
Mucinous cystic neoplasia (MCN) of the pancreas: systematic review and meta-analysis of sex differences in prevalence and malignancy risk between males and females
Highlight box
Key findings
• This systematic review of mucinous cystic neoplasia (MCN) in the pancreas found that, even if it is more common in female, the male rate is about 15%. The associated malignancy risk is considerably higher in men compared to female.
What is known and what is new?
• MCN is known to occur more often in female than males.
• This study demonstrates that about 14% of MCN occur in men. While MCNs are much rarer in men, the malignancy rate in men is reported to be about 3 times higher, with a pooled rate of 47.2%, in men compared to 16.6% in female.
What is the implication, and what should change now?
• Further investigation into the gender-disparities in MCN of the pancreas needs to be done, in particular to the putative molecular differences and malignancy risk associated with sex.
Introduction
Pancreatic cysts have become increasingly recognized as a clinical entity due to the overall increase and widespread in use of cross-sectional imaging in the population (1-3). Most cysts entities when detected are small in size and pose very little risk for malignancy, but some cyst types are described as premalignancies with a clear potential for invasive cancer development (2,4-6). Among the premalignant cysts are the mucinous cystic entities (4,6) (Figure 1), most commonly found as intraductal papillary mucinous neoplasia (IPMN) (3) and, considerably less common as mucinous cystic neoplasia (MCN) (7-9).
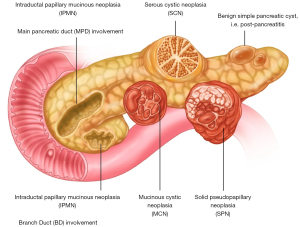
While an increasing knowledge base is developing for the more common IPMN lesions (3), there is less well-known data of MCNs as these are a much rarer to come by and hence not a well-described cyst entity overall. Many publications are still based on case reports (10-15), with larger case series being the exception rather than the rule (16-18). Notably, the MCNs have previously been thought to almost exclusively develop in females, but this has been controversial. For example, one report on MCN stated 0% in males (19), while another study suggests that up to 10% were reported to occur in males (2). In a recent, large multicenter study from Japan of 328 MCNs, only 7 (2.1%) where reported to occur in males (16). The diagnosis can be challenging (see Case presentation), as presentation in males is rare and hence often other differential diagnosis are considered as a priority initially. Findings on imaging (Figure 2) and histopathology (Figure 3) confirms the diagnosis.
Of note, several reports exist to the malignancy risks and findings of MCN in females (20-22), even during pregnancy which is a gender-specific finding (10,11,21,23,24). Further, the malignancy risk is considerable, with a reported variance in high-grade dysplasia (HGD) and invasive lesions between 10% and up to 25%, also for pancreatic MCNs found in females (17,25-27). Case reports have suggested a high risk in MCNs associated with male sex (13,15,28). Due to the rarity of pancreatic MCN in males, some reports of MCN have excluded all males with MCN in order to prevent bias from misclassification when investigating large databases (20,26), and hence the findings may not apply to males when only females are investigated for the condition (20,26).
Case presentation
A previously healthy 50-year-old male was referred to the hepatobiliary-pancreatic (HPB)-department at Karolinska University Hospital. The patient had undergone a laparoscopic cholecystectomy due to abdominal pain (at another department at Karolinska) two months prior to being referred to the HPB-department. The preoperative computed tomography (CT) had shown cholelithiasis and a pancreatic cyst.
After completion of and recovery from gallbladder surgery, the pancreatic cyst was further investigated with additional CT and magnetic resonance imaging (MRI) and the patient was discussed at a multidisciplinary team conference (MDT).
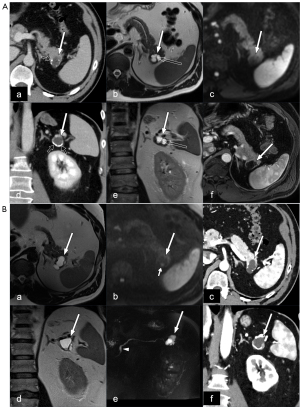
The radiological investigation showed a 3 cm lobulated cyst in the pancreatic tail with shell-like calcifications in the wall and internal septations (Figure 2A). There were no signs of impeded diffusion on MRI. The cyst had no evident communication with the main pancreatic duct (MPD). The lumen of the MPD had a variable width and was 4 mm in the pancreatic body downstream of the cyst. The differential diagnosis of the cystic lesion included oligocystic serous cystic neoplasia (SCN) and side-branch IPMN (sb-IPMN); due to the elevated carbohydrate antigen 19-9 (CA 19-9) levels (50 kE/L) and the slight variation of the MPD lumen, the patient was investigated further. The clinical investigation, including gastroscopy and coloscopy, could not find any pathology explaining the elevated CA 19-9. The patient was therefore followed up with pancreatic MRI. On follow-up four months later, the serum CA 19-9 was 38 kE/L. At the MRI follow-up examination, there were no changes in the imaging findings and the patient was therefore included in annual surveillance with MRI.
Five years later, the cyst had increased in size from 3 to 3.5 cm and two approximately 5 mm foci with signs of impeded diffusion were identified on MRI (Figure 2B). The serum CA 19-9 was 57 kE/L. Based on the above-mentioned changes of the imaging findings, the MDT concluded that resection is indicated and should be discussed with the patient. At the outpatient clinic, the decision to proceed with resection of the cyst was made with the consent of the patient.
The patient underwent a minimal invasive distal pancreatectomy with splenectomy. Perioperatively, it was clear that the cyst reached into the splenic hilum and it was not possible to separate the cyst from the splenic vessels. Therefore, the spleen was resected together with the distal pancreas. The procedure was uneventful, with a per-operative bleeding of 100 mL. The pancreas was divided with staple and a passive drain was placed (in line with institutional practice at that time). The initial postoperative course was also uneventful, and the patient was discharged to a rehabilitation clinic after three days. The patient was however readmitted five days later due to fever and abdominal pain. A CT showed a fluid collection, and a new percutaneous drain was placed with pancreatic amylase level of 145 mikrokat/L indicating a pancreatic fistula. The patient had a long-standing leakage that was internalised after two months using endoscopic ultrasound assisted internal drain placement.
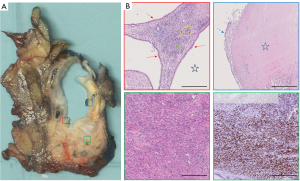
The pathological examination of the specimen was performed using a standardised protocol. The histological examination (Figure 3) showed a 32 mm multilocular cystic tumour lined by cubic epithelium with mild nuclear atypia. The epithelial cells exhibited eosinophilic cytoplasm, with some areas containing mucin. Furthermore, areas of regressive tissue changes were observed, characterized by denuded epithelium, infiltration of macrophages, a thickened wall with hyaline fibrosis, and focal osseous metaplasia. The underlying ovarian-type stroma consisted of densely packed spindle cells. The immunohistochemistry showed expression of oestrogen- and progesterone-receptors and positivity for smooth muscle antibodies (SMA) and Wilms tumor protein (WT1) in the ovarian-type stroma. There was sparse expression of tyrosine hydroxylase staining, highlighting luteinized stromal cells. The epithelium was positive for CA 19-9, CK7 and CK17 with minimal expression of MUC1, MUC6 and CA 125. It was negative for CDX2, mCEA, vimentin, MUC2, MUC5AC and IMP-3. The was no over-expression of p53. There was preserved expression of SMAD4 and low proliferation in Ki-67. In all, the immunohistochemistry indicated a MCN of the pancreas. The dysplasia was low-grade. There were no suggestions of malignant transformation. In total 22 regional lymph nodes were investigated with no signs of malignancy.
The final postoperative anatomical diagnosis was concluded to be a 32 mm MCN with low grade dysplasia, despite male gender. The closest margin was 1 mm from the posterior resection surface.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the patient for the publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Objective
The aim of this article is to present an updated and systematic review on the existing knowledge of pancreatic MCN and in particular what is known about the clinical, pathological and molecular-biological description found in this particular pancreatic cyst entity. We present this article in accordance with the PRISMA reporting checklist (available at https://cco.amegroups.com/article/view/10.21037/cco-24-124/rc).
Methods
Study design and data sources
A systematic review and meta-analysis were conducted on pre-existing literature to assess the prevalence and malignancy rates of MCN in male and female patients. The study protocol was prospectively registered in the PROSPERO database (CRD42024606793). Study protocol can be assessed by contacting the first author.
The systematic searches of PubMed, Embase, MEDLINE and Web of Science were performed, covering all studies published from January 2014 to August 2024. The search strategy included terms such as “pancreatic cyst”, “pancreatic cystic neoplasm”, “mucinous cystadenoma”, “mucinous cystadenocarcinoma”, “men” or “male”. Boolean operators “AND” and “OR” were incorporated to broaden the search and capture relevant studies. Reference lists of relevant articles were manually searched for additional studies. Non-English articles were excluded. All search results were exported into Rayyan software (https://www.rayyan.ai) for duplicate removal and initial screening.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (I) reported the incidence or malignancy rate of MCNs in male or female patients; and (II) included patients with histologically confirmed MCNs. Studies were excluded if they met any of the following criteria: (I) studies without sex-specific data on MCN; and (II) reviews, editorials, and non-original research articles.
Data extraction and quality assessment
Two independent reviewers extracted data from each included study, with discrepancies resolved by consensus. Data extracted included the number of male and female patients, study type, study duration, number of MCN cases, and the number of cases with HGD or invasive MCN (defined as ‘malignant MCN’ for the purpose of this study) and stratified by sex. The quality of studies was assessed using the methodological index for non-randomized studies (MINORS) tool (29).
Statistical analysis
Statistical analysis was conducted using R software (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria). Pooled estimates of the incidence and malignancy rates of MCNs in male and female patients were calculated using a random-effects model incorporating the DerSimonian-Laird method in order to account for expected heterogeneity across studies. Proportions and 95% confidence intervals (CIs) were calculated for each study. Between-study heterogeneity was evaluated using the I2 statistic and Cochran’s Q test, with I2 values above 50% indicating substantial heterogeneity and values above 75% considered high heterogeneity. A P value of <0.05 was considered statistically significant.
Results
The search is shown in the study flow chart presented in Figure 4 and included 51 studies, for a total of 3,519 patients. The included studies are presented in Tables 1,2. Eleven were multicenter studies (27,31,33,36,38,40,57,58,62,73,74), and the remainder single-center studies. The MINORS score for the 12 studies (27,28,31,39,42,46-48,53,58,61,63) used to calculate sex-differences in malignancy rate (Table 2) were between 8 and 19, as presented in Table S1.
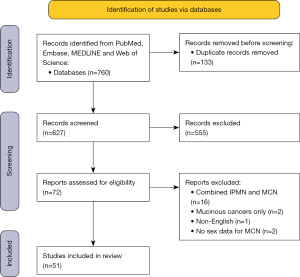
Table 1
| Study | Case report (Y/N) | Single vs. multicentre study | Number of MCN cases | ||
|---|---|---|---|---|---|
| Total | Male | Female | |||
| Agarwal A 2016 (30) | N | Single centre | 5 | 3 | 2 |
| Ahmad M 2020 (31) | N | Multicentre | 492 | 116 | 376 |
| Alkhateeb M 2020 (32) | Y | Single centre | 1 | 1 | 0 |
| An S 2019 (33) | N | Multicentre | 69 | 1 | 68 |
| Barutcuoglu B 2022 (34) | N | Single centre | 25 | 9 | 16 |
| Chang YT 2015 (35) | N | Single centre | 58 | 2 | 56 |
| Chaudhari V 2019 (36) | N | Multicentre | 128 | 21 | 107 |
| Chen Y 2018 (37) | N | Single centre | 32 | 5 | 27 |
| Chien C 2017 (38) | N | Multicentre | 47 | 6 | 41 |
| Choi J 2015 (39) | Y | Single centre | 1 | 1 | 0 |
| Chu L 2022 (40) | N | Multicentre | 33 | 1 | 32 |
| Dong Z 2022 (41) | N | Single centre | 33 | 4 | 29 |
| Ethun C 2017 (42) | N | Single centre | 349 | 39 | 310 |
| Fallahzadeh M 2014 (13) | Y | Single centre | 1 | 1 | 0 |
| Fan X 2019 (43) | Y | Single centre | 1 | 1 | 0 |
| Gao J 2021 (44) | N | Single centre | 55 | 5 | 50 |
| Gilani S 2020 (45) | N | Single centre | 33 | 2 | 31 |
| Griffin J 2017 (46) | N | Single centre | 142 | 5 | 137 |
| Gurzu S 2019 (47) | N | Single centre | 4 | 2 | 2 |
| Hui L 2018 (48) | N | Single centre | 63 | 3 | 60 |
| Hwang J 2018 (49) | N | Single centre | 8 | 1 | 7 |
| Jablonska B 2017 (50) | N | Single centre | 5 | 1 | 4 |
| Khoury T 2020 (51) | N | Single centre | 11 | 4 | 7 |
| Li C 2016 (52) | N | Single centre | 19 | 3 | 16 |
| Liang H 2021 (53) | N | Single centre | 165 | 10 | 155 |
| Lin X 2014 (54) | N | Single centre | 20 | 6 | 14 |
| Lipinski M 2018 (55) | N | Single centre | 3 | 1 | 2 |
| Oh H 2014 (56) | N | Single centre | 22 | 3 | 19 |
| Oh S 2017 (57) | N | Multicentre | 16 | 5 | 11 |
| Ohtsuka T 2020 (58) | N | Multicentre | 364 | 7 | 357 |
| Ohtsuka T 2014 (59) | N | Single centre | 21 | 1 | 20 |
| Paziewska A 2020 (60) | N | Single centre | 11 | 2 | 9 |
| Park J 2014 (61) | N | Single centre | 90 | 1 | 89 |
| Pezzilli R 2020 (62) | N | Multicentre | 38 | 6 | 32 |
| Postlewait L 2017 (27) | N | Multicentre | 349 | 39 | 310 |
| Revoredo-Rego F 2019 (63) | N | Single centre | 10 | 1 | 9 |
| Ribaldone D 2020 (64) | N | Single centre | 31 | 10 | 21 |
| Roch A 2017 (65) | N | Single centre | 108 | 8 | 100 |
| Scourtas A 2017 (66) | N | Single centre | 136 | 4 | 132 |
| Tamura S 2017 (67) | Y | Single centre | 2 | 2 | 0 |
| Tian H 2024 (68) | N | Single centre | 93 | 6 | 87 |
| Tomishima K 2020 (28) | Y | Single centre | 1 | 1 | 0 |
| Wang G 2021 (69) | N | Single centre | 14 | 2 | 12 |
| Wang GX 2020 (70) | N | Single centre | 44 | 11 | 33 |
| Xie H 2020 (71) | N | Single centre | 31 | 7 | 24 |
| Yang J 2019 (72) | N | Single centre | 32 | 5 | 27 |
| Yang Z 2022 (73) | N | Multicentre | 205 | 49 | 156 |
| Ye M 2023 (74) | N | Multicentre | 26 | 2 | 24 |
| Yuan Z 2024 (75) | N | Single centre | 15 | 3 | 12 |
| Zhang W 2017 (76) | N | Single centre | 21 | 4 | 17 |
| Zhang G 2023 (77) | N | Single centre | 36 | 8 | 28 |
MCN, mucinous cystic neoplasia; N, no; Y, yes.
Table 2
| Study | Number of male MCN cases | Number of female MCN cases | |||
|---|---|---|---|---|---|
| Total | With high grade dysplasia/malignancy | Total | With high grade dysplasia/malignancy | ||
| Ahmad M 2020 (31) | 116 | 96 | 376 | 272 | |
| Choi J 2015 (39) | 1 | 1 | 0 | 0 | |
| Ethun C 2017 (42) | 39 | 15 | 310 | 37 | |
| Griffin J 2017 (46) | 5 | 1 | 137 | 12 | |
| Gurzu S 2019 (47) | 2 | 2 | 2 | 1 | |
| Hui L 2018 (48) | 3 | 2 | 60 | 13 | |
| Liang H 2021 (53) | 10 | 0 | 155 | 15 | |
| Ohtsuka T 2020 (58) | 7 | 0 | 357 | 43 | |
| Park J 2014 (61) | 1 | 0 | 89 | 9 | |
| Postlewait L 2017 (27) | 39 | 15 | 310 | 24 | |
| Revoredo-Rego F 2019 (63) | 1 | 1 | 9 | 1 | |
| Tomishima K 2020 (28) | 1 | 1 | 0 | 0 | |
MCN, mucinous cystic neoplasia.
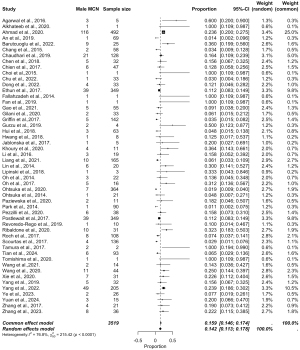
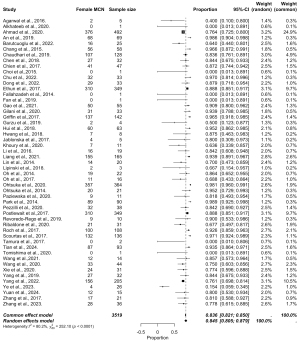
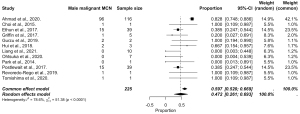
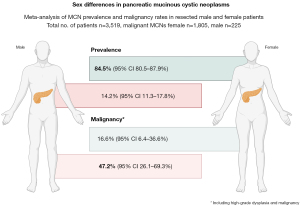
The pooled data are presented in Figures 5-8, respectively. Prevalence estimates of MCN in male patients (Figure 5) resulted in a pooled proportion of 14.2% (95% CI: 11.3–17.84%). The prevalence estimates of MCN in female patients (presented in Figure 6) produced a pooled proportion of 84.5% (95% CI: 80.5–87.9%). The prevalence estimates of malignant MCN in male patients (n=225) gave a pooled malignancy rate of 47.2% (95% CI: 26.1–69.3%), as shown in Figure 7. The prevalence estimates of malignant MCN in female patients (n=1,805; Figure 8) resulted in a pooled malignancy rate of 16.6% (95% CI: 6.4–36.6%). Overall, the main findings are summarized in Figure 9.

Discussion
This meta-analysis demonstrates notable sex-based differences in the prevalence and malignancy rates of MCN. MCNs were significantly more prevalent in female patients, with a pooled prevalence of 84.5% in females compared to an estimated pooled prevalence of 14.2% in males, consistent with previous observations that MCNs predominantly affect females. This sex-based disparity may be attributable to hormonal or genetic factors that promote cyst formation in female pancreatic tissue.
Despite the lower prevalence of MCNs in males, the malignancy rate among male patients was substantially higher at 47.2%, compared to 16.6% in females. These findings suggest that while rare, MCNs in male patients may exhibit a more aggressive course or may often remain undetected until later stages. However, this remains as speculative thoughts, as no robust data to support causes to the observed differences in relation to clinical or biological variation exists at this time. Clinically, the observed variation underscores the importance of vigilant evaluation and potential early intervention for pancreatic cystic lesions in males to mitigate malignancy risks. As noted by others, the misclassification risk is substantial in pancreatic cysts, even in the modern age with multiple imaging studies and endoscopic ultrasound at hand (26,78). Further research is warranted to elucidate the biological mechanisms underlying these sex-based differences and to refine sex-specific recommendations for MCN management.
Risk for malignancy in MCN has first and foremost been ascribed to size of the cyst, with cut-offs proposed at 30 mm (79) and at 40 mm (80) and some even at 50 mm (26). As for other pancreatic cysts, other worrisome features such as a mural nodule or cyst wall enhancement or thickening is often considered as indications for resection (17,80), in otherwise surgically fit patients. While MCN with malignancy tend to occur in somewhat elderly patients and with larger cyst size, size is not statistically significant between groups (likely owing to small sample size) (17). In one study, size up to 50 mm had no malignancy risk in the absence of other features, such as mural nodule or wall enhancement (26). A systematic review found only mural nodule to be predictive of presence of malignancy (81), while no other clinical or cyst features, including tumor biomarkers, were reliable in predicting malignancy (81). Compared to invasive IPMNs, malignant MCN is reported to have a better prognosis if no distant spread is found (73). Why and how MCNs degenerate into malignant transformation is not well understood.
For unknown reasons, the most common location of MCNs is in the distal pancreas (82), and more than 80–90% of resections are performed as left-sided pancreatectomies (27,82). This was also noted in the case presented in this paper, with a left-sided resection and splenectomy being performed. The practice of adding a splenectomy to a distal pancreatic resection has been highly variable in the past, with differences between hospitals (83-85). The contention in the past has been to the role of splenectomy with lymph node resection as a proper oncological procedure, at least for indications involving a suspicious risk of neoplasia or with risk for malignancy. Only recently have this routine practice been challenged, with a very low yield rate of node metastases found in the splenic hilum (86). Also, a splenectomy may often have been performed as a spleen-preserving procedure have been viewed as more difficult, particularly when done by minimal-invasive access in the past. Furthermore, the definition of a left-sided pancreatic resection has just recently been unified by consensus terminology (87), including splenectomy. When to leave the spleen and by what methods (vessel preserving or vessel-resecting) is still debated (88), as is the role of splenectomy for oncological purposes for malignancy (86,89). One study reports a higher rate of malignancy of lesions in the head (21 of 51 pancreatoduodenectomies; malignancy rate of 40%) (27), at least based on resectional series.
The role of surveillance for MCNs continues to be debated. A recent large, multicenter study from Japan found the 1-, 5-, and 10-year cumulative incidence rates of malignant MCN to be 0.8%, 5.6%, and 36.5%, respectively (16). Based on the findings they proposed that malignancy risk over time is too high to warrant surveillance, and surgery should be considered. However, a dual institution study from Italy suggest that surveillance can be entertained in most patients with size <50 mm in the absence of symptoms, mural nodule or wall enhancement, as the risk of malignancy is low (26). This is also supported by a more recent Chinese study (22), also reporting a favorable long-term survival in the few resected for malignancy, reporting 70% 5-year overall survival.
The molecular-biological differences underlying the MCNs is poorly understood. The ovarian-like stroma of the cysts and the positive staining of estrogens and progesterons, suggest a hormone-related tumorigenesis, which may explain the higher prevalence in women. However, androgens receptors are also expressed, as demonstrated by immunohistochemistry (90,91), and steroidogenesis in the ovarian-like stroma has been debated for more than a decade (92). Investigations into putative hormone expression or sexual dysfunctions of puberty have only produced very small, hypothesis-generating data (93). The most convincing data related to hormone-influence comes from the observed cases during pregnancy, with an overall younger age (around 30 years) and larger tumor size (over 10 cm) and reported growth spurt of the cyst during pregnancy (21,24).
Investigations into the mechanisms of neoplasia, has suggested activation of Wnt-signalling in the stroma (by means of beta-catenin staining) associated with MCN (94). Others have found mutations in ring finger protein 43 (RNF43) associated with cytological atypia in the ovarian-like stroma, and a high prevalence of KRAS mutations with increasing dysplasia and all with invasive cancer (92,95). Gradual telomere shortening has been found as a feature in MCN tumorigenesis (19). Several other molecular features of low- to high-grade dysplasia are found along the route of other premalignant mucinous lesions in the pancreas (96,97).
Some limitations should be mentioned. As noted, most data are accrued from case series, case reports and retrospective institutional series. Only more recently has multicenter reports emerged. Despite this, we believe the findings are robust and updated, based on the most recent available literature. The study may serve as a backdrop for further research into the genesis of MCN as a rather rare but a particular cyst of interest in the pancreas.
Conclusions
The prevalence of MCN in males is 14.2% in case series of surgical resections, which is a higher rate than previously reported. Of note, there is a much higher malignancy rate in males, almost 3 times that of females, with an unexplained biological rationale for the increased risk, but drivers in sex hormone-related pathways may be further interrogated.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://cco.amegroups.com/article/view/10.21037/cco-24-124/rc
Peer Review File: Available at https://cco.amegroups.com/article/view/10.21037/cco-24-124/prf
Funding: This work was supported in part by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cco.amegroups.com/article/view/10.21037/cco-24-124/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration and its subsequent amendments.. Written informed consent was obtained from the patient for the publication of this article and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vilela A, Quingalahua E, Vargas A, et al. Global Prevalence of Pancreatic Cystic Lesions in the General Population on Magnetic Resonance Imaging: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2024;22:1798-1809.e6. [Crossref] [PubMed]
- Gonda TA, Cahen DL, Farrell JJ. Pancreatic Cysts. N Engl J Med 2024;391:832-43. [Crossref] [PubMed]
- Søreide K. Burgeoning rise in intraductal papillary mucinous neoplasia (IPMN) - a blessing in disguise. Scand J Gastroenterol 2023;58:1101-4. [Crossref] [PubMed]
- Søreide K, Marchegiani G. Clinical Management of Pancreatic Premalignant Lesions. Gastroenterology 2022;162:379-84. [Crossref] [PubMed]
- Kim HS, Choi YH, Jo IH, et al. Tracking incidentally discovered pancreatic cysts smaller than 30 mm: Natural course and predictors of malignancy. Dig Liver Dis 2024;56:137-43. [Crossref] [PubMed]
- Aunan JR, Al-Saiddi MS, Stutchfield B, et al. Pancreatic Cystic Lesions and Risk of Cancer. In: Søreide K, Stättner S, editors. Textbook of Pancreatic Cancer: Principles and Practice of Surgical Oncology. Cham: Springer International Publishing; 2021. p. 777-97.
- Fernández-del Castillo C. Mucinous cystic neoplasms. J Gastrointest Surg 2008;12:411-3. [Crossref] [PubMed]
- Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol 2007;20:S71-93. [Crossref] [PubMed]
- Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg 2000;231:205-12. [Crossref] [PubMed]
- Musleh A, Abbadi K, Asbah M, et al. Rare clinical entity of diffuse mucinous cystic neoplasm of the pancreas: A case report and review of the literature. Int J Surg Case Rep 2023;111:108859. [Crossref] [PubMed]
- Al Shamousi K, Al-Busafi SA, Kashoob M, et al. Mucinous Cystic Neoplasm of the Pancreas in Pregnancy: A Case Report. Cureus 2023;15:e50446. [Crossref] [PubMed]
- Tang H, Shen Z, Lu B. Huge mucinous cystic neoplasms with adhesion to the left colon: A case report and literature review. Open Med (Wars) 2022;17:2130-7. [Crossref] [PubMed]
- Fallahzadeh MK, Zibari GB, Wellman G, et al. Mucinous cystic neoplasm of pancreas in a male patient: a case report and review of the literature. J La State Med Soc 2014;166:67-9. [PubMed]
- Murakami Y, Uemura K, Morifuji M, et al. Mucinous cystic neoplasm of the pancreas with ovarian-type stroma arising in the head of the pancreas: case report and review of the literature. Dig Dis Sci 2006;51:629-32. [Crossref] [PubMed]
- Suzuki M, Fujita N, Onodera H, et al. Mucinous cystic neoplasm in a young male patient. J Gastroenterol 2005;40:1070-4. [Crossref] [PubMed]
- Hozaka Y, Ohtsuka T, Nakamura M, et al. Feasibility of Surveillance for Mucinous Cystic Neoplasm of the Pancreas: A Multi-Institutional Retrospective Study of 328 Patients by the Japanese Pancreatic Society. Pancreas 2023;52:e288-92. [Crossref] [PubMed]
- Ramia JM, Del Rio Martín J, Blanco-Fernández G, et al. Pancreatic mucinous cystic neoplasms located in the distal pancreas: a multicenter study. Gland Surg 2022;11:795-804. [Crossref] [PubMed]
- Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas 2004;28:241-6. [Crossref] [PubMed]
- Sung YN, Stojanova M, Shin S, et al. Gradual telomere shortening in the tumorigenesis of pancreatic and hepatic mucinous cystic neoplasms. Hum Pathol 2024;152:105653. [Crossref] [PubMed]
- Wong P, Pollini T, Adam MA, et al. Distinct Indications for Adjuvant Therapy in Resected Invasive Mucinous Cystic Neoplasms of the Pancreas Compared with Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 2024;31:8276-86. [Crossref] [PubMed]
- Fogliati A, Crippa S, Marchegiani G, et al. Implications of pregnancy on MCN of the pancreas: A multicentric case-control study. Pancreatology 2024;24:747-52. [Crossref] [PubMed]
- Xia Q, Li F, Min R, et al. Malignancy risk factors and prognostic variables of pancreatic mucinous cystic neoplasms in Chinese patients. World J Gastroenterol 2023;29:3119-32. [Crossref] [PubMed]
- Oyama K, Iwagami Y, Kobayashi S, et al. A Ruptured Mucinous Cystadenocarcinoma of the Pancreas Extensively Evaluated Before and After the Rupture: A Case Report. Pancreas 2023;52:e163-7. [Crossref] [PubMed]
- Dhamor D, Irrinki S, Naik A, et al. Pregnancy-associated mucinous cystic neoplasms of the pancreas - A systematic review. Am J Surg 2023;225:630-8. [Crossref] [PubMed]
- Vullierme MP, Gregory J, Rebours V, et al. MRI is useful to suggest and exclude malignancy in mucinous cystic neoplasms of the pancreas. Eur Radiol 2022;32:1297-307. [Crossref] [PubMed]
- Marchegiani G, Andrianello S, Crippa S, et al. Actual malignancy risk of either operated or non-operated presumed mucinous cystic neoplasms of the pancreas under surveillance. Br J Surg 2021;108:1097-104. [Crossref] [PubMed]
- Postlewait LM, Ethun CG, McInnis MR, et al. Association of Preoperative Risk Factors With Malignancy in Pancreatic Mucinous Cystic Neoplasms: A Multicenter Study. JAMA Surg 2017;152:19-25. [Crossref] [PubMed]
- Tomishima K, Fujisawa T, Fukumura Y, et al. Mucinous Cystadenocarcinoma of the Pancreas with Cyst Infection in a Male Patient. Intern Med 2020;59:2383-9. [Crossref] [PubMed]
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Agarwal A, Scott FI, Ahmad NA, et al. Chronic immunosuppression does not potentiate the malignant progression of mucinous pancreatic cystic lesions. Pancreatology 2016;16:900-4. [Crossref] [PubMed]
- Ahmad M, Maegawa FB, De La Rosa E, et al. Mucinous cystic neoplasms of the pancreas in the modern era. Experience with 707 patients. Am J Surg 2020;220:1433-7. [Crossref] [PubMed]
- Alkhateeb MA, Boqari D, Mansi NK. Pancreatic acinar cystadenoma in a background of diffuse multifocal pancreatic cystic lesions: A case report. Int J Surg Case Rep 2020;73:223-7. [Crossref] [PubMed]
- An S, Kim MJ, Kim SJ, et al. Multiple KRAS mutations in the non-mucinous epithelial lining in the majority of mucinous cystic neoplasms of the pancreas. Histopathology 2019;75:559-67. [Crossref] [PubMed]
- Barutcuoglu B, Oruc N, Ak G, et al. Co-analysis of pancreatic cyst fluid carcinoembryonic antigen and glucose with novel cut-off levels better distinguishes between mucinous and non-mucinous neoplastic pancreatic cystic lesions. Ann Clin Biochem 2022;59:125-33. [Crossref] [PubMed]
- Chang YT, Tien YW, Jeng YM, et al. Overweight increases the risk of malignancy in patients with pancreatic mucinous cystic neoplasms. Medicine (Baltimore) 2015;94:e797. [Crossref] [PubMed]
- Chaudhari VA, Pradeep R, Ramesh H, et al. Surgery for cystic tumors of pancreas: Report of high-volume, multicenter Indian experience over a decade. Surgery 2019;166:1011-6. [Crossref] [PubMed]
- Chen Y, Guo C, Zhang Q, et al. Patients with pancreatic cystic neoplasms can benefit from management of multidisciplinary team: Experience from a Chinese academic center. Pancreatology 2018;18:799-804. [Crossref] [PubMed]
- Chien CY, Wang SY, Liao CH, et al. Evaluation of Clinical Parameters to Distinguish Mucinous Cystic Neoplasms from Serous Cystic Neoplasms of Pancreas- A Retrospective Study. Journal of Clinical and Diagnostic Research 2017;11:XC06-9. [Crossref]
- Choi JH, Kim JH, Kim CH, et al. Pancreatic mucinous cystadenoma of borderline malignancy associated with Clonorchis sinensis. Korean J Intern Med 2015;30:398-401. [Crossref] [PubMed]
- Chu LC, Park S, Soleimani S, et al. Classification of pancreatic cystic neoplasms using radiomic feature analysis is equivalent to an experienced academic radiologist: a step toward computer-augmented diagnostics for radiologists. Abdom Radiol (NY) 2022;47:4139-50. [Crossref] [PubMed]
- Dong Z, Chen X, Cheng Z, et al. Differential diagnosis of pancreatic cystic neoplasms through a radiomics-assisted system. Front Oncol 2022;12:941744. [Crossref] [PubMed]
- Ethun CG, Postlewait LM, McInnis MR, et al. The diagnosis of pancreatic mucinous cystic neoplasm and associated adenocarcinoma in males: An eight-institution study of 349 patients over 15 years. J Surg Oncol 2017;115:784-7. [Crossref] [PubMed]
- Fan X, Wang W, Li C, et al. An osteoclast-like giant cell tumor embedded in the mural nodule of a pancreatic mucinous cystic neoplasm: A case report and literature review. Medicine (Baltimore) 2019;98:e15246. [Crossref] [PubMed]
- Gao J, Han F, Wang X, et al. Multi-Phase CT-Based Radiomics Nomogram for Discrimination Between Pancreatic Serous Cystic Neoplasm From Mucinous Cystic Neoplasm. Front Oncol 2021;11:699812. [Crossref] [PubMed]
- Gilani SM, Adeniran AJ, Cai G. Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytologic Evaluation of Intraductal Papillary Mucinous Neoplasm and Mucinous Cystic Neoplasms of Pancreas. Am J Clin Pathol 2020;154:559-70. [Crossref] [PubMed]
- Griffin JF, Page AJ, Samaha GJ, et al. Patients with a resected pancreatic mucinous cystic neoplasm have a better prognosis than patients with an intraductal papillary mucinous neoplasm: A large single institution series. Pancreatology 2017;17:490-6. [Crossref] [PubMed]
- Gurzu S, Bara T, Molnar C, et al. The epithelial-mesenchymal transition induces aggressivity of mucinous cystic neoplasm of the pancreas with neuroendocrine component: An immunohistochemistry study. Pathol Res Pract 2019;215:82-9. [Crossref] [PubMed]
- Hui L, Rashid A, Foo WC, et al. Significance of T1a and T1b Carcinoma Arising in Mucinous Cystic Neoplasm of Pancreas. Am J Surg Pathol 2018;42:578-86. [Crossref] [PubMed]
- Hwang J, Kim YK, Min JH, et al. Comparison between MRI with MR cholangiopancreatography and endoscopic ultrasonography for differentiating malignant from benign mucinous neoplasms of the pancreas. Eur Radiol 2018;28:179-87. [Crossref] [PubMed]
- Jabłońska B, Braszczok Ł, Szczęsny-Karczewska W, et al. Surgical treatment of pancreatic cystic tumors. Pol Przegl Chir 2017;89:1-8. [Crossref] [PubMed]
- Khoury T, Kadah A, Mari A, et al. The Utility of Endoscopic Ultrasound Fine Needle Aspiration in Pancreatic Cystic Lesions Diagnosis. Diagnostics (Basel) 2020;10:507. [Crossref] [PubMed]
- Li C, Lin X, Hui C, et al. Computer-Aided Diagnosis for Distinguishing Pancreatic Mucinous Cystic Neoplasms From Serous Oligocystic Adenomas in Spectral CT Images. Technol Cancer Res Treat 2016;15:44-54. [Crossref] [PubMed]
- Liang H, Xie W, Lin X, et al. Pathologic T1 and T2 encapsulated invasive carcinomas arising from mucinous cystic neoplasms of the pancreas have favorable prognosis and might be treated conservatively. J Pathol Clin Res 2021;7:507-16. [Crossref] [PubMed]
- Lin XZ, Wu ZY, Li WX, et al. Differential diagnosis of pancreatic serous oligocystic adenoma and mucinous cystic neoplasm with spectral CT imaging: initial results. Clin Radiol 2014;69:1004-10. [Crossref] [PubMed]
- Lipinski M, Degowska M, Rydzewska G. Cystic fluid neutrophil gelatinase-associated lipocalin (NGAL) concentration in differential diagnosis of pancreatic cystic lesions: a new factor enters the scene? Prz Gastroenterol 2018;13:132-6. [Crossref] [PubMed]
- Oh HC, Kang H, Brugge WR. Cyst fluid amylase and CEA levels in the differential diagnosis of pancreatic cysts: a single-center experience with histologically proven cysts. Dig Dis Sci 2014;59:3111-6. [Crossref] [PubMed]
- Oh SH, Lee JK, Lee KT, et al. The Combination of Cyst Fluid Carcinoembryonic Antigen, Cytology and Viscosity Increases the Diagnostic Accuracy of Mucinous Pancreatic Cysts. Gut Liver 2017;11:283-9. [Crossref] [PubMed]
- Ohtsuka T, Nakamura M, Hijioka S, et al. Prediction of the Probability of Malignancy in Mucinous Cystic Neoplasm of the Pancreas With Ovarian-Type Stroma: A Nationwide Multicenter Study in Japan. Pancreas 2020;49:181-6. [Crossref] [PubMed]
- Ohtsuka T, Takahata S, Takanami H, et al. Laparoscopic surgery is applicable for larger mucinous cystic neoplasms of the pancreas. J Hepatobiliary Pancreat Sci 2014;21:343-8. [Crossref] [PubMed]
- Paziewska A, Polkowski M, Goryca K, et al. Mutational Mosaics of Cell-Free DNA from Pancreatic Cyst Fluids. Dig Dis Sci 2020;65:2294-301. [Crossref] [PubMed]
- Park JW, Jang JY, Kang MJ, et al. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology 2014;14:131-6. [Crossref] [PubMed]
- Pezzilli R, Buscarini E, Pollini T, et al. Epidemiology, clinical features and diagnostic work-up of cystic neoplasms of the pancreas: Interim analysis of the prospective PANCY survey. Dig Liver Dis 2020;52:547-54. [Crossref] [PubMed]
- Revoredo-Rego F, Reano-Paredes G, de Vinatea-de Cardenas J, et al. Characteristics of pancreatic mucinous cystic neoplasm in patients treated at a National Hospital in Peru. Rev Peru Med Exp Salud Publica 2019;36:670-5. [Crossref] [PubMed]
- Ribaldone DG, Bruno M, Gaia S, et al. Differential diagnosis of pancreatic cysts: A prospective study on the role of intra-cystic glucose concentration. Dig Liver Dis 2020;52:1026-32. [Crossref] [PubMed]
- Roch AM, Bigelow K, Schmidt CM 2nd, et al. Management of Undifferentiated Solitary Mucinous Cystic Lesion of the Pancreas: A Clinical Dilemma. J Am Coll Surg 2017;224:717-23. [Crossref] [PubMed]
- Scourtas A, Dudley JC, Brugge WR, et al. Preoperative characteristics and cytological features of 136 histologically confirmed pancreatic mucinous cystic neoplasms. Cancer Cytopathol 2017;125:169-77. [Crossref] [PubMed]
- Tamura S, Yamamoto H, Ushida S, et al. Mucinous cystic neoplasms in male patients: two cases. Rare Tumors 2017;9:7096. [Crossref] [PubMed]
- Tian H, Zhang B, Zhang Z, et al. DenseNet model incorporating hybrid attention mechanisms and clinical features for pancreatic cystic tumor classification. J Appl Clin Med Phys 2024;25:e14380. [Crossref] [PubMed]
- Wang G, Dang H, Yu P, et al. Multiparameter Analysis Using (18)F-FDG PET/CT in the Differential Diagnosis of Pancreatic Cystic Neoplasms. Contrast Media Mol Imaging 2021;2021:6658644. [Crossref] [PubMed]
- Wang GX, Wang ZP, Chen HL, et al. Discrimination of serous cystadenoma from mucinous cystic neoplasm and branch duct intraductal papillary mucinous neoplasm in the pancreas with CT. Abdom Radiol (NY) 2020;45:2772-8. [Crossref] [PubMed]
- Xie H, Ma S, Guo X, et al. Preoperative differentiation of pancreatic mucinous cystic neoplasm from macrocystic serous cystic adenoma using radiomics: Preliminary findings and comparison with radiological model. Eur J Radiol 2020;122:108747. [Crossref] [PubMed]
- Yang J, Guo X, Zhang H, et al. Differential diagnosis of pancreatic serous cystadenoma and mucinous cystadenoma: utility of textural features in combination with morphological characteristics. BMC Cancer 2019;19:1223. [Crossref] [PubMed]
- Yang Z, Shi G. Comparison of clinicopathologic characteristics and survival outcomes between invasive IPMN and invasive MCN: A population-based analysis. Front Oncol 2022;12:899761. [Crossref] [PubMed]
- Ye M, Zhang B, Han X, et al. Low-Pass Genomic Sequencing Reveals Novel Subtypes of Pancreatic Cystic Neoplasms. Ann Surg Oncol 2023;30:5804-12. [Crossref] [PubMed]
- Yuan Z, Li J, Yang L, et al. The differential diagnosis of pancreatic cystic neoplasms with conventional ultrasound and contrast-enhanced ultrasound. Quant Imaging Med Surg 2024;14:4304-18. [Crossref] [PubMed]
- Zhang W, Linghu E, Chai N, et al. New criteria to differentiate between mucinous cystic neoplasm and serous cystic neoplasm in pancreas by endoscopic ultrasound: A preliminarily confirmed outcome of 41 patients. Endosc Ultrasound 2017;6:116-22. [Crossref] [PubMed]
- Zhang G, Chen W, Wang Z, et al. Automated diagnosis of pancreatic mucinous and serous cystic neoplasms with modality-fusion deep neural network using multi-modality MRIs. Front Oncol 2023;13:1181270. [Crossref] [PubMed]
- Salvia R, Burelli A, Nepi A, et al. Pancreatic cystic neoplasms: Still high rates of preoperative misdiagnosis in the guidelines and endoscopic ultrasound era. Surgery 2023;174:1410-5. [Crossref] [PubMed]
- Nguyen D, Dawson DW, Hines OJ, et al. Mucinous cystic neoplasms of the pancreas: are we overestimating malignant potential? Am Surg 2014;80:915-9. [Crossref] [PubMed]
- Kim GH, Choi K, Paik N, et al. Diagnostic Concordance and Preoperative Risk Factors for Malignancy in Pancreatic Mucinous Cystic Neoplasms. Gut Liver 2022;16:637-44. [Crossref] [PubMed]
- Solis-Pazmino P, Pazmino C, Termeie O, et al. Predictors of malignant transformation in mucinous pancreatic cystic neoplasm: A systemic review and meta-analysis. Surg Oncol 2024;57:102153. [Crossref] [PubMed]
- Kim H, Kim JH, An J, et al. Comparison with surgically resected mucinous cystic neoplasm of pancreas and branch-duct type intraductal papillary mucinous neoplasm considering clinico-radiological high-risk features: a reassessment of current guidelines. Abdom Radiol (NY) 2024;49:2746-55. [Crossref] [PubMed]
- Søreide K, Olsen F, Nymo LS, et al. A nationwide cohort study of resection rates and short-term outcomes in open and laparoscopic distal pancreatectomy. HPB (Oxford) 2019;21:669-78. [Crossref] [PubMed]
- Søreide K, Nymo LS, Kleive D, et al. Variation in use of open and laparoscopic distal pancreatectomy and associated outcome metrics in a universal health care system. Pancreatology 2019;19:880-7. [Crossref] [PubMed]
- Dai MH, Shi N, Xing C, et al. Splenic preservation in laparoscopic distal pancreatectomy. Br J Surg 2017;104:452-62. [Crossref] [PubMed]
- Gorris M, van Bodegraven EA, Abu Hilal M, et al. Outcomes after distal pancreatectomy with or without splenectomy for intraductal papillary mucinous neoplasm: international multicentre cohort study. Br J Surg 2024;111:znad424. [Crossref] [PubMed]
- van Ramshorst TME, van Hilst J, Boggi U, et al. Standardizing definitions and terminology of left-sided pancreatic resections through an international Delphi consensus. Br J Surg 2024;111:znae039. [Crossref] [PubMed]
- Søreide K, Sparrelid E. Defining what is left in a left-sided pancreatectomy. Br J Surg 2024;111:znae096. [Crossref] [PubMed]
- Bruna CL, van Hilst J, Esposito A, et al. The value of splenectomy during left-sided pancreatectomy for pancreatic ductal adenocarcinoma: predefined subanalysis in the DIPLOMA randomized trial. Br J Surg 2024;111:znae236. [Crossref] [PubMed]
- Ishida K, Sasano H, Moriya T, et al. Immunohistochemical analysis of steroidogenic enzymes in ovarian-type stroma of pancreatic mucinous cystic neoplasms: Comparative study of subepithelial stromal cells in intraductal papillary mucinous neoplasms of the pancreas. Pathol Int 2016;66:281-7. [Crossref] [PubMed]
- Kumata H, Murakami K, Ishida K, et al. Steroidogenesis in ovarian-like mesenchymal stroma of hepatic and pancreatic mucinous cystic neoplasms. Hepatol Res 2018;48:989-99. [Crossref] [PubMed]
- Fukushima N, Zamboni G. Mucinous cystic neoplasms of the pancreas: update on the surgical pathology and molecular genetics. Semin Diagn Pathol 2014;31:467-74. [Crossref] [PubMed]
- Regi P, Salvia R, Cena C, et al. Cystic "feminine" pancreatic neoplasms in men. Do any clinical alterations correlate with these uncommon entities? Int J Surg 2013;11:157-60. [Crossref] [PubMed]
- Sano M, Driscoll DR, De Jesus-Monge WE, et al. Activated wnt signaling in stroma contributes to development of pancreatic mucinous cystic neoplasms. Gastroenterology 2014;146:257-67. [Crossref] [PubMed]
- Conner JR, Mariño-Enríquez A, Mino-Kenudson M, et al. Genomic Characterization of Low- and High-Grade Pancreatic Mucinous Cystic Neoplasms Reveals Recurrent KRAS Alterations in "High-Risk" Lesions. Pancreas 2017;46:665-71. [Crossref] [PubMed]
- Fischer CG, Wood LD. From somatic mutation to early detection: insights from molecular characterization of pancreatic cancer precursor lesions. J Pathol 2018;246:395-404. [Crossref] [PubMed]
- Naqvi AAT, Hasan GM, Hassan MI. Investigating the role of transcription factors of pancreas development in pancreatic cancer. Pancreatology 2018;18:184-90. [Crossref] [PubMed]


