not One Size Fits All. Plast Reconstr Surg Glob Open 2019;7:e2103. [Crossref] [PubMed]
- Torresan RZ, dos Santos CC, Okamura H, et al. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12:1037-44. [Crossref] [PubMed]
- Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg 2006;118:603-10; discussion 611-3. [Crossref] [PubMed]
- Jepsen C, Hallberg H, Pivodic A, et al. Complications, patient-reported outcomes, and aesthetic results in immediate breast reconstruction with a dermal sling: A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2019;72:369-80. [Crossref] [PubMed]
- de Oliveira-Junior I, da Costa Vieira RA, Biller G, et al. Factors associated with unsatisfactory cosmetic results in oncoplastic surgery. Front Oncol 2023;13:1071127. [Crossref] [PubMed]
- Piper ML, Nathan S, Henderson S, et al. The Impact of a Single Dual-Trained Surgeon in the Management of Mastectomy and Reconstruction. Plast Reconstr Surg 2022;149:820-8. [Crossref] [PubMed]
- Karamchandani MM, De La Cruz Ku G, Gaffney KA, et al. Single Versus Dual Surgeon Approaches to Oncoplastic Surgery: A Comparison of Outcomes. J Surg Res 2023;283:1064-72. [Crossref] [PubMed]
- Spring LM, Fell G, Arfe A, et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin Cancer Res 2020;26:2838-48. [Crossref] [PubMed]
- Clough KB, Lewis JS, Couturaud B, et al. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003;237:26-34. [Crossref] [PubMed]
- Almeida NR, Brenelli FP, Dos Santos CC, et al. Comparative study of surgical and oncological outcomes in oncoplastic versus non oncoplastic breast-conserving surgery for breast cancer treatment. JPRAS Open 2021;29:184-94. [Crossref] [PubMed]
- André C, Holsti C, Svenner A, et al. Recurrence and survival after standard versus oncoplastic breast-conserving surgery for breast cancer. BJS Open 2021;5:zraa013. [Crossref] [PubMed]
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol 2012;38:395-8. [Crossref] [PubMed]
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: Long term follow-up of a large series at a single institution: A matched-cohort analysis. Eur J Surg Oncol 2016;42:71-7. [Crossref] [PubMed]
- Fitoussi AD, Berry MG, Famà F, et al. Oncoplastic breast surgery for cancer: analysis of 540 consecutive cases Plast Reconstr Surg 2010;125:454-62. [outcomes article]. [Crossref] [PubMed]
- Fitzal F, Bolliger M, Dunkler D, et al. Retrospective, Multicenter Analysis Comparing Conventional with Oncoplastic Breast Conserving Surgery: Oncological and Surgical Outcomes in Women with High-Risk Breast Cancer from the OPBC-01/iTOP2 Study. Ann Surg Oncol 2022;29:1061-70. [Crossref] [PubMed]
- Hing JX, Kang BJ, Keum HJ, et al. Long-term oncological outcomes of oncoplastic breast-conserving surgery after a 10-year follow-up - a single center experience and systematic literature review. Front Oncol 2022;12:944589. [Crossref] [PubMed]
- Kelemen P, Pukancsik D, Újhelyi M, et al. Comparison of clinicopathologic, cosmetic and quality of life outcomes in 700 oncoplastic and conventional breast-conserving surgery cases: A single-centre retrospective study. Eur J Surg Oncol 2019;45:118-24. [Crossref] [PubMed]
- Rose M, Svensson H, Handler J, et al. Oncoplastic Breast Surgery Compared to Conventional Breast-Conserving Surgery With Regard to Oncologic Outcome. Clin Breast Cancer 2019;19:423-432.e5. [Crossref] [PubMed]
- Mansell J, Weiler-Mithoff E, Stallard S, et al. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast 2017;32:179-85. [Crossref] [PubMed]
- Clough KB, van la Parra RFD, Thygesen HH, et al. Long-term Results After Oncoplastic Surgery for Breast Cancer: A 10-year Follow-up. Ann Surg 2018;268:165-71. [Crossref] [PubMed]
- Carter SA, Lyons GR, Kuerer HM, et al. Operative and Oncologic Outcomes in 9861 Patients with Operable Breast Cancer: Single-Institution Analysis of Breast Conservation with Oncoplastic Reconstruction. Ann Surg Oncol 2016;23:3190-8. [Crossref] [PubMed]
- Chen JY, Huang YJ, Zhang LL, et al. Comparison of Oncoplastic Breast-Conserving Surgery and Breast-Conserving Surgery Alone: A Meta-Analysis. J Breast Cancer 2018;21:321-9. [Crossref] [PubMed]
- Kosasih S, Tayeh S, Mokbel K, et al. Is oncoplastic breast conserving surgery oncologically safe? A meta-analysis of 18,103 patients. Am J Surg 2020;220:385-92. [Crossref] [PubMed]
- Angarita FA, Acuna SA, Cordeiro E, et al. Does oncoplastic surgery increase immediate (30-day) postoperative complications? An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Breast Cancer Res Treat 2020;182:429-38. [Crossref] [PubMed]
- O'Connell RL, Baker E, Trickey A, et al. Current practice and short-term outcomes of therapeutic mammaplasty in the international TeaM multicentre prospective cohort study. Br J Surg 2018;105:1778-92. [Crossref] [PubMed]
- Nanda A, Hu J, Hodgkinson S, et al. Oncoplastic breast-conserving surgery for women with primary breast cancer. Cochrane Database Syst Rev 2021;10:CD013658. [PubMed]
- Witmer TJK, Kouwenberg CAE, Bargon CA, et al. Comparing costs of standard Breast-Conserving Surgery to Oncoplastic Breast-Conserving Surgery and Mastectomy with Immediate two-stage Implant-Based Breast Reconstruction. J Plast Reconstr Aesthet Surg 2022;75:2569-76. [Crossref] [PubMed]
- de Andrade Urban C. New classification for oncoplastic procedures in surgical practice. Breast 2008;17:321-2. [Crossref] [PubMed]
- Arnaout A, Ross D, Khayat E, et al. Position statement on defining and standardizing an oncoplastic approach to breast-conserving surgery in Canada. Curr Oncol 2019;26:e405-9. [Crossref] [PubMed]
- Chatterjee A, Gass J, Patel K, et al. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann Surg Oncol 2019;26:3436-44. [Crossref] [PubMed]
- Hoffmann J, Wallwiener D. Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of 1225 operations in 1166 patients. BMC Cancer 2009;9:108. [Crossref] [PubMed]
- Wormald JC, Wade RG, Figus A. The increased risk of adverse outcomes in bilateral deep inferior epigastric artery perforator flap breast reconstruction compared to unilateral reconstruction: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg 2014;67:143-56. [Crossref] [PubMed]
- Graziano FD, Plotsker EL, Rubenstein RN, et al. National Trends in Acellular Dermal Matrix Utilization in Immediate Breast Reconstruction. Plast Reconstr Surg 2024;153:25e-36e. [PubMed]
- Tierney BP, De La Garza M, Jennings GR, et al. Clinical Outcomes of Acellular Dermal Matrix (SimpliDerm and AlloDerm Ready-to-Use) in Immediate Breast Reconstruction. Cureus 2022;14:e22371. [Crossref] [PubMed]
- Liu J, Hou J, Li Z, et al. Efficacy of Acellular Dermal Matrix in Capsular Contracture of Implant-Based Breast Reconstruction: A Single-Arm Meta-analysis. Aesthetic Plast Surg 2020;44:735-42. [Crossref] [PubMed]
- Lee KT, Mun GH. A Meta-analysis of Studies Comparing Outcomes of Diverse Acellular Dermal Matrices for Implant-Based Breast Reconstruction. Ann Plast Surg 2017;79:115-23. [Crossref] [PubMed]
- Acea Nebril B, García Novoa A, García Jiménez L, et al. Immediate breast reconstruction by prepectoral polyurethane implant: Preliminary results of the prospective study PreQ-20. Cir Esp (Engl Ed) 2023;101:187-97. [Crossref] [PubMed]
- Correia-Pinto JM, Poleri F, Barbosa JP, et al. Comparing Polyurethane and Acellular Dermal Matrix Implant Cover in Prepectoral Breast Reconstruction: Short-term Complications. Plast Reconstr Surg Glob Open 2023;11:e4798. [Crossref] [PubMed]
- Coyette M, Coulie J, Lentini A, et al. Prepectoral immediate breast reconstruction with polyurethane foam-coated implants: Feasibility and early results in risk-reducing and therapeutic mastectomies. J Plast Reconstr Aesthet Surg 2021;74:2876-84. [Crossref] [PubMed]
- de Vita R, Villanucci A, Buccheri EM, et al. Extended Clinical Experience With Nipple-Sparing Mastectomy and Prepectoral Polyurethane Implant Positioning (BRAND4P method). Clin Breast Cancer 2022;22:e623-8. [Crossref] [PubMed]
- Nava MB, Blondeel P, Botti G, et al. International Expert Panel Consensus on Fat Grafting of the Breast. Plast Reconstr Surg Glob Open 2019;7:e2426. [Crossref] [PubMed]
- Strong AL, Cederna PS, Rubin JP, et al. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast Reconstr Surg 2015;136:897-912. [Crossref] [PubMed]
- Goto H, Shimono Y, Funakoshi Y, et al. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene 2019;38:767-79. [Crossref] [PubMed]
- Kronowitz SJ, Mandujano CC, Liu J, et al. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast Reconstr Surg 2016;137:385-93. [Crossref] [PubMed]
- Tukiama R, Vieira RAC, Moura ECR, et al. Oncologic safety of breast reconstruction with autologous fat grafting: A systematic review and meta-analysis. Eur J Surg Oncol 2022;48:727-35. [Crossref] [PubMed]
- Vieira RADC, Bailão-Junior A, de Oliveira-Junior I. Does breast oncoplastic surgery improve quality of life? Front Oncol 2022;12:1099125. [Crossref] [PubMed]
- Panayi AC, Knoedler S, Knoedler L, et al. Patient-reported Outcomes Utilizing the BREAST-Q Questionnaire After Breast-Conserving Surgery With and Without Oncoplastic Breast Surgery: A Systematic Review and Meta-analysis. Aesthet Surg J 2024;44:NP778-89. [Crossref] [PubMed]
- Vieira RADC, Paulinelli RR, de Oliveira-Junior I. Extreme oncoplasty: past, present and future. Front Oncol 2023;13:1215284. [Crossref] [PubMed]
- Gulcelik MA, Dogan L. Feasibility of level II oncoplastic techniques in the surgical management of locally advanced breast cancer after neoadjuvant treatment. Int J Clin Pract 2021;75:e13987. [Crossref] [PubMed]
- Paulinelli RR, de Oliveira VM, Bagnoli F, et al. Oncoplastic mammaplasty with geometric compensation--a technique for breast conservation. J Surg Oncol 2014;110:912-8. [Crossref] [PubMed]
- Silverstein MJ, Savalia N, Khan S, et al. Extreme oncoplasty: breast conservation for patients who need mastectomy. Breast J 2015;21:52-9. [Crossref] [PubMed]
- Deo SV, Purkayastha J, Shukla NK, et al. Myocutaneous versus thoraco-abdominal flap cover for soft tissue defects following surgery for locally advanced and recurrent breast cancer. J Surg Oncol 2003;83:31-5. [Crossref] [PubMed]
- Persichetti P, Brunetti B, Cagli B, et al. Chest wall reconstruction with the perforator-plus thoracoabdominal flap. Plast Reconstr Surg 2012;130:488e-9e. [Crossref] [PubMed]
- Vieira RADC, da Silva KMT, de Oliveira-Junior I, et al. ITADE flap after mastectomy for locally advanced breast cancer: A good choice for mid-sized defects of the chest wall, based on a systematic review of thoracoabdominal flaps. J Surg Oncol 2017;115:949-58. [Crossref] [PubMed]
- Vieira RADC, Ribeiro LM, Carrara GFA, et al. Effectiveness and Safety of Implant-Based Breast Reconstruction in Locally Advanced Breast Carcinoma: A Matched Case-Control Study. Breast Care (Basel) 2019;14:200-10. [Crossref] [PubMed]
- Vieira RA, Carrara GF, Scapulatempo Neto C, et al. The role of oncoplastic breast conserving treatment for locally advanced breast tumors. A matching case-control study. Ann Med Surg (Lond) 2016;10:61-8. [Crossref] [PubMed]
- Baroudi R, Pinotti JA, Keppke EM. A transverse thoracoabdominal skin flap for closure after radical mastectomy. Plast Reconstr Surg 1978;61:547-54. [Crossref] [PubMed]
- Lin YN, Ou-Yang F, Hsieh MC, et al. Use of Extended Pedicled Transverse Rectus Abdominis Myocutaneous Flap for Extensive Chest Wall Defect Reconstruction After Mastectomy for Locally Advanced Breast Cancer. Ann Plast Surg 2020;84:S34-9. [Crossref] [PubMed]
- Munhoz AM, Montag E, Arruda E, et al. Immediate locally advanced breast cancer and chest wall reconstruction: surgical planning and reconstruction strategies with extended V-Y latissimus dorsi myocutaneous flap. Plast Reconstr Surg 2011;127:2186-97. [Crossref] [PubMed]
- Song D, Liu D, Pafitanis G, et al. Extensive Microsurgical Reconstruction of Chest Wall Defects for Locally Advanced Breast Cancer: A 10-Year Single-Unit Experience. Ann Plast Surg 2020;84:293-9. [Crossref] [PubMed]
- De Lorenzi F, Di Bella J, Maisonneuve P, et al. Oncoplastic breast surgery for the management of ductal carcinoma in situ (DCIS): is it oncologically safe? A retrospective cohort analysis. Eur J Surg Oncol 2018;44:957-62. [Crossref] [PubMed]
- Mason EJ, Di Leone A, Franco A, et al. Oncoplastic Breast Surgery versus Conservative Mastectomy in the Management of Large Ductal Carcinoma In Situ (DCIS): Surgical, Oncological, and Patient-Reported Outcomes. Cancers (Basel) 2022;14:5624. [Crossref] [PubMed]
- Skjerven HK, Myklebust EM, Korvald C, et al. Long-term follow-up of complex oncoplastic breast-conserving surgery, standard breast conservation and skin-sparing mastectomy in DCIS - a register-based study. Eur J Surg Oncol 2024;50:107938. [Crossref] [PubMed]
- van la Parra RFD, Clough KB, Lejalle-Alaeddine C, et al. Oncoplastic Level 2 Mammoplasty for Large DCIS: 5-Year Results. Ann Surg Oncol 2019;26:2459-65. [Crossref] [PubMed]
- Adamson K, Chavez-MacGregor M, Caudle A, et al. Neoadjuvant Chemotherapy does not Increase Complications in Oncoplastic Breast-Conserving Surgery. Ann Surg Oncol 2019;26:2730-7. [Crossref] [PubMed]
- Varghese J, Gohari SS, Rizki H, et al. A systematic review and meta-analysis on the effect of neoadjuvant chemotherapy on complications following immediate breast reconstruction. Breast 2021;55:55-62. [Crossref] [PubMed]
- Ahmed GA, Baron DH, Agrawal A. Oncologic and cosmetic outcomes of oncoplastic breast-conserving surgery after neoadjuvant systemic therapy: systematic review and meta-analysis. Breast Cancer Res Treat 2025;209:229-52. [Crossref] [PubMed]
- Carstensen L, Rose M, Bentzon N, et al. Knowledge and opinions on oncoplastic surgery among breast and plastic surgeons. Dan Med J 2015;62:A5030. [PubMed]
- Challoner T, Skillman J, Wallis K, et al. Oncoplastic techniques: Attitudes and changing practice amongst breast and plastic surgeons in Great Britain. Breast 2017;34:58-64. [Crossref] [PubMed]
- Chatterjee A, Gass J, Burke MB, et al. Results from the American Society of Breast Surgeons Oncoplastic Surgery Committee 2017 Survey: Current Practice and Future Directions. Ann Surg Oncol 2018;25:2790-4. [Crossref] [PubMed]
- Davies C, Whisker L, Skillman J, et al. Current practice and provision of oncoplastic breast-conserving surgery in the UK: results of the ANTHEM national practice questionnaire. Breast Cancer Res Treat 2023;200:163-70. [Crossref] [PubMed]
- Emiroğlu M, Sert İ, İnal A, et al. The approach of general surgeons to oncoplastic and reconstructive breast surgery in Turkey: a survey of practice patterns. Balkan Med J 2014;31:307-12. [Crossref] [PubMed]
- Heil J, Riedel F, Solbach C, et al. Oncoplastic breast-conserving surgery: More relevant than ever? Results of a survey among breast surgeons. Arch Gynecol Obstet 2019;299:1109-14. [Crossref] [PubMed]
- Yang B, Ren G, Song E, et al. Current Status and Factors Influencing Surgical Options for Breast Cancer in China: A Nationwide Cross-Sectional Survey of 110 Hospitals. Oncologist 2020;25:e1473-80. [Crossref] [PubMed]
- Rubio IT, Wyld L, Esgueva A, et al. Variability in breast cancer surgery training across Europe: An ESSO-EUSOMA international survey. Eur J Surg Oncol 2019;45:567-72. [Crossref] [PubMed]
- Businaro Fernandes João T, de Oliveira VM, Bagnoli F, et al. How well are Brazilian mastologists (breast surgeons) trained in breast reconstruction and oncoplastic surgery? A study of the impact of a breast reconstruction and oncoplastic surgery improvement course. Front Oncol 2023;13:1139461. [Crossref] [PubMed]
- Karakatsanis A, Sund M, Rocco N, et al. Chest wall perforator flaps for breast reconstruction: international survey on attitudes and training needs. Br J Surg 2023;110:966-72. [Crossref] [PubMed]
- Sclafani LM, Bleznak A, Kelly T, et al. Training a new generation of breast surgeons: are we succeeding? Ann Surg Oncol 2012;19:1856-61. [Crossref] [PubMed]
- Cataliotti L, De Wolf C, Holland R, et al. Guidelines on the standards for the training of specialised health professionals dealing with breast cancer. Eur J Cancer 2007;43:660-75. [Crossref] [PubMed]
- Hassan AT, Urban CA, Facina G, et al. Training in oncoplastic surgery for mastologists. Rev Assoc Med Bras (1992) 2024;70:e2024S119.
- Marcasciano M, Kaciulyte J, Mori FLR, et al. Breast surgeons updating on the thresholds of COVID-19 era: results of a multicenter collaborative study evaluating the role of online videos and multimedia sources on breast surgeons education and training. Eur Rev Med Pharmacol Sci 2020;24:7845-54. [PubMed]
- Wilson AR, Marotti L, Bianchi S, et al. The requirements of a specialist Breast Centre. Eur J Cancer 2013;49:3579-87. [Crossref] [PubMed]
- Cavalcante FP, Novita GG, Paulinelli RR, et al. Factors Associated with the Practice of Oncoplastic Surgery among Breast Surgeons in Brazil: a National Survey. SABCS (Poster) 2024. Available online: https://sabcs.multilearning.com/sabcs/2024/breast-cancer-symposium/4148440/francisco.pimentel.cavalcante.p4.07.13.factors.associated.with.the.practice.of.html?f=listing%3D4%2Abrowseby%3D8%2Asortby%3D2%2Amedia%3D3%2Aspeaker%3D1026616
- Freitas-Júnior R, Gagliato DM, Moura Filho JWC, et al. Trends in breast cancer surgery at Brazil's public health system. J Surg Oncol 2017;115:544-9. [Crossref] [PubMed]
- Reid A, Thomas R, Pieri A, et al. The impact of advanced oncoplastic surgery on breast-conserving surgery rates: A retrospective cohort study of 3,875 breast cancer procedures at a tertiary referral centre. Breast 2024;78:103814. [Crossref] [PubMed]


