
Clinical significance of tumor infiltration length along the bile duct mucosa and submucosa in hilar cholangiocarcinoma
Highlight box
Key findings
• The average infiltration length in the proximal bile duct mucosa and submucosa in hilar cholangiocarcinoma (HCCA) patients were 8.5±5.2 and 8.6±4.9 mm, while the average infiltration length in the distal bile duct mucosa and submucosa were 12.8±7.5 and 11.5±7.2 mm, respectively. Preoperative imaging often underestimates the true extent of tumor invasion in HCCA. Extensive hepatectomy, involving the resection of more than half of the liver, can increases the R0 resection rate.
What is known and what is new?
• Radical resection remains the mainstay of treatment for patients with HCCA, however, the scope of resection remains controversial.
• This study introduces a novel ink marking method for accurate tumor infiltration assessment, revealing the limitations of imaging and the need for more aggressive surgery.
What is the implication, and what should change now?
• The findings suggest that surgeons should revise surgical strategies for HCCA based on a more comprehensive understanding of tumor infiltration, considering extensive hepatectomy as the preferred method to ensure negative margins (R0 resection). Larger, multi-center studies are needed to validate these findings and evaluate the impact of extensive resection on postoperative complications and survival rates.
Introduction
Hilar cholangiocarcinoma (HCCA) is the most common subtype of cholangiocarcinoma, with a high degree of malignancy. If untreated, the median overall survival is only 5–10 months (1-3). At present, radical resection is the only way to achieve long-term survival and potential cure (4). The past decade has witnessed unprecedented advances in surgical technology and imaging diagnosis; about 40% of patients with HCCA can undergo radical resection, and the 5-year survival rate has been significantly improved (5-7). Bile duct margin is an important risk factor for recurrence and survival of HCCA patients after resection (8). Computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP) are the most commonly used imaging method for preoperative evaluation of tumor location and extent to achieve a negative margin (R0) for HCCA. Although modern imaging techniques provide more details to identify the extent of tumors, they are only more sensitive to space-occupying lesions (9,10). However, imaging may seriously underestimate the longitudinal invasion of HCCA, which can be explained by the spread of the tumor along the bile duct wall with microinvasion (10). Although a frozen section of the bile duct margin is commonly performed during surgery to confirm that the margin is negative, overwhelming evidence substantiates that the accuracy of frozen sections is only between 57% and 90% compared with permanent histopathology (11,12).
Over the years, the surgical approach for HCCA has gradually changed from simple cholangiectomy to combined hepatectomy, although the optimal scope of resection remains controversial. An increasing body of evidence suggests that extensive hepatectomy, including more than half of the liver, should be performed for patients with Bismuth types I, II, III and IV (13,14). However, some researchers argue that liver tissue should be removed as little as possible, and partial hepatectomy should be adopted to reduce surgical complications and perioperative mortality (15). In this respect, for patients with Bismuth type I and type II, many surgeons think that visibility of the scope of the tumor is limited on imaging (16,17). For such cases, local or extrahepatic cholangiectomy is recommended by Chinese guidelines and experts consensus (18-21). According to the anatomical characteristics of the bile duct, the average lengths of the left, right and common hepatic ducts are 1.7, 0.9, and 2.5 cm, and the length of the common bile duct is 4–8 cm (22,23). Current evidence suggests that when HCCA infiltrates along the bile duct, it is likely to extend beyond the left and right hepatic ducts to the secondary bile duct in the liver and may also involve the pancreatic segment bile duct distally. A European study on the evaluation of the pathological report of HCCA also pointed out that the distance between the tumor and the resection margin of the bile duct should be described in the pathological report, which is helpful for a comprehensive assessment of the prognosis and selection of adjuvant treatment (24). However, there is a lack of accurate measurement methods during pathological sampling of HCCA domestically and internationally.
Clinically, surgeons place much emphasis on the boundary of HCCA infiltrating along the bile duct and the scope of surgical resection to achieve R0 resection. However, few studies have comprehensively reported the infiltration length of HCCA along the bile duct mucosa and submucosa, and many errors have been reported with previous measurement methods. Herein, we adopted original ink marking and measurement methods to make the results more objective and accurate and discussed the effect of the infiltration length of HCCA along the bile duct mucosa and submucosa on radical surgery. We present this article in accordance with the STROBE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-123/rc).
Methods
Patients
Thirty-one patients with HCCA who underwent en bloc and extended liver resection in the Department of Biliary-Pancreatic Surgery of Sun Yat-sen Memorial Hospital from January 2020 to December 2021 were included in this study. The inclusion criteria were as follows: (I) HCCA was diagnosed by preoperative examination and postoperative pathology, (II) the first operation was performed in the Sun Yat-sen Memorial Hospital, and all patients underwent en bloc and extended liver resection combined with total caudate lobe and extrahepatic bile duct. Patients were excluded for the following reasons: (I) preoperative radiotherapy, chemotherapy and other anti-tumor treatments; (II) the postoperative specimens were not marked and measured in accordance with the indicated methods; (III) missing clinical or pathological data. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. SYSKY-2022-124-01) and individual consent for this retrospective analysis was waived.
Perioperative period and surgical procedure
The ratio of residual liver volume to standard liver volume was routinely calculated by CT before operation (25). After the serum total bilirubin concentration was restored to nearly normal levels by biliary drainage, the liver function was evaluated by the indocyanine green clearance test (26). Selective portal vein embolization (PVE) was performed preoperatively in patients without sufficient residual liver volume or liver function for safe resection (27).
All operations were performed by the same experienced surgeon. The basic surgical procedures included en bloc and extended liver resection, including at least half of the liver volume combined with total caudate lobe and extrahepatic bile duct, regional lymph node dissection and Roux-en-Y choledochojejunostomy. Resection and reconstruction of the portal vein and/or hepatic artery were conducted when hilar vessels adhered to the tumor and could not be separated from the tumor (28). If the pancreatic segment of the common bile duct was involved and the peripancreaticoduodenal metastatic lymph nodes invaded the head of the pancreas, combined pancreaticoduodenectomy was indicated (29).
Specimen handling and pathological evaluation
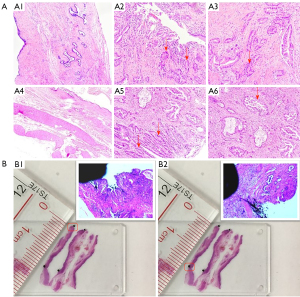
All the resected specimens were treated immediately after removal from the patient. In order to determine the location of the tumor, the main bile duct in the hilar region was dissected longitudinally along the anterior wall (Figure 1A). The branches of intrahepatic bile ducts were named according to the classification system of bile ducts (30). Then, the proximal and distal bile ducts were sampled, including the gross tumor margin and the bile duct resection margin. The ink was absorbed with a 2 mL syringe to mark the gross tumor boundary and bile duct resection margin (Figure 1B). Each segment of the bile duct was fixed on a separate foam plate with a No. 3 insect nail to prevent curling and deformation in the formalin solution. Then, it was placed in a specimen box filled with 10% formalin solution and the box was labeled with the specimen name. The procedure was performed by the surgeon, who took photos and videos of the whole process. After 24 hours of fixation, the pathologist characterized the gross findings of the tumor and measured the length (t1) of the gross tumor boundary from the proximal and distal bile ducts, respectively. The proximal and distal bile duct specimens was embedded in paraffin and serially sectioned longitudinally at intervals of 1–2 mm along the two ink marks. After staining, the length (t2; Figure 1C) between the microscopic margin of the tumor and the margin of the proximal and distal bile duct marked with ink was measured. The length (d = t1 − t2; Figure 1D) of HCCA infiltration along the proximal and distal bile ducts beyond the gross tumor boundary could be calculated by the difference value. The bile duct wall was divided into the mucous and the submucous layers. According to the above method, the infiltration distance of each proximal and distal bile duct wall layer could be obtained (Figure 2A). To assess whether the specimens fixed with insect nails would undergo significant shortening and deformation, we calculated the ratio between the length of the two inks under a microscope. The ratio of the length of the two inks under the naked eye was between 0.9 and 0.98 (Figure 2B). The above measurements were performed by the same two pathologists.
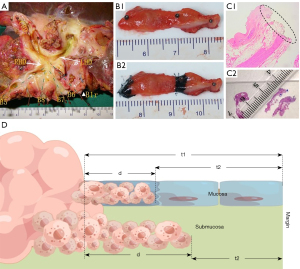
Clinical parameters and definitions
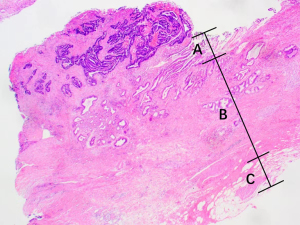
The bile duct wall is divided into mucosal epithelium, lamina propria and adventitia layer (Figure 3). The mucosal epithelium is mainly composed of monolayer columnar epithelial cells, and the lamina propria and adventitia are loose connective tissue, including blood vessels, lymphatic vessels, nerves and elastic fibers. Some smooth muscle layers are scattered or continuously distributed between the lamina propria and the adventitia, mostly in the extrahepatic bile duct, especially in the lower segment of the common bile duct, while there is little or no muscle layer in the intrahepatic secondary bile duct (31). We defined the mucous layer in this study as the mucosal epithelium; the submucous layer implied the lamina propria and adventitia beneath the mucosal epithelium, including the smooth muscle layer, if present.
Statistical analysis
The data were analyzed and processed by SPSS statistical software (IBM Statistics 25.0). The categorical variables were described as frequency and percentage, and continuous variables as mean, standard deviation, minimum and maximum. A P value <0.05 was statistically significant.
Results
Baseline data
A total of 31 patients with HCCA were included in the study, including 15 males and 16 females, with a mean age of 61.7±11.1 years. Table 1 summarizes the clinicopathological features of the patients, including the extent of hepatectomy, gross type, degree of differentiation and TNM stage, etc.
Table 1
| Variables | Results |
|---|---|
| Age (years) | 61.7±11.1 |
| Male/female | 15/16 |
| Maximum diameter of tumor (cm) | 3.1±1.3 |
| Underwent preoperative biliary drainage | 23 (74.2) |
| Total bilirubin before preoperative biliary drainage (μmol/L) | 170.6±145.9 |
| Duration of preoperative biliary drainage (days) | 23.0±21.5 |
| Preoperative portal vein embolism | 9 (29.0) |
| Extent of hepatectomy | |
| Left hemihepatectomy | 3 (9.7) |
| Right hemihepatectomy | 2 (6.5) |
| Extended left hemihepatectomy | 13 (41.9) |
| Extended right hemihepatectomy | 10 (32.3) |
| Left trisectionectomy | 1 (3.2) |
| Right trisectionectomy | 2 (6.5) |
| Combined vascular resection and reconstruction | 12 (38.7) |
| Combined pancreaticoduodenectomy | 2 (6.5) |
| Gross type | |
| Sclerotic type | 14 (45.2) |
| Nodular/mass + sclerotic type | 14 (45.2) |
| Mass type | 1 (3.2) |
| Nipple type | 2 (6.5) |
| Degree of differentiation | |
| Low | 13 (41.9) |
| Medium | 14 (45.2) |
| High | 4 (12.9) |
| TNM staging† | |
| I + II | 8 (25.8) |
| III + IV | 23 (74.2) |
| Preoperative Bismuth-Corlette classification | |
| II | 1 (3.2) |
| III | 19 (61.3) |
| IV | 11 (35.5) |
| Margin status | |
| R0/R1/R2 | 23 (74.2)/6 (19.4)/2 (6.5) |
Data are presented as mean ± standard deviation, n or n (%). †, according to the American Joint Committee on Cancer 8th edition staging manual (32).
Tumor infiltrating length along the bile duct
A total of 42 proximal and 31 distal bile ducts were evaluated in 31 surgically resected specimens, with an average of 1.4 proximal bile ducts per patient (range, 1–3 proximal bile ducts). If the bile duct margin was positive, it was calculated according to the maximum length of infiltration that could be observed. If there were two or more proximal bile ducts, the bile duct with the longest tumor invasion length was analyzed.
As shown in Figure 4, in most cases, the infiltration length along the proximal bile duct mucosa and submucosa and distal bile duct mucosa were within the 5–10 mm range. In most cases, the infiltration length of distal bile duct submucosa was within the 10–15 mm range. It was found that the average tumor infiltration length along the mucosa and the submucosa of the proximal bile duct was 8.5±5.2 and 8.6±4.9 mm, respectively, and the maximum length was 20.0 mm in both layers. The average infiltration length of the tumor along the mucous layer of the distal bile duct was 12.8±7.5 mm, with a maximum of 33.0 mm. In contrast, the average infiltration length along the submucosa of the distal bile duct was 11.5±7.2 mm, with a maximum of 32.0 mm. Table 2 summarizes the relationship between different pathological features and infiltration length.
Table 2
| Category | Proximal bile duct infiltration | Distal bile duct infiltration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mucosa | Submucosa | Mucosa | Submucosa | ||||||||
| N | Length (mm) | N | Length (mm) | N | Length (mm) | N | Length (mm) | ||||
| Resection range | |||||||||||
| Left liver† (n=17) | 12 | 8.8±6.7 (2.0–20.0) | 13 | 9.5±5.4 (4.0–20.0) | 8 | 13.2±7.5 (5.0–24.0) | 12 | 12.4±6.4 (3.0–24.0) | |||
| Right liver‡ (n=14) | 11 | 8.1±3.4 (5.0–16.6) | 11 | 7.6±4.2 (2.0–17.0) | 10 | 12.5±7.8 (5.0–33.0) | 11 | 10.5±8.3 (3.0–32.0) | |||
| Gross type | |||||||||||
| Sclerotic type (n=14) | 12 | 7.7±5.5 (2.0–20.0) | 11 | 9.0±5.0 (4.0–19.0) | 8 | 14.9±9.1 (6.0–33.0) | 10 | 12.7±8.4 (3.0–32.0) | |||
| Mixed type§ (n=14) | 9 | 9.0±4.9 (3.0–20.0) | 11 | 7.9±4.8 (2.0–20.0) | 8 | 11.1±5.6 (5.0–22.0) | 10 | 11.5±6.5 (4.0–24.0) | |||
| TNM staging | |||||||||||
| I + II (n=8) | 6 | 9.3±6.3 (3.0–16.6) | 6 | 9.7±5.5 (4.0–17.0) | 5 | 20.6±9.8 (6.0–33.0) | 5 | 18.4±9.4 (6.0–32.0) | |||
| III + IV (n=23) | 17 | 8.2±5.0 (2.0–20.0) | 18 | 8.3±4.8 (2.0–20.0) | 13 | 9.8±3.4 (5.0–17.0) | 18 | 9.6±5.4 (3.0–24.0) | |||
| Differentiation | |||||||||||
| Low (n=13) | 10 | 8.2±5.2 (2.0–20.0) | 9 | 8.7±5.0 (2.0–20.0) | 6 | 12.2±6.5 (5.0–22.0) | 8 | 9.6±4.7 (4.0–18.0) | |||
| Medium/high (n=18) | 13 | 8.7±5.5 (3.0–20.0) | 15 | 8.5±5.0 (4.0–19.0) | 12 | 13.2±8.2 (5.0–33.0) | 15 | 12.5±8.2 (3.0–32.0) | |||
Data are presented as mean ± SD (minimum to maximum). †, including left hemihepatectomy, enlarged left hemihepatectomy and left trefoil hepatectomy; ‡, including right hemihepatectomy, enlarged right hemihepatectomy and right trefoil hepatectomy; §, nodular/mass + sclerotic type. SD, standard deviation.
Changes in the Bismuth-Corlette classification
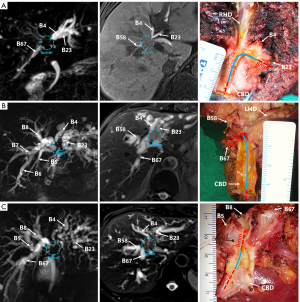
During preoperative imaging examination, according to the Bismuth-Corlette classification, 31 patients were classified as type II (n=1), type IIIa (n=10), type IIIb (n=9) and type IV (n=11). However, postoperative pathological results showed that 1 case of type II, 3 cases of type IIIa and 3 cases of type IIIb lesions were type IV lesions. Overall, the accuracy of preoperative imaging diagnosis for type III lesions was 68.4% (13/19). Figure 5 shows the extent of tumor infiltration during preoperative imaging examination and in resected specimens.
Margin status
Of the 31 patients in this study, 23 (74.2%) had histological evidence of non-invasive cancer at the incision margins (R0). Of the 17 patients who underwent extensive left hepatectomy [left hemihepatectomy (n=3), enlarged left hemihepatectomy (n=13) and left trisectionectomy (n=1)], 11 (64.7%) underwent R0 resection. Of the 14 patients who underwent extensive right hepatectomy [right hemihepatectomy (n=2), enlarged right hemihepatectomy (n=10) and right trisectionectomy (n=2)], 12 (85.7%) underwent R0 resection. Of the 18 patients with Bismuth IV confirmed by pathology, 12 (66.7%) achieved R0 resection after extensive hepatectomy. Table 3 summarizes the margin status of extensive hepatectomy. The main reason for non-R0 resection was positive margins of bile ducts and/or vessels.
Table 3
| Margin status | Extensive hepatectomy (n=31), n (%) | Left hepatectomy (n=17), n (%) | Right hepatectomy (n=14), n (%) | Extensive hepatectomy for Bismuth IV (n=18), n (%) |
|---|---|---|---|---|
| R0 | 23 (74.2) | 11 (64.7) | 12 (85.7) | 12 (66.7) |
| R1 | 6 (19.4) | 4 (23.5) | 2 (14.3) | 4 (22.2) |
| R2 | 2 (6.5) | 2 (11.8) | 0 | 2 (11.1) |
Discussion
Surgical resection represents the main curative approach for HCCA at present. It is well-established that the efficacy of surgical resection is closely related to the status of the bile duct margin. A negative bile duct margin can improve the survival rate of patients and reduce tumor recurrence (8). However, due to the biological characteristics of HCCA infiltration along the bile duct wall, especially the mucosa and submucosa, preoperative imaging and intraoperative frozen section examination cannot accurately judge the extent of the tumor (10,11). Therefore, it is necessary to explore the length of HCCA infiltration along the bile duct to select the surgical approach and the extent of resection.
In prior studies on the infiltration length of HCCA, the sampling or measurement methods were not described in detail, which led to doubts about the robustness of their conclusions (33-35). In this study, the original ink marking and measurement method was adopted; i.e., the surgeon marked the macroscopic tumor boundary and bile duct resection margin with ink before the fresh specimen was sent to the pathology department. Given that the surgeon could more clearly visualize the location of the macroscopic tumor boundary during the operation, he was responsible for the marking task. This marking method has two benefits: first, for short or irregular bile duct tissue, marking is more helpful for pathologists to distinguish the longitudinal axis of the bile duct to achieve full-length sampling and longitudinal section along the bile duct, which is the basis for measuring the length of infiltration. Moreover, it enables accurate recognition of the end of the cutting edge under the microscope to measure the length from the boundary of the microscopic tumor to the cutting edge. Since it is difficult to directly measure the length of tumor infiltration along the mucosa and submucosa of the bile duct beyond the macroscopic boundary under the microscope, we calculated the difference.
Sakamoto et al. found that the average infiltration lengths of proximal bile duct along the mucosa and submucosa were 11.5 and 6 mm, respectively (33), which is different from our findings. This discrepancy may be due to the different definitions used for the level and length of bile duct infiltration. In their study, the bile duct was divided into mucosal, submucosal-intramural, or submucosal-extramural layers. The latter two layers were equivalent to the submucosa referred to in our study. In addition, they defined the submucosal infiltration length as a cancerous extension of the submucosa without mucosal extension, and the average length measured was shorter than ours because the mucosal extension length was not taken into account. In a study by Ebata et al., the average infiltration lengths along the superficial and intramural proximal bile duct were 14 and 4.6 mm; the corresponding values for the distal bile duct were 13.4 and 4.4 mm, respectively (34). However, all types of cholangiocarcinoma, including HCCA, were analyzed in that study. Yamaguchi et al. reported that the average length between macroscopic and microscopic tumor boundaries was 6.1±6.1 mm for the proximal bile duct and 6.2±9.1 mm for the distal bile duct (36). However, they estimated the length by cholangiography without pathological evaluation, which is relatively less accurate. Hayashi et al. also pointed out that the average length of extramucosal infiltration of proximal and distal bile ducts was 12.8 and 6.1 mm, respectively (35). They measured the length of extramucosal infiltration beyond the extent of intramucosal carcinogenesis, and 5 mm-thick sections were obtained perpendicular to the longitudinal axis of the bile duct, which led to significant errors. This approach was significantly different from our method, involving the starting point of measurement and sectioning method. In this study, the length of infiltration was further compared between different sides of liver resection, gross types, TNM stages, and degrees of differentiation. However, more data are needed to verify the conclusion. Herein, we preliminarily substantiated the correlation between infiltration length and pathological features.
It was found that the maximum infiltration length of the proximal bile duct was 2 cm, the maximum infiltration length of the distal bile duct was 3.3 cm, and the average length of the left and right hepatic ducts were 1.7 and 0.9 cm, respectively. Since the common hepatic duct length was about 2.5 cm, and the common bile duct was about 4–8 cm, the tumor may invade the bile duct proximal to the secondary bile duct or distal to the pancreatic segment bile duct. From a surgical point of view, a study measured the length of resectable hepatic ducts in four types of hepatectomy (37). The average length of the resected hepatic duct was 14.1±5.7, 14.9±5.7, 21.3±6.4, and 25.1±6.4 mm during left hemihepatectomy, right hemihepatectomy, left trilobar hepatectomy and right trilobar hepatectomy, respectively. It can be seen that the length of hepatic duct resected by left and right hepatectomy was comparable, while the length of the hepatic duct was significantly shorter during hemihepatectomy than in trisectionectomy. Interestingly, right trisectionectomy was associated with the longest proximal hepatic duct margin. It was suggested that when the liver function is good and the remaining liver volume is estimated to be sufficient, right trisectionectomy should be the first choice for Bismuth IV, followed by left trisectionectomy (37). In our study, the maximum infiltration length of the mucosa and submucosa of the proximal bile duct was 20.0 mm. Accordingly, only trisectionectomy could ensure negative margins. In addition, combination with pancreaticoduodenectomy is indicated if the tumor infiltrates distal to the pancreatic segmental common bile duct.
The Bismuth-Corlette classification based on preoperative imaging is important for the selection of HCCA surgery (38). Among the 31 patients in this study, 7 initially classified as type II and III by preoperative imaging were classified as type IV by postoperative pathology. The accuracy of preoperative imaging diagnosis of type III was only 68.4% (13/19). It is conceivable that some patients diagnosed with type I or II on imaging may progress to type III or even type IV. Accordingly, extensive hepatectomy combined with hemihepatectomy is necessary, even for type I and II HCCA. It is widely thought that right hemihepatectomy combined with caudate lobectomy or extended right hemihepatectomy is indicated for patients with Bismuth type I, II and IIIa, left hemihepatectomy with caudate lobectomy for Bismuth IIIb, and extended hemihepatectomy with caudate lobectomy or trisectionectomy for Bismuth IV (13,14,39). Overwhelming evidence substantiates that patients with Bismuth type I and type II who undergo limited resection have a high local recurrence rate and a poor prognosis even after R0 resection (2,5). van Gulik et al. showed that the prognosis of patients with extended resection was significantly better than patients with local resection with similar tumor infiltration (40). While extended liver resection significantly improves survival rates, it also carries a higher risk of postoperative complications. Postoperative liver failure is one of the most common and fatal complications, particularly in patients with underlying liver diseases (25). Additionally, bile leakage is another important complication that may require drainage or reoperation (39). The incidence of postoperative infections is also high, especially in the context of liver failure, where the weakened immune function makes infection control more challenging (25). Studies have shown that preoperative PVE combined with biliary drainage can significantly reduce the incidence of liver failure, thus decreasing the risk of postoperative mortality (39,41,42). Therefore, balancing surgical outcomes with the risk of postoperative complications is crucial, requiring thorough preoperative evaluation and the implementation of preventive measures.
A series of studies have shown that the R0 resection rate of HCCA increases significantly with combined hepatectomy (43,44). Tsao et al. reported that 89% of patients undergoing hepatectomy at Nagoya University had an R0 resection rate of 79%, whereas only 16% of patients undergoing hepatectomy at Lahey Clinical Center had an R0 resection rate of 28% (45). Of the 386 cases of HCCA surgically resected at Nagoya University from 2001 to 2010, nearly 95% underwent extensive hepatectomy, and the R0 resection rate was about 77.5% (46). In our study, 31 patients underwent extensive hepatectomy, and the R0 resection rate was 74.2%, comparable to Nagoya University’s findings. For HCCA with positive margins, the significance of further resection remains subject to debate. Ribero et al. and Ma et al. reported a significant survival benefit with further resection (47,48). However, Endo et al. and Shingu et al. pointed out that further resection did not improve survival (49,50). Considering that the prognosis of re-resection is not optimistic, it is difficult to re-resect the proximal bile duct over 5 mm from an anatomical and technical point of view (34). Therefore, in patients with a good liver function reserve, direct extensive hepatectomy is indicated to minimize the risks of positive resection margins.
There are some limitations in this study. First, this was a single-center study, and there may be confounding factors that were not considered. Moreover, the number of patients in this study was limited. However, because HCCA is relatively rare, a small sample size is common in most studies of HCCA. Finally, our study focused on the proximal and distal margins of the bile duct and did not evaluate the radial margin of HCCA (the margin of the circumferential tumor adjacent to the liver, hilar plate, and blood vessels).
Conclusions
This study revealed the infiltration characteristics of HCCA along the bile duct mucosa and submucosa, and the infiltration length was measured. Indeed, preoperative imaging examination often underestimates the extent of HCCA. With extensive hepatectomy, longer bile ducts can be removed, and the R0 resection rate can be improved.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-123/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-123/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-123/prf
Funding: This work was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-123/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. SYSKY-2022-124-01) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-88. [Crossref] [PubMed]
- Padmanaban V, Ruff SM, Pawlik TM. Multi-Disciplinary Care of Hilar Cholangiocarcinoma: Review of Guidelines and Recent Advancements. Cancers (Basel) 2023;16:30. [Crossref] [PubMed]
- Gaspersz MP, Buettner S, van Vugt JLA, et al. Conditional survival in patients with unresectable perihilar cholangiocarcinoma. HPB (Oxford) 2017;19:966-71. [Crossref] [PubMed]
- van Keulen AM, Franssen S, van der Geest LG, et al. Nationwide treatment and outcomes of perihilar cholangiocarcinoma. Liver Int 2021;41:1945-53. [Crossref] [PubMed]
- Lauterio A, De Carlis R, Centonze L, et al. Current Surgical Management of Peri-Hilar and Intra-Hepatic Cholangiocarcinoma. Cancers (Basel) 2021;13:3657. [Crossref] [PubMed]
- Park MJ, Kim YK, Lim S, et al. Hilar cholangiocarcinoma: value of adding DW imaging to gadoxetic acid-enhanced MR imaging with MR cholangiopancreatography for preoperative evaluation. Radiology 2014;270:768-76. [Crossref] [PubMed]
- Ito F, Cho CS, Rikkers LF, et al. Hilar cholangiocarcinoma: current management. Ann Surg 2009;250:210-8. [Crossref] [PubMed]
- Saxena A, Chua TC, Chu FC, et al. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg 2011;202:310-20. [Crossref] [PubMed]
- Manfredi R, Barbaro B, Masselli G, et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis 2004;24:155-64. [Crossref] [PubMed]
- Senda Y, Nishio H, Oda K, et al. Value of multidetector row CT in the assessment of longitudinal extension of cholangiocarcinoma: correlation between MDCT and microscopic findings. World J Surg 2009;33:1459-67. [Crossref] [PubMed]
- Yamaguchi K, Shirahane K, Nakamura M, et al. Frozen section and permanent diagnoses of the bile duct margin in gallbladder and bile duct cancer. HPB (Oxford) 2005;7:135-8. [Crossref] [PubMed]
- Okazaki Y, Horimi T, Kotaka M, et al. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepatogastroenterology 2002;49:625-7. [PubMed]
- Nagino M, Kamiya J, Arai T, et al. "Anatomic" right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg 2006;243:28-32. [Crossref] [PubMed]
- Ikeyama T, Nagino M, Oda K, et al. Surgical approach to bismuth Type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg 2007;246:1052-7. [Crossref] [PubMed]
- Miyazaki M, Ito H, Nakagawa K, et al. Parenchyma-preserving hepatectomy in the surgical treatment of hilar cholangiocarcinoma. J Am Coll Surg 1999;189:575-83. [Crossref] [PubMed]
- Zhang XF, Zhang N, Tsilimigras DI, et al. Surgical Strategies for Bismuth Type I and II Hilar Cholangiocarcinoma: Impact on Long-Term Outcomes. J Gastrointest Surg 2021;25:3084-91. [Crossref] [PubMed]
- Chen RX, Li CX, Luo CH, et al. Surgical Strategies for the Treatment of Bismuth Type I and II Hilar Cholangiocarcinoma: Bile Duct Resection with or Without Hepatectomy? Ann Surg Oncol 2020;27:3374-82. [Crossref] [PubMed]
- Zheng SG, Xiang CH, Feng XB, et al. Guidelines for diagnosis and treatment of hilar cholangiocarcinoma (version 2013). Chinese Journal of Surgery 2013;51:865-71.
- Anti-cancer Association of Chin. Guideline for the diagnosis and therapy of hilar cholangim carcinoma (2015). Chinese Journal of Hepatobiliary Surgery 2015;21:505-11.
- Liang H, Qin S, Shen F, et al. CSCO Expert Consensus on Diagnosis and Treatment of Biliary Tract Tumors (2019). Chinese Clinical Oncology 2019;24:828-38.
- Chinese Chapter of International Hepato-Pancreato-Biliary Association. Diagnosis and treatment of cholangiocarcinoma: surgical expert consensus. Journal of Clinical Hepatology 2015;31:12-6.
- Healey JE Jr, Schroy PC. Anatomy of the biliary ducts within the human liver; analysis of the prevailing pattern of branchings and the major variations of the biliary ducts. AMA Arch Surg 1953;66:599-616. [Crossref] [PubMed]
- Skandalakis JE, Skandalakis LJ, Skandalakis PN, et al. Hepatic surgical anatomy. Surg Clin North Am 2004;84:413-35. viii. [Crossref] [PubMed]
- Chatelain D, Farges O, Fuks D, et al. Assessment of pathology reports on hilar cholangiocarcinoma: the results of a nationwide, multicenter survey performed by the AFC-HC-2009 study group. J Hepatol 2012;56:1121-8. [Crossref] [PubMed]
- Hammond JS, Guha IN, Beckingham IJ, et al. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188-200. [Crossref] [PubMed]
- Yokoyama Y, Ebata T, Igami T, et al. The Predictive Value of Indocyanine Green Clearance in Future Liver Remnant for Posthepatectomy Liver Failure Following Hepatectomy with Extrahepatic Bile Duct Resection. World J Surg 2016;40:1440-7. [Crossref] [PubMed]
- Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364-72. [Crossref] [PubMed]
- Ebata T, Ito T, Yokoyama Y, et al. Surgical technique of hepatectomy combined with simultaneous resection of hepatic artery and portal vein for perihilar cholangiocarcinoma (with video). J Hepatobiliary Pancreat Sci 2014;21:E57-61. [Crossref] [PubMed]
- Ebata T, Yokoyama Y, Igami T, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg 2012;256:297-305. [Crossref] [PubMed]
- Ohkubo M, Nagino M, Kamiya J, et al. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg 2004;239:82-6. [Crossref] [PubMed]
- Hong SM, Kang GH, Lee HY, et al. Smooth muscle distribution in the extrahepatic bile duct: histologic and immunohistochemical studies of 122 cases. Am J Surg Pathol 2000;24:660-7. [Crossref] [PubMed]
- Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7.
- Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg 1998;227:405-11. [Crossref] [PubMed]
- Ebata T, Watanabe H, Ajioka Y, et al. Pathological appraisal of lines of resection for bile duct carcinoma. Br J Surg 2002;89:1260-7. [Crossref] [PubMed]
- Hayashi S, Miyazaki M, Kondo Y, et al. Invasive growth patterns of hepatic hilar ductal carcinoma. A histologic analysis of 18 surgical cases. Cancer 1994;73:2922-9. [Crossref] [PubMed]
- Yamaguchi K, Chijiiwa K, Saiki S, et al. Carcinoma of the extrahepatic bile duct: mode of spread and its prognostic implications. Hepatogastroenterology 1997;44:1256-61. [PubMed]
- Hirose T, Igami T, Ebata T, et al. Surgical and Radiological Studies on the Length of the Hepatic Ducts. World J Surg 2015;39:2983-9. [Crossref] [PubMed]
- Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology 2011;53:1363-71. [Crossref] [PubMed]
- Kawasaki S, Imamura H, Kobayashi A, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg 2003;238:84-92. [Crossref] [PubMed]
- van Gulik TM, Kloek JJ, Ruys AT, et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol 2011;37:65-71. [Crossref] [PubMed]
- Sano T, Shimada K, Sakamoto Y, et al. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg 2006;244:240-7. [Crossref] [PubMed]
- Olthof PB, Aldrighetti L, Alikhanov R, et al. Portal Vein Embolization is Associated with Reduced Liver Failure and Mortality in High-Risk Resections for Perihilar Cholangiocarcinoma. Ann Surg Oncol 2020;27:2311-8. [Crossref] [PubMed]
- Dinant S, Gerhards MF, Rauws EA, et al. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol 2006;13:872-80. [Crossref] [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [Crossref] [PubMed]
- Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg 2000;232:166-74. [Crossref] [PubMed]
- Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013;258:129-40. [Crossref] [PubMed]
- Ribero D, Amisano M, Lo Tesoriere R, et al. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg 2011;254:776-81; discussion 781-3. [Crossref] [PubMed]
- Ma WJ, Wu ZR, Shrestha A, et al. Effectiveness of additional resection of the invasive cancer-positive proximal bile duct margin in cases of hilar cholangiocarcinoma. Hepatobiliary Surg Nutr 2018;7:251-69. [Crossref] [PubMed]
- Endo I, House MG, Klimstra DS, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol 2008;15:2104-12. [Crossref] [PubMed]
- Shingu Y, Ebata T, Nishio H, et al. Clinical value of additional resection of a margin-positive proximal bile duct in hilar cholangiocarcinoma. Surgery 2010;147:49-56. [Crossref] [PubMed]



