Advances in evidence-based cancer adoptive cell therapy
Introduction
Cancer is a major global public health problem and the number of newly diagnosed cases continues to increase due to aging and growth of worldwide populations. Although advancement in early detection, prevention, and treatment options, cancer is still the second cause of human mortality. To date, most cancers are clinically diagnosed at the advanced stages of diseases, which result in curable surgery not an option, while chemotherapy and radiotherapy are not effective in cure of most cancer patients. During the past decade, development of modern medicine, such as target therapy has still relatively short-term benefits for selected patients (1).
In recent years, different studies demonstrated that enhancement of cytotoxicity to target the cellular immune system could help clinicians to fight human cancer (2). For instance, sipuleucel-T, the first therapeutic cancer vaccine approved by US FDA, is an autologous active cell immunotherapy to improve overall survival (OS) of patients with metastatic castration-resistant prostate cancer in phase III clinical trials (3). Ipilimumab (4), a human monoclonal antibody that activates the immune system by cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), has increased the OS rate of patients with melanoma by 45.6% at 12 months, 33.2% at 18 months, and 23.5% at 24 months. Programmed cell death 1 (PD-1) is another negative co-stimulatory receptor expressed primarily on the surface of activated T cells (5,6) and anti-PD-1 antibodies (pembrolizumab and nivolumab) were able to not only increase OS of patients with metastatic melanoma (7,8), but also to reach better response rate and progression-free survival (PFS) of patients with advanced, previously treated squamous-cell NSCLC (9-11). Thus, immunotherapy shows encouraging in control of human cancers.
Adoptive cell therapy (ACT) is another potentially useful approach to treat human cancers (12) and previous studies showed that such a cancer immunotherapy can include non-specific cell therapy [lymphokine-activated killer (LAK) cells, cytokine-induced killer (CIK) cells and natural killer cells (NK)], specific cell therapy [cytotoxic T lymphocytes (CTLs), tumor-infiltrating lymphocytes (TILs), T cell therapy with modified T cell receptor (TCR) genes, T cell therapy with modified chimeric antigen receptors (CAR) genes] and so on. ACT is to stimulate body own immune system in order to trigger antitumor immune response, and eventually enable natural abilities to better recognize, target, and, finally, eliminate cancer cells from human body. Compared to other forms of cancer immunotherapy, ACT has multiple advantages, e.g., long-term benefit after short-term treatment and slight fewer adverse events. Although dendritic cells (DCs) and CTLs therapy have made a great progress, clinical applications are still somewhat limited. Recently, the genetic-modified T cells expressing specific TCRs or CARs are just now entering the clinical trials and the data have shown a great potential for high avidity to tumor-associated antigens and long-lasting anti-tumor responses, which encourages researchers to continuously study feasibility of this therapy in human cancers (13-15). Thus, our current review summarized the most recent advances and discussed and predicted the future research directions in this field.
Non-specific cell therapy
Non-specific cell therapy is an immunotherapy to non-specifically activate immune cells to induce their “non”-specific antitumor immune response to reject and destroy cancer cells. Based on different types of immune cells involved, non-specific cell therapy can be divided into LAK cell, CIK cell, and NK cell-mediated antitumor therapies. This non-specific cell therapy was used to treat different human cancers clinically (16-18).
LAK cell-mediated non-specific cell therapy
Grimm and his colleagues first reported in 1982 (19) non-specific killer cells generated by culture of peripheral blood mononuclear cells (PBMC) with high dose of interleukin-2 (IL-2), which was named as LAK cells. LAK cells contain a mixture of T cells and NK cells, both of which are not restricted by the major histocompatibility complex (MHC) against a broad range of tumor cells in vitro (16,20) .
Thereafter, Rosenberg and associates (16) showed effects of autologous LAK cells and IL-2 on patients with advanced cancers in whom standard therapy had failed. The objective tumor regression achieved in 11 out of 25 patients, i.e., a complete tumor regression (CR) occurred in 1 patient with metastatic melanoma and partial responses (PRs) occurred in 9 patients with pulmonary or hepatic metastases from melanoma, colon cancer, or renal-cell cancer and in patients with primarily unresectable lung adenocarcinoma. In 1988, another study summarized a series of clinical trials using high-dose of IL-2 alone or in combination with LAK (17). Of 221 patients, 16 had a CR in patients with metastatic cancer and an additional 26 had a partial tumor regression (PR). Based on these studies, LAK cell therapy has been considered to be effective against metastatic melanoma, renal cell carcinoma, and other advanced solid tumors.
However, in another randomized clinical trial, the result suggested a trend toward improving survival when IL-2 was given together with LAK cells to melanoma patients, but not occur in patients with renal cell carcinoma (21). Moreover, a randomized phase III trial of IL-2 with or without LAK cells in treatment of patients with advanced renal cell carcinoma demonstrated that there was no difference in treatment response (P=0.61) and survival (P=0.67) between these two treatment arms and more patients on the LAK arm experienced pulmonary toxicity (22). In addition, several other studies showed the safety and efficacy of LAK cell therapy in patients with malignant gliomas (23-25). In one study, a median OS of 31 patients with glioblastoma multiforme (GBM) was 17.5 months versus 13.6 months in control group (23). Boiardi et al. reported that the focal injection of LAK cells and IL-2 in 9 recurrent GBM patients were well-tolerated and the response rate was 33%, although the median OS of patients didn’t show significant improvement (26).
Thus, although LAK cell therapy seemed to effectively kill some tumor cells, high toxicity caused by high dose of IL-2 (such as vascular leakage and severe hypotension) (26,27) limited its clinical usage.
CIK cell-mediated non-specific cell therapy
CIK cells are obtained by isolated from PBMCs and then stimulated with a cocktail of interferon-gamma (IFN-γ), anti-CD3 monoclonal antibody, and IL-2 in a stepwise in a time-dependent ex vivo culturing process for approximately 2 weeks (28). CIK cells are a mixture of cells with non-MHC-restricted cytolytic activity, including CD3+CD56−T cells and CD3+CD56+NK-T cells, and a relatively minor population of CD3−CD56+NK cells (29-31). Compared to standard IL-2 stimulated LAK cells, CIK cells have enhanced antitumor cytotoxic activity by over 70-fold (32). The lytic activity can be further enhanced by addition of IL-1, IFN-γ, IL-7, IL-15, and other cytokines (33-36). To date, CIK cells have been evaluated as an adoptive cell immunotherapy for cancer patients in numerous clinical trials (summarized in Table 1). In the first phase I clinical study, autologous immunological effector cells were transfected with the IL-2 gene and to treat patients with metastatic renal cancer, colorectal cancer and lymphoma and data showed that 6 patients remained in disease progression, 3 showed stable disease (SD), and only 1 lymphoma patient had a complete response (CR) (37). PubMed search of the international registry on CIK cells (IRCC) (18) for “CIK cells clinical trials” found 11 such clinical trials in 2011 that contained 384 patients treated with autologous CIK cell immunotherapy, of which 24 patients had a CR, 27 patients had a PR, and 40 patients had a minor response. The total response rate (RR) was 23.7% (91/384), while 161 patients (41.9%) had a SD and 129 patients (33.6%) had a progressive disease (PD). Only 3 patients had tumor volume decreased. The side effects of CIK cell treatment were minimal and at final data analysis, which indicated that adjuvant immunotherapy with CIK cells could prevent tumor recurrence and improve quality of life and progression-free survival (PFS) rate in patients. A latest study published in Gastroenterology (62) showed the efficacy and safety of a multicenter, randomized, open-label, phase III trial using activated CIK cells as the adjuvant immunotherapy that included 230 hepatocellular carcinoma patients after surgical resection, radiofrequency ablation, or percutaneous ethanol injection in South Korea. The median time of recurrence-free survival (RFS) was 44.0 months in the immunotherapy group vs. 30.0 months in the control group. However, patients in the immunotherapy group had higher proportion of adverse events than in the control group, although the proportion of serious adverse events did not differ significantly between these two groups of patients.
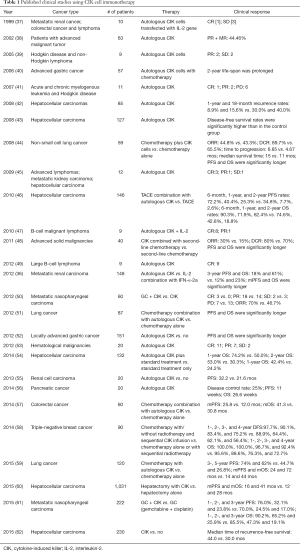
Full table
In China, there have been numbers of such clinical trials using CIK cell immunotherapy of human cancers. For example, Liu et al. (36) reported 148 patients with metastatic renal clear cell carcinoma randomized to autologous CIK cell immunotherapy (arm 1, n=74) or IL-2 treatment combination with IFN-α-2a (arm 2, n=74). The 3-year PFS and OS in arm 1 were 18% and 61%, respectively, as compared to 12% and 23%, respectively, in arm 2. The median PFS and OS in arm 1 were significantly longer than those in arm 2. Pan et al. (58) reported 90 patients with post-mastectomy triple-negative breast cancer in a retrospective study and 45 patients received chemotherapy alone or with sequential radiotherapy and 45 patients received chemotherapy with/without radiotherapy and sequential CIK infusion. The 1-, 2-, 3-, and 4-year disease-free survival (DFS) rates in the CIK group were 97.7%, 90.1%, 83.4%, and 75.2%, respectively, vs. 88.9%, 64.4%, 62.1%, and 56.4%, respectively, in the control group. Also the 1-, 2-, 3-, and 4-year OS rates were significantly higher in treatment group (100.0%, 100.0%, 96.7%, and 92.4%, respectively, vs. 95.6%, 88.6%, 76.3%, and 72.7%, respectively, in the control group). In subgroup analyses, CIK adjuvant therapy increased DFS rate of patients with pathologic grade III disease and significantly increased the OS rate of patients with N1, N2, N3, IIB, or III TNM disease. These data indicate that adjuvant CIK treatment combined with chemotherapy was an effective therapeutic strategy.
Thus, overall, these trials certainly demonstrated that CIK cells therapy was feasible and safe in patients with hepatocellular carcinoma, renal cell carcinoma, non-small cell lung cancer, gastric cancer, and other solid tumors. In most cancer patients, adjuvant CIK cell therapy combination with conventional treatment had better clinical outcomes than standard therapy alone.
NK cell-mediated non-specific cell therapy
NK cells were first identified and characterized by Herberman et al. (63) and Kiessling et al. (64) in 1975 as a unique subset of lymphocytes that are larger in size than regular T and B lymphocytes and contain distinctive cytoplasmic granules. In human beings, NK cells are defined by expression of the surface marker CD56 and lack of the T cell markers, such as CD3 or TCR (65) and account for approximately 5% to 15% of human peripheral blood lymphocytes (66). NK cells are innate lymphocytes with the capacity to target foreign, damaged, malignant, and virally infected cells without prior immunization or MHC restriction. The cytotoxic granules released by NK cells up on targeting cells are largely composed of perforin and granzyme. Beyond their cytotoxicity, NK activation also leads to release of cytokines IFN-γ, TNF-α, G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3 and others (67,68).
As NK cells are found primarily in blood, NK cell therapy has been most successful in hematopoietic malignancies (69-73). Currently, NK cells can be used to treat patients with refractory leukemia before conventional hematopoietic cell transplantation (HCT) for induction of remission, after HCT as consolidation, or replace of HCT. A number of studies have shown encouraging results of NK adoptive infusion in patients with acute myeloid leukemia (AML) (74-77). At the same time, NK cells have also been assessed in many non-hematopoietic forms of cancer. Clinical trials showed transfusion of autologous NK cells was safe with no negative side effects on patients with metastatic colorectal cancer, non-small cell lung cancer, metastatic melanoma or renal cell carcinoma, although there was no significant clinical response observed (78). Thus, due to the limited clinical response associated with autologous NK cell therapy, adoptive transfusion of allogeneic NK cells was explored as an alternative.
Furthermore, allogeneic NK cells are thought to be of non-cross-resistant mechanisms and minimal overlapping toxicities for cancer therapy. Safety and efficacy of allogeneic NK cell transfusions were established in patients with metastatic melanoma, renal cell carcinoma, refractory Hodgkin’s disease, and refractory AML (74,79-81). In a study of allogeneic NK cell therapy of AML patients, complete remissions were observed in 26% of patients and nearly all patients showed an expansion in NK cells after IL-2 therapy (74). In a NKAML pilot study (82), children with high risk of AML who achieved first complete remission after conventional chemotherapy received infusion of haploidentical NK cells. Non-hematologic toxicity was limited with no graft-versus-host disease (GVHD). The 2-year event-free survival was 100%. In another study, 5 of 19 adults with advanced AML achieved a complete hematological response.
NK-92 cells, a pure allogeneic activated NK cell line (83), have been successfully used as effector cells for cancer therapy. Arai’s group (83) first used NK-92 cells in patients with advanced renal cell carcinoma or melanoma and showed the safety and efficacy of a large-scale NK-92 expansion and 2 patients’ experienced transient minor decreases in tumor size. Although NK-92 therapy was reported to be safe, limited data were accumulated to date on the efficacy of this approach. In summary, NK cells could be promising cancer therapy approach, especially allogeneic NK cell infusion, which may become a new area of novel cell-based immunotherapy against human cancer.
Specific immunotherapy
Compared to non-specific cell therapy, specific immunotherapy is to specifically activate or modulate lymphocytes for target particular genes that are activated tumor cells (12); therefore, to destroy these tumor cells.
TIL-mediating specific immunotherapy
More than 100 years ago, it was noted that malignant tumors contain variable numbers of lymphocytes (84), which have come to be known as tumor infiltrating lymphocytes (TILs), which represent the local immune response directed against tumor growth and metastasis. TILs are composed of a mixture of lymphocytes with multiple phenotypic and functional properties, such as CD4+ and CD8+ lymphocytes. Several previous studies demonstrated that CD8+ TILs are generally associated with tumor regression, whereas the role of CD4+ TILs in cancer is controversial. Generally, both CD4+ and CD8+ TILs are necessary for effective tumor elimination (85,86). TILs grade was an independent predictor of sentinel lymph node status and survival of patients with cutaneous melanoma (87). Recently, TILs were also recognized to be associated with pathologic response to neoadjuvant therapy and DFS and OS after adjuvant chemotherapy of triple-negative and human epidermal growth factor receptor 2 (HER2)-positive breast cancers (88).
Rosenberg’s group first described the expansion of human TILs as immunotherapy in 1987 (89). TILs were successfully expanded from 24 of 25 consecutive human tumors, including 6 melanomas, 10 sarcomas, and 8 adenocarcinomas and used for immunotherapy of human cancers. In 1988, they further showed the adoptive transfusion of autologous TILs in treatment of patients with metastatic melanoma (90) and objective regression of tumor occurred in 9 of 15 patients (60%) who had not previously been treated with IL-2 and in 2 of 5 patients (40%) in whom previous failed with IL-2 therapy. In 1994 (91), this group increased number of the patients to 86, and showed that treatment with TILs and IL-2 with or without cyclophosphamide could result in objective responses in approximately one third of patients with metastatic melanoma. These data illustrated the potential value of lymphocytes in treatment of melanoma.
In the past few years, an increasing number of TILs infusion in combination with high-dose IL-2 and non-myeloablative (NMA) lymphodepletion chemotherapy has been reported and metastatic melanoma patients after such therapy had clinical responses up to 50% (92-94). For instance, a clinical trial (94) of 93 refractory melanoma with NMA and with or without 2 to 12 Gy of total-body irradiation (TBI) showed 48%, 52%, and 72% of the overall response rates (ORR) and 13%, 20%, and 40% of the CR, respectively. Data from Besser et al. (95) showed 29% and 9.8 months of the ORR and median survival in control patients vs. 40% and 15.2 months, respectively, in stage IV melanoma patients after treated with TILs and a high-dose of IL-2 following NMA. Five patients achieved CR and 18 PR and the 3-year survival of responding patients was 78%. Thus, ACT using autologous TILs is considered to be the most effective approach to induce ORR in metastatic melanoma patients.
However, TIL therapy may not be effective on other cancer types and the major limitation is the difficulty to identify antigen-specific T cells in those cancers, although it is now being increase in developing modifications for treatment of other solid tumors, such as cervical, pancreatic, lung, and head and neck cancers (http://www.clinicaltrials.gov/). For example, in a phase I trial of patients with locoregionally advanced nasopharyngeal carcinoma using adoptively transferred TILs following concurrent chemoradiotherapy (96), 19 of 20 patients exhibited an objective antitumor response, while 18 patients displayed DFS longer than 12 months after TILs infusion. There were only mild adverse events (AEs) observed and 1 patient had Grade 3 neutropenia (1/23, 5%).
Although TIL therapy of different human cancers developed slowly, a continuing progress has been made during the past several decades. The main advantage of TILs is the ability to specifically recognize tumor antigens, which is unfortunately also the disadvantage since most solid tumors don’t display such tumor antigens and those naturally occurring TILs fail to eliminate malignant cells. Thus, the main objectives of TIL-ACT are enhancing the immunogenicity and enlarging the numbers of activated tumor-specific T cells in tumor lesions.
Genetically engineered T cells-mediating specific immunotherapy
As described in the above, TILs have been shown to induce a durable tumor regression in melanoma patients. However, TILs therapy may not be effective in other types of cancers. Moreover, TILs therapy requires a surgical resection of tumor lesions from each patient to isolate and generate T cells with antitumor activity. Advances in genetically engineered T cells have overcome such obstacles by introducing tumor-antigen-targeting receptors into human peripheral blood T cells. During the past two decades, genetically engineered T cells expressed highly active T-cell receptors (TCRs) or CARs have translated from a laboratory technology to clinical evaluation.
T cell immunotherapy with modified TCR genes
TCR is a molecule found on the surface of T lymphocytes and be responsible for recognizing antigens bound to MHC, which contains two different protein chains α and β. These TCR α and β chains can be isolated from T cells of the rare patients who responded to tumors (97-99). Using the expression vectors, retrovirus, or lentivirus, we can genetically engineer TCRs into T cells (100,101) and then introduce these genetically modified T cells back to cancer patients. Thus, this novel strategy can produce a large amount of antigen-specific T cells and target tumor cells that express the target tumor-associated antigens (TAAs) presented by MHC molecules and release Th1 cytokines, including IFN-γ, GM-CSF, and TNF-a (102); therefore, to eliminate tumor lesions in patients.
The first specific gene target-transferred TCR-clinical trial was reported in 1999 that utilized a melanoma-antigen specific TCR MART-1 to introduce genetically modified T cell immunotherapy of patients with metastatic melanoma (103). They first prepared autologous lymphocytes from peripheral blood of patients and then successfully encoded a TCR through the retrovirus carrying genetically modified TCR chains. The data showed that 2 out 15 (13%) patients had responded after infusion of autologous TCRs, which was low than predicted 50% of such an approach; however, this method has potential for cancer patients for whom TILs are not available. Afterwards, the same team (104) showed the data on a subsequent clinical trial using newly established MART-1 and gp100-specific TCR genes to modified T cells for treatment of patients with metastatic melanoma. Objective cancer regression rates were 30% (6 of 20) and 19% (3 of 16) in patients who received the MART-1 and gp100-modified TCR, respectively. Another promising TAA was carcinoembryonic antigen (CEA), which is frequently overexpressed in many human cancers, most notably in colorectal adenocarcinoma. One study reported (104) that infusion of T cells after modified to target CEA cDNA resulted in decrease in serum CEA levels by 74–99% in patients and 1 patient had an objective regression of cancer metastatic to the lung and liver after T cells infusion. Another clinical trial (105) enrolled patients with positive NY-ESO-1, which is expressed in 80% of patients with synovial cell sarcoma and in approximately 25% of patients with melanoma and common epithelial tumors, for treatment with autologous TCR-transduced T cells plus 720,000 IU/kg of IL-2. Objective clinical responses were observed in 4 of 6 patients with synovial cell sarcoma and 5 of 11 melanoma patients. Two of 11 melanoma patients demonstrated complete tumor regression for more than one year. Moreover, a synovial cell sarcoma patient showed a PR lasting 18 months. In 2013, Morgan et al. (106) reported another study of 9 patients using autologous anti-MAGE-A3 TCR-engineered T cells and 5 patients experienced clinical regression of their cancers including 2 on-going responders. Furthermore, other cancer antigens, such as LAGE-1, MAGE-A4, and SSX-2, have also been investigated as tumor target antigens for genetically modified T cell immunotherapy. Some of their anti-tumor activities against different tumor cell lines and tumor models have also shown promising (107,108), although there is no report thus far in clinical trials.
However, although clinical response rate of infusion of genetically modified T cells is promising and the data supported more clinical usage of this approach, there have also been a number of reported toxicities related to “on target” toxicity to normal tissues but “off tissue” autoimmune toxicities effects on patients. The common side effects included exhibition of destruction in the skin, eye, and earod patients after infusion MART-1 and gp100-transduced T cells, while patients may also develop a severe transient inflammatory colitis after infusion of CEA-targeted T cells (104) and 3 of 9 patients had severe neurological toxicity in MAGE-A3 clinical trial (106). Thus, further investigation is needed to reduce side effects of genetically modified T cell immunotherapy but maximally to maintain their antitumor activities in clinic.
T cell therapy with modified CAR genes
T cell targeting specificity can be altered after expressing a single-chain CAR (109). The latter is composed of a specific antigen-binding motif derived from a monoclonal antibody that links VH with VL sequences to recognize a single chain fragment variable (scFv) region and signaling components derived from the ζ chain of the TCR/CD3 complex on co-stimulatory molecules T lymphocytes (110,111). To date, CAR research reached to four generations (112), i.e., the first-generation CAR contains a single signaling domain most commonly derived from the CD3ζ chain of the CD3/TCR complex. The second-generation CAR incorporates an additional intracellular co-stimulatory endodomains (such as CD28, OX40, or 4-1BB) to the basic first-generation receptor configuration in order to improve T-cell effector function. The third generation CAR includes a combination of CD28, 4-1BB, and CD3ζ signaling moieties. The fourth generation CAR or TRUCKS employ a vector or vectors to encode a CAR and also a CAR-responsive promoter (e.g., nuclear factor of activated T cells) to respond upon successful signaling of the CAR after the transgenic production of cytokines, such as IL-12. In preclinical models, T cells engrafted with the second and third generation CARs possessed greater effector functions and had potent non-cross-resistant clinical activity after infusion in three of three patients treated with advanced chronic lymphocytic leukemia (CLL) (112). Moreover, clinical protocols for CAR-T cells immunotherapy usually involve previously conditioned NMA and high dose of IL-2 therapy after CAR-T infusions, which facilitated the engraftment and persistence of CAR-T cells in tumor lesions. To date, previous studies demonstrated some successful pre-clinical models and phase I clinical trials in ovarian cancer (111), renal cell carcinoma (113,114), neuroblastoma (115,116), B-cell non-Hodgkin lymphoma (NHL), and mantle cell lymphoma (MCL) (117) with the first-generation CARs. The early phase clinical trials indicated this approach was feasible, but the ORR was mild and most patients did not have visibly or significantly clinical benefit. However, this CAR-modified T cell immunotherapy did show great response rate in NHL and MCL patients using genetically modified CD20 autologous T cell electroporation. Of the 7 treated patients, 2 had a CR, 1 achieved a PR, and 4 had SD (117).
Thus far, both second- and the third-generation CARs are in progress in the clinical trials and the third generation CAR-T cells showed to be more effective, although there is no study reporting the comparison to the effectiveness of the second generation CARs. Most clinical trials focused on CD19 or CD20 antigen in hematologic malignancies, such as NHL and lymphocytic leukemia (Table 2).
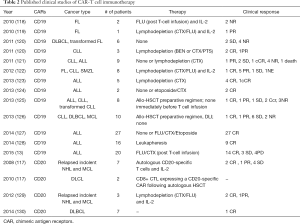
Full table
Indeed, the CAR-T cells have been successful in treating hematological malignancies, but their application for solid tumors has been greatly hampered. Clinical studies with CAR-T cells targeting HER2, CEA, VEGF-R2, EGFR, or GD2 in solid tumors (111,115,116,131-133) have demonstrated the feasibility, but the clinical effectiveness was generally disappointed.
In addition, different clinical trials indicated that CAR-T cell-based therapy was associated with noticeable side effects. To date, there were 2 death cases reported (CAR T cells targeted ERBB2 in 1 patient with widely metastatic colon cancer and another 1 with bulky chronic lymphocytic leukemia by targeting CD19). Significant toxicity such as hepatic toxicity (113), fatal pulmonary dysfunction (113), systemic inflammatory response syndrome (SIRS) or even “cytokine storm” (109,124) has been also reported in patients. These obvious toxicities were usually associated with the lack of discrimination between tumor and normal cells by CAR-T cells. However, the technology will continue to improve, the side effects will be overcome, and future directions will likely include combination therapies.
DC-based immunotherapy
DCs are antigen-presenting cells in the mammalian immune system and display an extraordinary capacity to stimulate antigen-specific cytolytic and memory T-cell responses to antigens by processing antigen material and presenting it on the cell surface to T-lymphocytes (134). Immature DCs are particularly efficient in uptake of tumor derived material, while mature DCs can activate tumor-reactive CD8+ CTLs and CD4+ T cells (135,136). Moreover, DCs also can induce NK cell cytotoxicity and the latter essentially contribute to eliminating tumor cells (137-139). DCs can also directly mediate tumor-directed cytotoxicity (140,141). Due to the numerous antitumor effects, DCs evolved as promising candidates in cancer immunotherapy (142). For example, DCs can be used for antitumor vaccination through various means, including tumor lysates, tumor antigen-derived peptides, synthetic MHC class I—restricted peptides, and whole protein. The first DC vaccine was used in 4 B-cell lymphoma patients using autologous antigen-pulsed DCs and the data were published in 1996 (143). In this pilot study, all patients developed measurable antitumor cellular immune responses. Since then, several DC vaccine clinical trials in patients with prostate cancer (144,145), melanoma (146,147), renal cell carcinoma (148,149), glioma (150), hepatocellular carcinoma (151-153), and pediatric solid tumor (154,155) have been reported. Although some of these trials did not reach the end point of primary study, others have reported positive results. In one notable trial, Provenge, monocyte-derived dendritic cells (moDCs) pulsed with fusion antigen protein consisting of prostatic acid phosphatase (PAP) and GM-CSF, the first therapeutic cancer vaccine to be approved by the U.S. Food and Drug Administration in 2010, showed to prolong median OS by 4.1 months for metastatic castration resistant prostate cancer (4). Tecemotide vaccine, DCs pulsed with MUC1 for inoperable stage III NSCLC as a maintenance therapy following either concurrent or sequential chemoradiotherapy (156) showed 25.6 months of median OS in tecemotide versus 22.3 months in placebo, but the OS had no significant difference in administration of tecemotide after chemoradiotherapy compared to placebo. Tecemotide might have a role in patients who initially receive concurrent chemoradiotherapy. NY-ESO-1 protein to target DCs vaccine was assessed in 45 patients with advanced malignancies and 13 patients experienced stabilization of disease, with a median duration of 6.7 months and 2 patients had tumor regression. There was no dose-limiting or grade 3 toxicity observed (157).
Since 2001, numerous DC vaccine clinical trials have been reported. Schadendorf et al. (158) had demonstrated that DC vaccine could not be more effective than DTIC chemotherapy in stage IV melanoma patients, but the follow-up data confirmed that melanoma patients did get clinical benefits after DC vaccines. Other two phase II clinical studies showed a clinical benefit (PR + SD) in 55.5% of evaluable cases to date (159,160). Furthermore, beyond large sample size clinical trials with autogeneic DC vaccines, numerous early phase clinical studies using autogeneic/allogeneic DCs with allogeneic tumor cells continue to be progress (161-164). It has been suggested that allogeneic DCs are more effective in both in vitro and in vivo. For example, in a phase I/II trial of metastatic melanoma patients undergoing DCs loaded with an allogeneic tumor cell lysate (165), 4 out of 9 patients survived for more than 20 months, 2 patients showed signs of clinical response, all of whom didn’t show any grade 3 or 4 adverse events related to the vaccines. In a sequential clinical trial reported allogeneic tumor cell vaccine with TGF-β in IV NSCLC patients, OS was 562 days and median survival was 660 days. Patients didn’t have significant toxic effect (166,167). However, the majority of such studies still remain in the pre-clinical models or Phase I/II clinical trials.
Genetic modification is another way to improve the effective of DC vaccines, which includes overexpression of positive regulators (e.g., cytokine, chemokine and co-stimulatory molecules) and inhibition of negative regulators [e.g., suppressor of cytokine signaling-1 (SOCS1), programmed death ligand 1 (PD-L1), or A20]. For example, GVAX, a GM-CSF gene-transfected tumor cell vaccine (168) can extant the time to progression and the median OS and improve quality of life of patients with metastatic melanoma, pancreatic cancer, prostate cancer, or other tumors (169-172). DC vaccine would clearly enhance host antitumor immune responses. Nevertheless, numerous phase I/II studies had an overall limited clinical benefit. Therefore, further improvement is required, which may be achieved by understanding of DC biology and combination of DC-based vaccination with traditional therapy.
Summary and future directions
In this review, we summarized up to date advancement in ACT, which indeed improves PFS, OS, DFS, and/ or quality of life of cancer patients, especially melanoma patients. Compared to other standard therapies, ACT can trigger immune response within cancer lesion to elicit persistent antitumor immune response for eliminating tumor cells and such treatment has nearly no serious side effect, although improvement of the clinical efficacy is warranted in future studies. Most recently, antitumor immunotherapy is very hot and different investigators have evaluated artificial antigen presenting cells, enhanced immunogenicity or generate genetically engineered T cells in preclinical models and in clinical trials. Moreover, combination of immune therapy together with surgery, chemotherapy and radiotherapy could be an effective way in control cancer progression.
Acknowledgements
Funding: This study was supported in part by grants from National High Technology Research and Development 863 Program of China (#2012AA02A201), National Natural Science Foundation of China (#81602029, #81260307, #81060185 and #81470005), National Clinical Key Specialty Construction Projects of Oncology of National Health and Family Planning Commission of China (Awarding to Tumor Hospital of Yunnan Province_#2013-2014), and the Yunnan Province Leading Talent Program of Health System (#L-201213).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Payne KK, Bear HD, Manjili MH. Adoptive cellular therapy of cancer: exploring innate and adaptive cellular crosstalk to improve anti-tumor efficacy. Future Oncol 2014;10:1779-94. [Crossref] [PubMed]
- Nabhan C. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:1966-7; author reply 1968.
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews. Cancer 2012;12:252-64. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62-8. [Crossref] [PubMed]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Davis JL, Theoret MR, Zheng Z, et al. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin Cancer Res 2010;16:5852-61. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
- Rosenberg SA. Immunotherapy of cancer by systemic administration of lymphoid cells plus interleukin-2. J Biol Response Mod 1984;3:501-11. [PubMed]
- Rosenberg SA, Lotze MT, Mule JJ. NIH conference. New approaches to the immunotherapy of cancer using interleukin-2. Ann Intern Med 1988;108:853-64. [Crossref] [PubMed]
- Hontscha C, Borck Y, Zhou H, et al. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol 2011;137:305-10. [Crossref] [PubMed]
- Grimm EA, Mazumder A, Zhang HZ, et al. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982;155:1823-41. [Crossref] [PubMed]
- Mulé JJ, Shu S, Schwarz SL, et al. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science 1984;225:1487-9. [Crossref] [PubMed]
- Rosenberg SA, Lotze MT, Yang JC, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst 1993;85:622-32. [Crossref] [PubMed]
- Law TM, Motzer RJ, Mazumdar M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995;76:824-32. [Crossref] [PubMed]
- Dillman RO, Duma CM, Schiltz PM, et al. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother 2004;27:398-404. [Crossref] [PubMed]
- Hayes RL, Koslow M, Hiesiger EM, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer 1995;76:840-52. [Crossref] [PubMed]
- Hayes RL, Arbit E, Odaimi M, et al. Adoptive cellular immunotherapy for the treatment of malignant gliomas. Crit Rev Oncol Hematol 2001;39:31-42. [Crossref] [PubMed]
- Boiardi A, Silvani A, Ruffini PA, et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother 1994;39:193-7. [Crossref] [PubMed]
- Papa MZ, Mule JJ, Rosenberg SA. Antitumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo: successful immunotherapy of established pulmonary metastases from weakly immunogenic and nonimmunogenic murine tumors of three district histological types. Cancer Res 1986;46:4973-8. [PubMed]
- Bonanno G, Iudicone P, Mariotti A, et al. Thymoglobulin, interferon-gamma and interleukin-2 efficiently expand cytokine-induced killer (CIK) cells in clinical-grade cultures. J Transl Med 2010;8:129. [Crossref] [PubMed]
- Franceschetti M, Pievani A, Borleri G, et al. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol 2009;37:616-628.e2. [Crossref] [PubMed]
- Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol 1994;153:1687-96. [PubMed]
- Sangiolo D, Martinuzzi E, Todorovic M, et al. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: implications for their infusion across major HLA barriers. Int Immunol 2008;20:841-8. [Crossref] [PubMed]
- Shablak A, Hawkins RE, Rothwell DG, et al. T cell-based immunotherapy of metastatic renal cell carcinoma: modest success and future perspective. Clin Cancer Res 2009;15:6503-10. [Crossref] [PubMed]
- Ochoa AC, Gromo G, Alter BJ, et al. Long-term growth of lymphokine-activated killer (LAK) cells: role of anti-CD3, beta-IL 1, interferon-gamma and -beta. J Immunol 1987;138:2728-33. [PubMed]
- Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med 2013;11:83. [Crossref] [PubMed]
- Yu J, Ren X, Li H, et al. Synergistic effect of CH-296 and interferon gamma on cytokine-induced killer cells expansion for patients with advanced-stage malignant solid tumors. Cancer Biother Radiopharm 2011;26:485-94. [Crossref] [PubMed]
- Liu L, Zhang W, Qi X, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res 2012;18:1751-9. [Crossref] [PubMed]
- Schmidt-Wolf IG, Finke S, Trojaneck B, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer 1999;81:1009-16. [Crossref] [PubMed]
- Chen FX, Liu JQ, Zhang NZ, et al. Clinical observation on adoptive immunotherapy with autologous cytokine-induced killer cells for advanced malignant tumor. Ai Zheng 2002;21:797-801. [PubMed]
- Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2005;11:181-7. [Crossref] [PubMed]
- Jiang J, Xu N, Wu C, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res 2006;26:2237-42. [PubMed]
- Introna M, Borleri G, Conti E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica 2007;92:952-9. [Crossref] [PubMed]
- Weng DS, Zhou J, Zhou QM, et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother 2008;31:63-71. [Crossref] [PubMed]
- Hui D, Qiang L, Jian W, et al. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis 2009;41:36-41. [Crossref] [PubMed]
- Wu C, Jiang J, Shi L, et al. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res 2008;28:3997-4002. [PubMed]
- Olioso P, Giancola R, Di Riti M, et al. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol 2009;27:130-9. [Crossref] [PubMed]
- Hao MZ, Lin HL, Chen Q, et al. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer 2010;29:172-7. [Crossref] [PubMed]
- Yang B, Lu XC, Zhu HL, et al. Clinical study of autologous cytokine induced killer cells combined with IL-2 for therapy of elderly patients with B-cell malignant lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2010;18:1244-9. [PubMed]
- Niu Q, Wang W, Li Y, et al. Cord blood-derived cytokine-induced killer cells biotherapy combined with second-line chemotherapy in the treatment of advanced solid malignancies. Int Immunopharmacol 2011;11:449-56. [Crossref] [PubMed]
- Lu XC, Yang B, Yu RL, et al. Clinical study of autologous cytokine-induced killer cells for the treatment of elderly patients with diffuse large B-cell lymphoma. Cell Biochem Biophys 2012;62:257-65. [Crossref] [PubMed]
- Li JJ, Gu MF, Pan K, et al. Autologous cytokine-induced killer cell transfusion in combination with gemcitabine plus cisplatin regimen chemotherapy for metastatic nasopharyngeal carcinoma. J Immunother 2012;35:189-95. [Crossref] [PubMed]
- Li R, Wang C, Liu L, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother 2012;61:2125-33. [Crossref] [PubMed]
- Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother 2012;61:2251-9. [Crossref] [PubMed]
- Yang B, Lu XC, Yu RL, et al. Repeated transfusions of autologous cytokine-induced killer cells for treatment of haematological malignancies in elderly patients: a pilot clinical trial. Hematol Oncol 2012;30:115-22. [Crossref] [PubMed]
- Yu X, Zhao H, Liu L, et al. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J Clin Immunol 2014;34:194-203. [Crossref] [PubMed]
- Zhang Y, Wang J, Wang Y, et al. Autologous CIK cell immunotherapy in patients with renal cell carcinoma after radical nephrectomy. Clin Dev Immunol 2013;2013:195691.
- Chung MJ, Park JY, Bang S, et al. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother 2014;63:939-46. [Crossref] [PubMed]
- Zhang J, Zhu L, Zhang Q, et al. Effects of cytokine-induced killer cell treatment in colorectal cancer patients: a retrospective study. Biomed Pharmacother 2014;68:715-20. [Crossref] [PubMed]
- Pan K, Guan XX, Li YQ, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res 2014;20:3003-11. [Crossref] [PubMed]
- Zhang J, Zhu L, Du H, et al. Autologous cytokine-induced killer cell therapy in lung cancer patients: a retrospective study. Biomed Pharmacother 2015;70:248-52. [Crossref] [PubMed]
- Pan QZ, Wang QJ, Dan JQ, et al. A nomogram for predicting the benefit of adjuvant cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Sci Rep 2015;5:9202. [Crossref] [PubMed]
- Li Y, Pan K, Liu LZ, et al. Sequential Cytokine-Induced Killer Cell Immunotherapy Enhances the Efficacy of the Gemcitabine Plus Cisplatin Chemotherapy Regimen for Metastatic Nasopharyngeal Carcinoma. PLoS One 2015;10:e0130620. [Crossref] [PubMed]
- Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology Gastroenterology 2015;148:1383-91.e6. [Crossref] [PubMed]
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer 1975;16:216-29. [Crossref] [PubMed]
- Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5:112-7. [Crossref] [PubMed]
- Liu D, Xu L, Yang F, et al. Rapid biogenesis and sensitization of secretory lysosomes in NK cells mediated by target-cell recognition. Proc Natl Acad Sci U S A 2005;102:123-7. [Crossref] [PubMed]
- De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proc Natl Acad Sci U S A 2011;108:728-32. [Crossref] [PubMed]
- Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol 2010;28:57-78. [Crossref] [PubMed]
- Li S, Yan Y, Lin Y, et al. Rapidly induced, T-cell independent xenoantibody production is mediated by marginal zone B cells and requires help from NK cells. Blood 2007;110:3926-35. [Crossref] [PubMed]
- Leung W. Use of NK cell activity in cure by transplant. Br J Haematol 2011;155:14-29. [Crossref] [PubMed]
- Leung W. Immunotherapy in acute leukemia. Semin Hematol 2009;46:89-99. [Crossref] [PubMed]
- Lowdell MW, Craston R, Samuel D, et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol 2002;117:821-7. [Crossref] [PubMed]
- Farnault L, Sanchez C, Baier C, et al. Hematological malignancies escape from NK cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol 2012;2012:421702.
- Farhan S, Lee DA, Champlin RE, et al. NK cell therapy: targeting disease relapse after hematopoietic stem cell transplantation. Immunotherapy 2012;4:305-13. [Crossref] [PubMed]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105:3051-7. [Crossref] [PubMed]
- Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant 2006;12:876-84. [Crossref] [PubMed]
- Lowe EJ, Turner V, Handgretinger R, et al. T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Br J Haematol 2003;123:323-26. [Crossref] [PubMed]
- Kröger N, Binder T, Zabelina T, et al. Low number of donor activating killer immunoglobulin-like receptors (KIR) genes but not KIR-ligand mismatch prevents relapse and improves disease-free survival in leukemia patients after in vivo T-cell depleted unrelated stem cell transplantation. Transplantation 2006;82:1024-30. [Crossref] [PubMed]
- Krause SW, Gastpar R, Andreesen R, et al. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: a clinical phase i trial. Clin Cancer Res 2004;10:3699-707. [Crossref] [PubMed]
- Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002;295:2097-100. [Crossref] [PubMed]
- Ruggeri L, Mancusi A, Perruccio K, et al. Natural killer cell alloreactivity for leukemia therapy. J Immunother 2005;28:175-82. [Crossref] [PubMed]
- Iliopoulou EG, Kountourakis P, Karamouzis MV, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother 2010;59:1781-9. [Crossref] [PubMed]
- Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010;28:955-9. [Crossref] [PubMed]
- Arai S, Meagher R, Swearingen M, et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 2008;10:625-32. [Crossref] [PubMed]
- Richters A, Kaspersky CL. Surface immunoglobulin positive lymphocytes in human breast cancer tissue and homolateral axillary lymph nodes. Cancer 1975;35:129-33. [Crossref] [PubMed]
- Caldwell CC, Kojima H, Lukashev D, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 2001;167:6140-9. [Crossref] [PubMed]
- Lukashev D, Klebanov B, Kojima H, et al. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol 2006;177:4962-5. [Crossref] [PubMed]
- Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 2012;30:2678-83. [Crossref] [PubMed]
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959-66. [Crossref] [PubMed]
- Topalian SL, Muul LM, Solomon D, et al. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods 1987;102:127-41. [Crossref] [PubMed]
- Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988;319:1676-80. [Crossref] [PubMed]
- Schwartzentruber DJ, Hom SS, Dadmarz R, et al. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J Clin Oncol 1994;12:1475-83. [Crossref] [PubMed]
- Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res 2010;16:2646-55. [Crossref] [PubMed]
- Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005;23:2346-57. [Crossref] [PubMed]
- Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008;26:5233-9. [Crossref] [PubMed]
- Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 2013;19:4792-800. [Crossref] [PubMed]
- Li J, Chen QY, He J, et al. Phase I trial of adoptively transferred tumor-infiltrating lymphocyte immunotherapy following concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Oncoimmunology 2015;4:e976507. [Crossref] [PubMed]
- Li LP, Lampert JC, Chen X, et al. Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat Med 2010;16:1029-34. [Crossref] [PubMed]
- Dembić Z, Haas W, Weiss S, et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature 1986;320:232-8. [Crossref] [PubMed]
- Leisegang M, Wilde S, Spranger S, et al. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J Clin Invest 2010;120:3869-77. [Crossref] [PubMed]
- Provasi E, Genovese P, Lombardo A, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med 2012;18:807-15. [Crossref] [PubMed]
- Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 2011;117:72-82. [Crossref] [PubMed]
- Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol 2003;171:3287-95. [Crossref] [PubMed]
- McKee MD, Clay TM, Rosenberg SA, et al. Quantitation of T-cell receptor frequencies by competitive PCR: generation and evaluation of novel TCR subfamily and clone specific competitors. J Immunother 1999;22:93-102. [Crossref] [PubMed]
- Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009;114:535-46. [Crossref] [PubMed]
- Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917-24. [Crossref] [PubMed]
- Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 2013;36:133-51. [Crossref] [PubMed]
- McCormack E, Adams KJ, Hassan NJ, et al. Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother 2013;62:773-85. [Crossref] [PubMed]
- Hiasa A, Hirayama M, Nishikawa H, et al. Long-term phenotypic, functional and genetic stability of cancer-specific T-cell receptor (TCR) alphabeta genes transduced to CD8+ T cells. Gene therapy 2008;15:695-9. [Crossref] [PubMed]
- Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Mede 2011;3:95ra73.
- Grada Z, Hegde M, Byrd T, et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids 2013;2:e105. [Crossref] [PubMed]
- Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006;12:6106-15. [Crossref] [PubMed]
- Cheadle EJ, Gornall H, Baldan V, et al. CAR T cells: driving the road from the laboratory to the clinic. Immunological reviews 2014;257:91-106. [Crossref] [PubMed]
- Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006;24:e20-22. [Crossref] [PubMed]
- Lamers CH, Langeveld SC, Groot-van Ruijven CM, et al. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother 2007;56:1875-83. [Crossref] [PubMed]
- Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther 2007;15:825-33. [Crossref] [PubMed]
- Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008;14:1264-70. [Crossref] [PubMed]
- Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 2008;112:2261-71. [Crossref] [PubMed]
- Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant 2010;16:1245-56. [Crossref] [PubMed]
- Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010;116:4099-102. [Crossref] [PubMed]
- Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822-6. [Crossref] [PubMed]
- Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817-28. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709-20. [Crossref] [PubMed]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Mede 2013;5:177ra38.
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 2013;122:2965-73. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 2013;122:4129-39. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Mede 2014;6:224ra25.
- Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 2012;119:3940-50. [Crossref] [PubMed]
- Wang Y, Zhang WY, Han QW, et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clinical immunologym 2014;155:160-75.
- Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013;21:904-12. [Crossref] [PubMed]
- Craddock JA, Lu A, Bear A, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother 2010;33:780-8. [Crossref] [PubMed]
- Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011;118:6050-6. [Crossref] [PubMed]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007;449:419-26. [Crossref] [PubMed]
- Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today 1997;18:175-82. [Crossref] [PubMed]
- Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol 2001;22:269-76. [Crossref] [PubMed]
- Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999;5:405-11. [Crossref] [PubMed]
- Wehner R, Lobel B, Bornhauser M, et al. Reciprocal activating interaction between 6-sulfo LacNAc+ dendritic cells and NK cells. Int J Cancer 2009;124:358-66. [Crossref] [PubMed]
- Ferlazzo G, Tsang ML, Moretta L, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 2002;195:343-51. [Crossref] [PubMed]
- Fanger NA, Maliszewski CR, Schooley K, et al. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Exp Med 1999;190:1155-64. [Crossref] [PubMed]
- Schmitz M, Zhao S, Deuse Y, et al. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol 2005;174:4127-34. [Crossref] [PubMed]
- Wang S, Hong S, Wezeman M, et al. Dendritic cell vaccine but not idiotype-KLH protein vaccine primes therapeutic tumor-specific immunity against multiple myeloma. Front Biosci 2007;12:3566-75. [Crossref] [PubMed]
- Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996;2:52-8. [Crossref] [PubMed]
- Xi HB, Wang GX, Fu B, et al. Survivin and PSMA Loaded Dendritic Cell Vaccine for the Treatment of Prostate Cancer. Biol Pharm Bull 2015;38:827-35. [Crossref] [PubMed]
- Reyes D, Salazar L, Espinoza E, et al. Tumour cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patients. Br J Cancer 2013;109:1488-97. [Crossref] [PubMed]
- Oshita C, Takikawa M, Kume A, et al. Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial. Oncol Rep 2012;28:1131-8. [PubMed]
- de Rosa F, Ridolfi L, Ridolfi R, et al. Vaccination with autologous dendritic cells loaded with autologous tumor lysate or homogenate combined with immunomodulating radiotherapy and/or preleukapheresis IFN-alpha in patients with metastatic melanoma: a randomised "proof-of-principle" phase II study. J Transl Med 2014;12:209. [Crossref] [PubMed]
- Flörcken A, Kopp J, van Lessen A, et al. Allogeneic partially HLA-matched dendritic cells pulsed with autologous tumor cell lysate as a vaccine in metastatic renal cell cancer: a clinical phase I/II study. Hum Vaccin Immunother 2013;9:1217-27. [Crossref] [PubMed]
- Schwaab T, Schwarzer A, Wolf B, et al. Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with Aldesleukin (Interleukin 2) and IFN-{alpha}2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res 2009;15:4986-92. [Crossref] [PubMed]
- Chang CN, Huang YC, Yang DM, et al. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci 2011;18:1048-54. [Crossref] [PubMed]
- Wang X, Bayer ME, Chen X, et al. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J Surg Oncol 2015;111:862-7. [Crossref] [PubMed]
- Tada F, Abe M, Hirooka M, et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol 2012;41:1601-9. [PubMed]
- Palmer DH, Midgley RS, Mirza N, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 2009;49:124-32. [Crossref] [PubMed]
- Dohnal AM, Witt V, Hugel H, et al. Phase I study of tumor Ag-loaded IL-12 secreting semi-mature DC for the treatment of pediatric cancer. Cytotherapy 2007;9:755-70. [Crossref] [PubMed]
- Geiger JD, Hutchinson RJ, Hohenkirk LF, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res 2001;61:8513-9. [PubMed]
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [Crossref] [PubMed]
- Dhodapkar MV, Sznol M, Zhao B, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Mede 2014;6:232ra51.
- Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006;17:563-70. [Crossref] [PubMed]
- Ridolfi L, Petrini M, Granato AM, et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med 2013;11:135. [Crossref] [PubMed]
- Ridolfi L, Petrini M, Fiammenghi L, et al. Dendritic cell-based vaccine in advanced melanoma: update of clinical outcome. Melanoma research 2011;21:524-9. [Crossref] [PubMed]
- Hus I, Rolinski J, Tabarkiewicz J, et al. Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia 2005;19:1621-7. [Crossref] [PubMed]
- Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001;19:145-56. [Crossref] [PubMed]
- Michael A, Ball G, Quatan N, et al. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res 2005;11:4469-78. [Crossref] [PubMed]
- Neves AR, Ensina LF, Anselmo LB, et al. Dendritic cells derived from metastatic cancer patients vaccinated with allogeneic dendritic cell-autologous tumor cell hybrids express more CD86 and induce higher levels of interferon-gamma in mixed lymphocyte reactions. Cancer Immunol Immunother 2005;54:61-6. [Crossref] [PubMed]
- Salcedo M, Bercovici N, Taylor R, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother 2006;55:819-29. [Crossref] [PubMed]
- Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol 2006;24:4721-30. [Crossref] [PubMed]
- Nemunaitis J, Nemunaitis M, Senzer N, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther 2009;16:620-4. [Crossref] [PubMed]
- Nemunaitis J. Vaccines in cancer: GVAX, a GM-CSF gene vaccine. Expert review of vaccines 2005;4:259-74. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
- Lipson EJ, Sharfman WH, Chen S, et al. Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. J Transl Med 2015;13:214. [Crossref] [PubMed]
- Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol 2006;24:419-24. [Crossref] [PubMed]
- Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther 2006;13:555-62. [Crossref] [PubMed]


