
Risk factors for bone metastasis in patients with prostate cancer: a retrospective study based on single-center data and SEER database
Highlight box
Key findings
• The present study ascertained that prostate-specific antigen (PSA), Gleason score and N stage served as prognostic indicators for bone metastasis in prostate cancer (PCa). Percentage of monocyte (M%) was an independent risk factor for bone metastasis in PCa. The primary histological grade in Gleason score played an important role in bone metastasis in PCa.
What is known and what is new?
• Gleason score, PSA, androgen level, obesity, genetics and age, etc., were found to be related to bone metastasis in PCa.
• M% was an independent risk factor for bone metastasis in PCa. The primary histological grade in Gleason score played an important role in bone metastasis in PCa.
What is the implication, and what should change now?
• The investigation of the significance of M% and the primary histological grade in the diagnosis of bone metastasis in PCa warranted further exploration.
Introduction
Prostate cancer (PCa) has consistently ranked as the most prevalent malignancy affecting the male urogenital system on a global scale (1,2). Despite notable progress in the realms of diagnosis and treatment, PCa continues to impose a substantial burden on public health, primarily due to its propensity for bone metastasis, which carries a heightened risk of mortality (3). Although bone metastasis in PCa is infrequent, its potential ramifications are severe, encompassing intense pain, pathological fractures, and a diminished quality of life (4,5). Consequently, it is imperative to investigate the risk factors associated with the development of bone metastasis in order to enhance our understanding of this phenomenon.
Notably, PCa bone metastasis typically arises from either local recurrence or distant metastasis (6). The spine, hips, ribs, and proximal femur bones were found to be the most frequently affected sites of bone metastasis (7,8). It was observed that bone metastasis often remained asymptomatic until reaching advanced stages, at which point it had a significant impact on both patient survival and quality of life (9). Numerous factors were found to contribute to the progression of this disease, including androgen level, obesity, genetics, age, ethnicity, and clinical stage at diagnosis (10-12). Furthermore, higher Gleason scores and elevated levels of prostate-specific antigen (PSA) at diagnosis were indicative of a more aggressive tumor biology and an increased likelihood of bone metastasis (13,14). Additionally, the neutrophil to lymphocyte ratio (NLR) and the platelet to lymphocyte ratio (PLR), both parameters of systemic inflammation, have recently been found to be linked to bone metastasis in PCa (15). However, further extensive research is required to gain a more comprehensive understanding of the risk factors involved.
In this study, data were collected at our center (The First Affiliated Hospital of University of Science and Technology of China) to investigate the independent risk factors for PCa with bone metastases. Additionally, an external validation set from the Surveillance, Epidemiology, and End Results (SEER) database, which includes a large population of PCa cases, was also analyzed to confirm these findings. We present this article in accordance with the STROBE reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-78/rc).
Methods
Study design and patients
A total of 177 patients presented to the medical services at the Department of Urology, The First Affiliated Hospital of University of Science and Technology of China, from January 2015 to June 2020 were enrolled according to the inclusion and exclusion criteria. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The research protocol was approved by the Ethics Review Committee of The First Affiliated Hospital of University of Science and Technology of China (No. 2023-RE-189), and informed consent was taken from all the patients. Data of PSA, Gleason score, body mass index (BMI), pathological T-stage, N-stage and hypertension were collected. Bone metastasis in PCa patients was diagnosed according to the results of whole-body bone emission computed tomography (ECT) scanning. Inclusion criteria: (I) patients who underwent radical prostatectomy at our center and were clearly diagnosed as PCa; (II) age less than 75 years. Exclusion criteria: (I) patients with acute infectious diseases or haematological diseases that may cause alterations in peripheral blood; (II) patients with acute urinary retention, bladder stones, prostate or urethra-related operations within 2 weeks; (III) patients taking regular drug such as finasteride; (IV) patients combined with tumor of other systems; (V) incomplete clinical data.
Data from SEER database
We searched PCa patients between 2010 and 2015 from the SEER database (http://seer.cancer.gov), which is the largest publicly available cancer dataset, based on the 6th American Joint Committee on Cancer (AJCC) edition. The inclusion criteria and exclusion criteria are as follows: Inclusion criteria: (I) Adolescents and young adults site recode 2020 revision: PCa; (II) diagnosed from 2010 to 2015; (III) complete TNM stage. Exclusion criteria: (I) Gleason Patterns Clinical Recode was missing; (II) PSA value was missing. Finally, we searched 96,497 patients included in the study. The continuous variables including age, PSA vaule were transformed to the categorical variables: age at diagnosis (<45, 45–59, 60–75 and >75 years); PSA value (<10, 10–19, 20–49, 50–99 and ≥100 ng/mL); The race was listed as white, black, and others including American Indian, AK Native, Asian, Pacific Islander.
Statistical analysis
Continuous variables were presented by mean ± standard deviation or median (interquartile range). Categorical variables were described by counts (percentages). Normally distributed data were analyzed by one-way analysis of variance (ANOVA) for differences between groups with or without bone metastasis, non-normally distributed data were compared by Kruskal-Wallis (KW) test, Chi-squared test was used for comparison of rates. Logistic regression analysis to find independent risk factors. The receiver operating characteristic (ROC) curve was used to assess the diagnostic efficacy of bone metastasis of PCa. The statistical analysis was performed using SPSS 19.0 software (IBM, Armonk, USA) and MedCalc (18.9.1, MedCalc Software Ltd., Ostend, Belgium), P value of less than 0.05 was considered statistically significant.
Results
Clinical characteristics of bone metastasis and non-bone metastasis in PCa patients
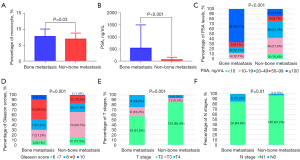
The demographic and clinical characteristics of the patients are presented in Table 1. Statistical analysis revealed significant differences between patients with bone metastasis and those without bone metastasis in terms of PSA levels (P<0.001) and percentage of monocytes (M%, P=0.03), as depicted in Figure 1A-1C. Additionally, significant differences were observed in the incidence rates of different Gleason scores, T stages, and regional (pelvic) lymph node metastasis, as illustrated in Figure 1D-1F. Notably, no significant differences were found in BMI or systemic inflammation parameters such as NLR and PLR between the two groups.
Table 1
| Variables | All patients (N=177) | Bone metastasis (N=33) | Non-bone metastasis (N=144) | P value |
|---|---|---|---|---|
| Age (years) | 69 [65–75] | 69 [66–75] | 69 [64–75] | 0.71 |
| BMI (kg/m2) | 23.27±3.24 | 23.12±3.30 | 23.30±3.24 | 0.77 |
| WBC (109/L) | 5.96±1.31 | 5.75±1.13 | 6.01±1.35 | 0.32 |
| Neu (%) | 60.84±7.53 | 60.72±6.86 | 60.87±7.70 | 0.92 |
| L (%) | 29.00±7.13 | 28.78±6.25 | 29.06±7.33 | 0.84 |
| M% | 7.07±1.98 | 7.73±2.31 | 6.92±1.87 | 0.03* |
| PLT (109/L) | 189.45±62.74 | 188.45±52.04 | 189.67±65.10 | 0.92 |
| Neu (109/L) | 3.64±0.99 | 3.51±0.86 | 3.67±1.01 | 0.39 |
| L (109/L) | 1.72±0.57 | 1.64±0.47 | 1.74±0.59 | 0.41 |
| M (109/L) | 0.42±0.14 | 0.44±0.16 | 0.41±0.13 | 0.27 |
| NLR | 2.07 (1.67–2.82) | 2.10 (1.65–2.88) | 2.05 (1.70–2.84) | 0.96 |
| LMR | 3.93 (3.15–5.50) | 3.77 (3.04–4.99) | 4.02 (3.20–6.11) | 0.25 |
| NMR | 8.71 (7.03–11.20) | 7.41 (6.59–11.23) | 8.82 (7.27–11.18) | 0.08 |
| PWR | 30.45 (24.72–37.31) | 31.49 (24.72–41.20) | 30.23 (24.48–36.44) | 0.29 |
| PSA (ng/mL) | 32.36 (14.77–94.60) | 200.0 (50.69–298.75) | 25.89 (13.32–49.62) | <0.001* |
| Hypertension | 0.01* | |||
| Yes | 41 (23.2) | 2 (6.1) | 39 (27.1) | |
| No | 136 (76.8) | 31 (93.9) | 105 (72.9) | |
| Diabetes | 0.49 | |||
| Yes | 13 (7.3) | 1 (3.0) | 12 (8.3) | |
| No | 164 (92.7) | 32 (97.0) | 132 (91.7) | |
| Gleason score | 0.001* | |||
| 6 | 25 (14.1) | 3 (9.1) | 22 (15.3) | |
| 7 | 80 (45.2) | 7 (21.2) | 73 (50.7) | |
| 8 | 32 (18.1) | 7 (21.2) | 25 (17.4) | |
| 9 | 35 (19.8) | 13 (39.4) | 22 (15.3) | |
| 10 | 5 (2.8) | 3 (9.1) | 2 (1.4) | |
| pT stage | 0.001* | |||
| pT2 | 142 (80.2) | 19 (57.6) | 123 (85.4) | |
| pT3 | 18 (10.2) | 6 (18.2) | 12 (8.3) | |
| pT4 | 17 (9.6) | 8 (24.2) | 9 (6.3) | |
| N stage | 0.01* | |||
| N0 | 168 (94.9) | 28 (84.8) | 140 (97.2) | |
| N1 | 9 (5.1) | 5 (15.2) | 4 (2.8) |
Data are presented as mean ± standard deviation, median (IQR) or n (%). *, significant values. BMI, body mass index; IQR, interquartile range; L, lymphocyte; LMR, lymphocyte-monocyte ratio; M%, percentage of monocyte; Neu, neutrophil; NLR, neutrophil-lymphocyte ratio; NMR, neutrophil-monocyte ratio; N0, regional (pelvic) lymph lode negative; N1, regional (pelvic) lymph lode positive; PLT, platelet; PSA, prostate-specific antigen; pT stage, pathological tumor stage; PWR, platelet-white blood cell ratio; WBC, white blood cell.
Univariate and multivariate logistic analysis for screening the independent predictors of bone metastasis
The results of the univariate logistic analysis indicated that factors, including PSA, Gleason score, M%, T stage, N stage, and hypertension, were associated with an increased risk of bone metastasis in patients with PCa. However, upon conducting a multivariate analysis of these clinical indicators, it was found that only PSA [odds ratio (OR): 2.282, 95% confidence interval (CI): 1.504–3.463, P<0.001], Gleason score (OR: 1.879, 95% CI: 1.169–3.019, P=0.009), M% (OR: 1.283, 95% CI: 1.008–1.632, P=0.04), and N stage (OR: 5.493, 95% CI: 1.005–30.024, P=0.04) remained as independent risk factors for bone metastasis in PCa patients. Conversely, hypertension and T stage were not found to be significant risk factors after applying a correction (Table 2). The ROC curves demonstrated that PSA (AUC =0.795) exhibited greater efficacy in the diagnosis of bone metastasis in PCa patients compared to Gleason score (AUC =0.700). Furthermore, the combination of PSA and Gleason score resulted in an enhanced diagnostic effectiveness (AUC =0.829) (Figure 2). PSA displayed a higher specificity (85.42%) in comparison to Gleason score (65.97%), while Gleason score exhibited a higher sensitivity (69.70%) than PSA (66.67%). The combination of PSA and Gleason score also improved diagnostic sensitivity (75.76%), albeit with a slight reduction in diagnostic specificity (81.25%) (Table 3).
Table 2
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | B | OR | 95% CI | P | ||
| Age (years) | 1.008 | 0.951–1.069 | 0.78 | |||||
| BMI (kg/m2) | 0.982 | 0.874–1.104 | 0.76 | |||||
| PSA group | 2.748 | 1.872–4.034 | <0.001* | 0.825 | 2.282 | 1.504–3.463 | <0.001* | |
| Gleason score | 2.092 | 1.432–3.057 | <0.001* | 0.631 | 1.879 | 1.169–3.019 | 0.009* | |
| WBC (109/L) | 0.317 | 0.642–1.154 | 0.35 | |||||
| Neu (%) | 0.997 | 0.948–1.049 | 0.92 | |||||
| L (%) | 0.995 | 0.943–1.049 | 0.84 | |||||
| M% | 1.225 | 1.014–1.480 | 0.04* | 0.249 | 1.283 | 1.008–1.632 | 0.04* | |
| PLT (109/L) | 1.000 | 0.994–1.006 | 0.92 | |||||
| Neu (109/L) | 0.840 | 0.566–1.248 | 0.39 | |||||
| L (109/L) | 0.741 | 0.366–1.502 | 0.41 | |||||
| M (109/L) | 4.416 | 0.309–63.026 | 0.27 | |||||
| NLR | 0.943 | 0.620–1.437 | 0.79 | |||||
| LMR | 0.862 | 0.689–1.078 | 0.19 | |||||
| NMR | 0.906 | 0.791–1.038 | 0.16 | |||||
| PWR | 1.014 | 0.979–1.050 | 0.43 | |||||
| pT | 2.495 | 1.496–4.162 | <0.001* | 0.327 | 1.387 | 0.753–2.556 | 0.29 | |
| N | 6.250 | 1.579–24.743 | 0.009* | 1.703 | 5.493 | 1.005–30.024 | 0.04* | |
| Hypertension | 0.174 | 0.040–0.760 | 0.02* | –1.647 | 0.193 | 0.037–0.998 | 0.05 | |
| Diabetes | 0.344 | 0.043–2.741 | 0.31 | |||||
*, significant values. BMI, body mass index; CI, confidence interval; L, lymphocyte; LMR, lymphocyte-monocyte ratio; M%, percentage of monocyte; Neu, neutrophil; N0, regional (pelvic) lymph lode negative; N1, regional (pelvic) lymph lode positive; NLR, neutrophil-lymphocyte ratio; NMR, neutrophil-monocyte ratio; OR, odds ratio; PLT, platelet; PSA, prostate-specific antigen; pT stage, pathological tumor stage; PWR, platelet-white blood cell ratio; WBC, white blood cell.
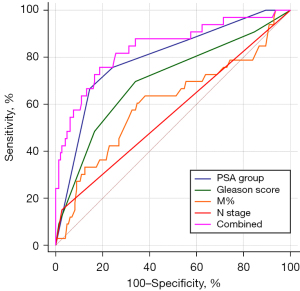
Table 3
| Variables | AUC | 95% CI | Sensitivity, % | Specificity, % | P value |
|---|---|---|---|---|---|
| PSA group | 0.795 | 0.728–0.852 | 66.67 | 85.42 | 0.02* |
| Gleason score | 0.700 | 0.627–0.767 | 69.70 | 65.97 | 0.003* |
| M% | 0.605 | 0.529–0.678 | 60.61 | 65.28 | <0.001* |
| N stage | 0.562 | 0.485–0.636 | 15.15 | 97.22 | <0.001* |
| Combined model | 0.829 | 0.765–0.881 | 75.76 | 81.25 | Reference |
*, significant values. AUC, area under the curve; CI, confidence interval; M%, percentage of monocyte; N, regional (pelvic) lymph lode; PSA, prostate-specific antigen.
Univariate and multivariate logistic analysis for screening the independent predictors of bone metastasis in the PCa patients from SEER database
A total of 96,497 patients from the SEER database were included in the study. The clinical characteristics of the patients are showed in Table S1. The clinical characteristics of the patients were presented in Table S1. Chi-squared tests were used to assess the significant differences in age, race, T stage, N stage, Gleason score, and PSA between patients with and without bone metastasis. Univariate logistic analysis revealed that age, T stage, N stage, Gleason score, and PSA were identified as risk factors for bone metastasis in patients with PCa, while race did not show a significant association. Subsequent multivariate logistic analysis demonstrated that age (OR: 1.227, 95% CI: 1.147–1.313, P<0.001), N stage (OR: 2.836, 95% CI: 2.523–3.188, P<0.001), Gleason score (OR: 2.345, 95% CI: 2.241–2.455, P<0.001) and PSA (OR: 2.890, 95% CI: 2.796–2.986, P<0.001) were independently associated with bone metastasis (Table 4). However, it is worth noting that T stage was not found to be an independent risk factor, which aligned with the findings from our center as previously mentioned.
Table 4
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | B | OR | 95% CI | P | ||
| Age (years) | 2.016 | 1.900–2.139 | <0.001* | 0.205 | 1.227 | 1.147–1.313 | <0.001* | |
| Race | 0.979 | 0.908–1.056 | 0.59 | |||||
| T | 1.751 | 1.676–1.830 | <0.001* | −0.005 | 0.995 | 0.944–1.048 | 0.84 | |
| N | 14.870 | 13.600–16.259 | <0.001* | 1.042 | 2.836 | 2.523–3.188 | <0.001* | |
| Gleason score | 3.995 | 3.849–4.147 | <0.001* | 0.852 | 2.345 | 2.241–2.455 | <0.001* | |
| PSA group | 3.905 | 3.790–4.023 | <0.001* | 1.061 | 2.890 | 2.796–2.986 | <0.001* | |
*, significant values. CI, confidence interval; OR, odds ratio; PSA, prostate-specific antigen; SEER, Surveillance, Epidemiology, and End Results.
Chi-squared test demonstrated the important role of primary histological grade in bone metastasis in PCa patients
A notable disparity in the occurrence of bone metastasis was observed among patients with PCa who had varying Gleason scores, as indicated in Table 1. However, it remains uncertain whether there are any variations in PCa patients with different primary histological grades, when their Gleason scores are identical. To investigate this further, a Chi-squared test was conducted, as depicted in Figure S1 (Figure S1A presented the data from the SEER database, while Figure S1B-S1D presented the data from our center). In Figure S1A, it is demonstrated that a significant difference in the rate of bone metastasis was observed when comparing Gleason score 7 with different primary histological grades (3+4 vs. 4+3, 0.55% vs. 2.19%, Chi-squared =228.015, P<0.001). Similar findings were observed for Gleason score 8 (3+5 vs. 4+4, 3.34% vs. 7.28%, Chi-squared =17.194, P<0.001; 3+5 vs. 5+3, 3.34% vs. 8.38%, Chi-squared =9.509, P=0.002; 4+4 vs. 5+3, 7.28% vs. 8.38%, Chi-squared =0.355, P=0.56) and Gleason score 9 (4+5 vs. 5+4, 16.11% vs. 25.60%, Chi-squared =83.273, P<0.001). These findings suggest the significant role of primary histological grades in bone metastasis. However, it is regrettable that no significant difference was observed in Gleason scores of 7 or 9 at our center due to the limited sample size (Figure S1C,S1D). Additionally, a Chi-squared test was not conducted for Gleason score 8 due to the small sample size.
Discussion
Approximately 10% of newly diagnosed patients with PCa present with bone metastasis (1). However, a considerable number of PCa patients in China already exhibited bone metastasis at the time of their initial medical consultation (14). The present study unveiled an incidence rate of 18.64% (33/177) for bone metastasis in this cohort. The association between bone metastasis and bone pain, pathological fractures, and poor prognosis has been widely acknowledged. Consequently, it is imperative for patients diagnosed with PCa to ascertain the presence of bone metastasis and promptly initiate treatment to prevent bone-related adverse events. At our center, several independent risk factors for PCa bone metastasis have been identified, including PSA levels, Gleason score, M%, and N stage. Furthermore, a comprehensive analysis revealed a positive correlation between the incidence of bone metastasis and the primary histological grade (with identical Gleason scores) in the SEER database, thus highlighting the significant role of primary histological grade in the metastasis of PCa. Conversely, no significant influence was observed for T stage at our institution or in the SEER database.
Numerous studies have extensively investigated the impact of PSA on PCa metastasis, consistently demonstrating a higher incidence with elevated PSA levels (13-17). Additionally, related studies have reported a bone metastasis rate ranging from 41.4% to 79.9% when PSA levels exceeded 100 ng/mL (18-20). The European Association of Urology recommended a standard value of PSA >20 ng/mL for bone scan (21). The current study demonstrated that PSA was an independent risk factor for bone metastasis in PCa, with a sensitivity of 66.67% and a specificity of 85.42% as presented in Tables 2-4, consistent with previous research findings.
Gleason score has been widely recognized as a significant indicator for distant metastasis, tumor recurrence post-surgery, and patient prognosis in PCa (22,23). The results of this study align with previous research, as multivariate logistic regression analysis confirmed that Gleason score was an independent risk factor for bone metastasis. It is widely recognized that the Gleason score is determined by combining the dominant primary histology grade with the dominant secondary histology grade. It has been conventionally understood that a Gleason score of 3+4 is less aggressive compared to 4+3 (24). Nevertheless, limited research has been conducted on the impact of the dominant primary histology grade on bone metastasis in PCa cases where the Gleason score remains the same. The distinctive aspect of this study lies in the evaluation of the rate of bone metastasis across various dominant primary histology grades, utilizing data sourced from the SEER database. The findings of this study demonstrated a significant association between a higher rate of bone metastasis and a higher dominant primary histology grade. However, it is unfortunate that no significant difference was observed in the dominant primary histology grade at our center, possibly due to the limited sample size. These results underscore the significance of considering the Gleason score and dominant primary histology grade in the context of PCa.
Numerous studies have highlighted the potential role of inflammation in the initiation and progression of PCa, yet there remains a dearth of research investigating the link between systemic inflammation and bone metastasis of PCa (25-27). The present study revealed a substantial disparity of M% between the bone metastasis and non-bone metastasis groups, with further analysis through multivariate logistic regression indicating that M% serves as an independent risk factor for bone metastasis in PCa. However, no significant distinction was observed in relation to NLR and PLR, which are recognized indicators of systemic inflammation and have been previously linked to PCa bone metastasis (15). Consequently, additional investigations are warranted to assess the correlation between systemic inflammation and bone metastasis in PCa.
The findings of this study indicate a notably higher proportion of individuals with pathological T3 or T4 in the bone metastasis group as compared to the non-bone metastasis group. However, the multivariate regression analysis revealed that pathological T stage did not emerge as a risk factor for bone metastasis, aligning with prior research (13,14). This outcome was further supported by data from the SEER database, albeit limited to clinical T stage. It is plausible that factors such as PSA and Gleason score surpass pathological T stage in their potential as risk factors.
Conclusions
The analysis of data from our center and the SEER database revealed that PSA, Gleason score and N stage are significant risk factors for bone metastasis in PCa. The intriguing findings of the M% value and dominant primary histology grade in PCa bone metastasis warrant further investigation. However, it is important to acknowledge the limitations of our study, including its retrospective nature and small sample size, which may introduce inherent biases. Furthermore, the diagnosis of bone metastasis was based on the use of ECT, which presents a limitation and potential confounding factor in the data. This is due to the exclusion of patients with disseminated disease manifesting as micrometastases, as these would not be detected and thus not included in the metastasis group due to the limitations of ECT.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-78/rc
Data Sharing Statement: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-78/dss
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-78/prf
Funding: The current study was supported in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-78/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The research protocol was approved by the Ethics Review Committee of The First Affiliated Hospital of University of Science and Technology of China (No. 2023-RE-189) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feng H, Deng Z, Peng W, et al. Circular RNA EPHA3 suppresses progression and metastasis in prostate cancer through the miR-513a-3p/BMP2 axis. J Transl Med 2023;21:288. [Crossref] [PubMed]
- Jeong SH, Raman JD. Impact of the evolving United States Preventative Services Task Force policy statements on incidence and distribution of prostate cancer over 15 years in a statewide cancer registry. Prostate Int 2021;9:12-7. [Crossref] [PubMed]
- Huang J, Freyhult E, Buckland R, et al. Osteoclasts directly influence castration-resistant prostate cancer cells. Clin Exp Metastasis 2022;39:801-14. [Crossref] [PubMed]
- Sun D, Lin A, Sun Z, et al. Nomograms predict survival benefits of radical prostatectomy and chemotherapy for prostate cancer with bone metastases: A SEER-based study. Front Oncol 2022;12:1020898. [Crossref] [PubMed]
- Vieira C, Fragoso M, Pereira D, et al. Pain prevalence and treatment in patients with metastatic bone disease. Oncol Lett 2019;17:3362-70. [Crossref] [PubMed]
- Kim DY, Lee WW, Song YS, et al. Detection of recurrence sites using (18)F-fluorocholine PET/CT in prostate cancer patients with PSA failure. Prostate Int 2023;11:69-75. [Crossref] [PubMed]
- Gilbert A, Tudor M, Montanari J, et al. Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions. Cancers (Basel) 2023;15:1962. [Crossref] [PubMed]
- Pala E, Procura A, Trovarelli G, et al. Intramedullary nailing for impending or pathologic fracture of the long bone: titanium vs carbon fiber peek nailing. EFORT Open Rev 2022;7:611-7. [Crossref] [PubMed]
- Lampe JB, Desai PP, Tripathi AK, et al. Cabazitaxel-Loaded Nanoparticles Reduce the Invasiveness in Metastatic Prostate Cancer Cells: Beyond the Classical Taxane Function. Pharmaceutics 2023;15:662. [Crossref] [PubMed]
- Yan B, Chen B, Min S, et al. iTRAQ-based Comparative Serum Proteomic Analysis of Prostate Cancer Patients with or without Bone Metastasis. J Cancer 2019;10:4165-77. [Crossref] [PubMed]
- Suhaimi SH, Hasham R, Hafiz Idris MK, et al. Optimization of Ultrasound-Assisted Extraction Conditions Followed by Solid Phase Extraction Fractionation from Orthosiphon stamineus Benth (Lamiace) Leaves for Antiproliferative Effect on Prostate Cancer Cells. Molecules 2019;24:4183. [Crossref] [PubMed]
- Tyson MD, Alvarez J, Koyama T, et al. Racial Variation in Patient-Reported Outcomes Following Treatment for Localized Prostate Cancer: Results from the CEASAR Study. Eur Urol 2017;72:307-14. [Crossref] [PubMed]
- Chaoying L, Chao M, Xiangrui Y, et al. Risk factors of bone metastasis in patients with newly diagnosed prostate cancer. Eur Rev Med Pharmacol Sci 2022;26:391-8. [PubMed]
- Peng C, Juan C, Mao W, et al. Retrospective analysis of risk factors for bone metastasis in newly diagnosed prostate cancer patients. Eur Rev Med Pharmacol Sci 2022;26:3832-9. [PubMed]
- Zhang JY, Ge P, Zhang PY, et al. Role of Neutrophil to Lymphocyte Ratio or Platelet to Lymphocyte Ratio in Prediction of Bone Metastasis of Prostate Cancer. Clin Lab 2019; [Crossref] [PubMed]
- Briganti A, Suardi N, Gallina A, et al. Predicting the risk of bone metastasis in prostate cancer. Cancer Treat Rev 2014;40:3-11. [Crossref] [PubMed]
- Chien TM, Lu YM, Geng JH, et al. Predictors of Positive Bone Metastasis in Newly Diagnosed Prostate Cancer Patients. Asian Pac J Cancer Prev 2016;17:1187-91. [Crossref] [PubMed]
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71:630-42. [Crossref] [PubMed]
- Walter LC, Fung KZ, Kirby KA, et al. Five-year downstream outcomes following prostate-specific antigen screening in older men. JAMA Intern Med 2013;173:866-73. [Crossref] [PubMed]
- Tanaka N, Fujimoto K, Shinkai T, et al. Bone scan can be spared in asymptomatic prostate cancer patients with PSA of <=20 ng/ml and Gleason score of <=6 at the initial stage of diagnosis. Jpn J Clin Oncol 2011;41:1209-13. [Crossref] [PubMed]
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 2014;65:124-37. [Crossref] [PubMed]
- Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol 2014;15:e484-92. [Crossref] [PubMed]
- Buyyounouski MK, Choyke PL, McKenney JK, et al. Prostate cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:245-53.
- Shao N, Zhu Y, Wan FN, et al. Identification of seven long noncoding RNAs signature for prediction of biochemical recurrence in prostate cancer. Asian J Androl 2019;21:618-22. [Crossref] [PubMed]
- Rundle AG, Sadasivan SM, Chitale DA, et al. Racial differences in the systemic inflammatory response to prostate cancer. PLoS One 2021;16:e0252951. [Crossref] [PubMed]
- Huang L, LaBonte MJ, Craig SG, et al. Inflammation and Prostate Cancer: A Multidisciplinary Approach to Identifying Opportunities for Treatment and Prevention. Cancers (Basel) 2022;14:1367. [Crossref] [PubMed]
- Messex JK, Byrd CJ, Thomas MU, et al. Macrophages Cytokine Spp1 Increases Growth of Prostate Intraepithelial Neoplasia to Promote Prostate Tumor Progression. Int J Mol Sci 2022;23:4247. [Crossref] [PubMed]


