Adjuvant and neoadjuvant therapy for resectable pancreatic adenocarcinoma
Introduction
Pancreatic adenocarcinoma continues to be a vexing medical problem. While mortality rates from many cancers are improving, clinical outcomes in pancreatic cancer remain abysmally poor (1). Combined with slowly rising incidence, it is growing into a leading cause of cancer-related death in the United States (1,2). Worldwide as well, it is the seventh-most common cause of cancer-related death (3). The arrest and eventual reversal of these trends depend heavily on increase in cure rates. Surgical resection is necessary for cure, but not usually sufficient. Multimodality therapy is required, and in this review, current standards and emerging trends in adjuvant and neoadjuvant therapy for resectable pancreatic adenocarcinoma are discussed. The terms pancreatic adenocarcinoma and pancreatic cancer are synonymous, and used interchangeably depending on context.
Definition of resectable disease
The first question for a potentially curative approach to a patient diagnosed with pancreatic cancer is resectability of the primary tumor (after establishing absence of metastatic disease, and patient’s fitness to undergo curative therapy). The definitions of resectability are several, and continue to evolve. The primary reason to define resectability is to assess anatomy as it pertains to the technical feasibility of a surgical operation that will yield, ideally, an R0 (margin-negative) resection. Various consensus definitions are available, including those from the MD Anderson Cancer Center, National Comprehensive Cancer Network, and the American Society of Surgical Oncology (4-6). These definitions often refer to borderline resectable disease and focus on the relationship of the primary tumor to surrounding blood vessels—key ones being celiac artery, common hepatic artery, superior mesenteric artery, superior mesenteric vein and portal vein. Traditionally, relationships (usually evaluated on contrast-enhanced cross-sectional computed tomographic or magnetic resonance imaging scans) have been described using subjective terms such as involvement, abutment, impingement, encasement, etc. There is an evolving shift toward the use of objective geometric descriptions of the tumor-vessel interface, using degrees of vessel circumference in contact with the primary tumor as the measure of vascular involvement (7). A consensus statement from the Society of Abdominal Radiology endorsed this method (8). This approach is the basis of the Intergroup definition that is used in most cooperative group trials of multimodality therapy for resectable and borderline resectable pancreatic adenocarcinoma (9). According to this definition, for a tumor to be classified as resectable, there should be no tumor-artery interface, less than 180° tumor-vein interface, and no abnormal lymph nodes outside the surgical basin. Using this definition, approximately 15% of all cases of pancreatic cancer are considered resectable. Another 15% present with borderline resectable disease, 20% with locally advanced (unresectable) disease, and 50% with metastatic disease (1,10).
Approach to resectable disease
Pancreatic resection has remained the sine qua non of a curative approach to pancreatic cancer. Usually, this entails a pancreatoduodenectomy—the Whipple procedure—since 70% of all pancreatic cancers arise in the head of the pancreas. Distal, subtotal, or total pancreatectomy may be the chosen option instead, depending on the location of the primary tumor. Perioperative mortality in experienced hands and high-volume centers has declined to less than 2% (11). Following surgical resection, adjuvant therapies—chemotherapy and radiation—have been employed. Results remain suboptimal; more aggressive regimens and neoadjuvant approaches are being evaluated. These are discussed in detail here.
Role of adjuvant chemotherapy
Adjuvant chemotherapy has been evaluated in a few large randomized controlled trials (Table 1). The first noteworthy trial was the Gastrointestinal Tumor Study Group (GITSG) study published in 1985, albeit using chemotherapy in conjunction with adjuvant radiation. A total of two years of adjuvant 5-fluorouracil, with radiation added for the first few weeks, was compared with surgical resection alone. Overall survival, the primary outcome, was improved from a median of 11 months with surgery alone to 20 months with the addition of adjuvant therapy (12). The study comprised only 43 patients, however, and predated modern surgery, chemotherapy and radiation approaches. The next major advance was the European Study Group for Pancreatic Cancer (ESPAC)-1 study, published in 2004. In a 2×2 design, adjuvant chemotherapy and radiation were compared with surgery alone. The use of adjuvant 5-fluorouracial for 6 months was associated with an improvement in median overall survival, the primary outcome, from 15.5 months in the no chemotherapy arms to 20.1 months in the chemotherapy arms (14). While the results of this study remain controversial, given the complicated design, it added to the extant literature that adjuvant chemotherapy could improve overall survival in this setting.

Full table
The success of gemcitabine in metastatic disease eventually led to the adjuvant Charité Onkologie (CONKO)-001 study, first published in 2007. In this study with 368 patients, adjuvant gemcitabine for 6 months was shown to improve disease-free survival—the primary outcome—from a median of 6.7 months in the surgery-alone arm to 13.4 months (15). Further follow-up from this study noted five-year overall survival improvement from 10.4% to 20.7%, albeit this was not the primary statistical outcome (18). Based on these results, adjuvant gemcitabine for 6 months has remained the de facto standard of care for resected pancreatic cancer over the last decade or so. It should be noted that 5-fluorouracil appears to be an acceptable alternative, given results of Radiation Therapy Oncology Group (RTOG) 9704, which tested 5-fluorouracil versus gemcitabine—with radiation added to both arms. This study showed that there was no difference in overall survival between the two arms (16). The ESPAC-3—albeit a periampullary trial with only 55% of patients with pancreatic adenocarcinoma—also showed that there was no overall survival difference between adjuvant gemcitabine and 5-fluorouracil (19).
In the Japanese population, S-1 was compared with gemcitabine for adjuvant therapy of resected pancreatic cancer in the Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC)-01 study, and led to a remarkable median overall survival of 46.5 vs. 25.5 months (20). S-1 has not been used widely in non-Asian patients, so it is difficult to extrapolate these findings to such patients. Initial studies of S-1 in the United States and Europe indicated a higher toxicity burden (mainly gastrointestinal toxicity in the form of nausea, vomiting, diarrhea) (21,22). A subsequent pharmacologic study found that while dose exposures of 5-fluorouracil were no different, gastrointestinal toxicities and fatigue were more common in Caucasian patients, compared with Asian patients. These results indicated that a combination of factors, including CYP2A6 enzyme polymorphisms and dihydropyrimidine dehydrogenase enzyme activities, may alter tissue exposure characteristics and toxicity profiles (23). Therefore, while S-1 is a useful standard of care in Asian populations, its utility in Caucasian populations has not been evaluated further.
A network meta-analysis of various trials of adjuvant therapy discussed above showed that the use of either 5-fluorouracil or gemcitabine was associated with improvement in overall survival (24). Along the lines of this finding, recently, the ESPAC-4 study results were published. This trial randomized patients with resected pancreatic cancer to six months of adjuvant chemotherapy with either gemcitabine plus capecitabine or gemcitabine alone. The primary outcome was overall survival, and it improved from a median of 25.5 months in the gemcitabine arm to 28 months in the combination arm (17). Of note, the effect was more pronounced in the margin-negative population (median overall survival, combination versus gemcitabine, was 39.5 vs. 27.9 months) and not so in the margin-positive population (23.7 vs. 23 months). While more mature results on 5-year overall survival are awaited, these findings are encouraging, and adjuvant gemcitabine plus capecitabine for 6 months is emerging as the new standard of care for resected pancreatic adenocarcinoma. It is also noteworthy that the median overall survival even in the gemcitabine arm was more than 2 years—better surgical and medical care, and patient selection are possible reasons.
Ongoing clinical studies are evaluating more aggressive adjuvant chemotherapy regimens: key trials include the UNICANCER study of adjuvant FOLFIRINOX (5-fluorouracil, irinotecan, oxaliplatin) compared with gemcitabine (NCT01526135), and APACT study of adjuvant gemcitabine plus nab-paclitaxel compared with gemcitabine (NCT01964430) (Table 2). Both studies use disease-free survival as the primary outcome, and will establish if these regimens that have improved overall survival in the metastatic setting provide similar benefit in the adjuvant setting as well. Since these trials—along with ESPAC-4—test different adjuvant regimens, it remains to be seen which approach will be accepted as the standard of care, if more than one shows overall survival improvement. Akin to the metastatic setting, where FOLFIRINOX and gemcitabine/nab-paclitaxel are two competing options, the landscape of adjuvant therapy may improve, while at the same time generating more questions about which might be the best regimen to study as a backbone in future trials.
Full table
Role of adjuvant radiation
While adjuvant chemotherapy use is well-established as the standard of care for resected pancreatic cancer, the role of adjuvant radiation remains controversial. The aforementioned GITSG study included radiation in the adjuvant therapy regimen, and demonstrated improved overall survival, setting the stage for the use of radiation in this setting (12). Results thereafter, however, have not shown similar benefit. The EORTC 40891 study, first published in 1999, tested adjuvant 5-fluorouracil-based radiation for resected pancreatic head and periampullary cancer, and found no improvement in overall survival, the primary outcome, compared with surgery alone (13). While the results are difficult to interpret, as only about 55% of the study population had pancreatic cancer (the rest had periampullary cancer), they certainly raise doubts about the utility of adjuvant radiation. More importantly, subsequently the ESPAC-1 trial mentioned above showed that the use of any adjuvant radiation was associated with worse overall survival compared with no adjuvant radiation (14). Again, criticism of ESPAC-1 abounds: the 2x2 trial design, questionable quality control, and marginal statistics. Nonetheless, these studies call into question the benefit of adjuvant radiation. This was also highlighted by a meta-analysis of these clinical trials of adjuvant therapy, which showed that the use of chemoradiation was not associated with improvement in overall survival (24). It is to be noted, however, that there continue to be geographic differences in the use of adjuvant radiation—European data from EORTC 40891 and ESPAC-1 guide low use, whereas in the United States, RTOG-9704 continues to guide adjuvant radiation use.
To answer this question definitively, the RTOG 0848 study (NCT01013649) was opened. It randomizes patients with resected pancreatic cancer to adjuvant chemotherapy with or without radiation. A large study (950 patients), it is accruing patients and results will hopefully inform us if adjuvant radiation should be a part of standard of care. Furthermore, data from stereotactic body radiation therapy (SBRT) studies in locally advanced pancreatic cancer indicate good safety and efficacy profiles. Outcomes data from ongoing studies may define a role for this refined method of administering radiation in the adjuvant setting as well (25).
Neoadjuvant therapy
Transition from a surgery-first to a systemic approach, in the form of neoadjuvant therapy, for management of resectable pancreatic adenocarcinoma is being investigated. The impetus for this shift is the growing recognition about the systemic nature of this disease from its early origins (26). Early control of systemic disease with neoadjuvant chemotherapy may lead to improved outcomes. This is especially true because administration of aggressive chemotherapy regimens is often not feasible after major abdominal surgery, making the preoperative platform an ideal setting. This has been seen in esophagogastric cancers, for example (27). In the ESPAC-4 study as well, only 65% of patients in the gemcitabine arm and 54% of patients in the gemcitabine plus capecitabine arm completed all adjuvant therapy (17). A propensity score-matched analysis on over 8,000 patients with resectable pancreatic cancer from the National Cancer Database showed that neoadjuvant therapy was associated with an improved overall survival compared with upfront resection (26 vs. 21 months) (28). There are other advantages to the neoadjuvant approach. It allows downstaging of tumors, facilitating a higher margin-negative resection rate. It allows evaluation of response in vivo. Furthermore, we can study biomarkers—both blood and tissue-based, at baseline, preoperative and intra-operative time-points. Importantly, for patients with resistant cancer or intolerance to chemotherapy, inconsequential surgery can be spared. The concern, of course, is that if chemotherapy toxicities preclude surgical resection, then the opportunity for a potential cure can be lost.
A handful of studies have evaluated neoadjuvant therapy in the resectable setting. Most of these are small, single-institution, phase II trials employing usually gemcitabine-based chemotherapy or chemoradiation regimens, and focus on resection rate and survival (Table 3), making it difficult to draw meaningful conclusions. For example, a randomized phase II study of neoadjuvant therapy in 50 patients, comparing gemcitabine plus cisplatin versus gemcitabine alone showed a resection rate of 70% and median overall survival of 15.6 months in the combination arm (30). However, another study of neoadjuvant gemcitabine plus cisplatin achieved a resection rate of 89% and a median overall survival of 26.5 months (31). Even two contemporaneous studies from a single institution showed lower resectability and survival outcomes using gemcitabine plus cisplatin with radiation (33), compared with the gemcitabine alone with radiation study (32). It is to be noted that in recent studies, the proportion of patients unable to reach surgical resection due to toxicities or clinical deterioration during neoadjuvant therapy is less than 5% (0/59 in Van Buren study, and 3/38 in O’Reilly study). Most cases not undergoing surgical resection are due to progressive disease (35,36). This alleviates, to some degree at least, the concern that neoadjuvant therapy may preclude curative surgical resection due to chemotherapy toxicities.

Full table
Since cross-study comparisons are not very meaningful, meta-analyses have been performed and they indicate that irrespective of the regimen used, 80–90% of patients with resectable tumors go on to surgical resection after neoadjuvant therapy, with a median overall survival of 20–24 months. In patients where the tumor is not resected, median overall survival is 8–10 months (37,38). Therefore, ability to reach surgical resection remains the main driver of clinical outcome. It is to be noted that all these studies used regimens that predate more successful combinations (FOLFIRINOX and gemcitabine/nab-paclitaxel) that we now have (39,40). It remains to be seen if these regimens as well as any novel agents can improve outcomes.
The role of radiation therapy (RT) also continues to be defined in the neoadjuvant setting. Prior studies utilized conventional RT (32-34), which is slowly being supplanted by modern methods of intensity-modulated RT (IMRT) and SBRT. Both these techniques use precise image guidance, improving target organ doses while sparing normal tissues. They also lead to shortening of treatment courses, by allowing larger dose fractions (41). Studies of IMRT in the locally advanced and adjuvant settings indicate good safety and toxicity profiles, with less than 10% of patients experiencing any grade 3 or higher acute and late toxicities (42,43). Data from early SBRT studies for locally advanced disease also show a good safety profile: a phase II study showed only 2% and 11% of patients with acute and late toxicities, respectively, with good pain relief and encouraging overall survival (25).
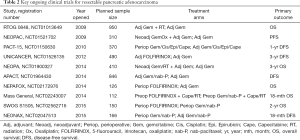
Some key clinical trials are testing these approaches (Table 2). The NEOPAC trial (NCT01521702) is evaluating neoadjuvant gemcitabine plus oxaliplatin followed by adjuvant gemcitabine, with a control arm of adjuvant gemcitabine. Disease-free survival is the primary outcome. The NEPAFOX study (NCT02172976) tests perioperative FOLFIRINOX versus adjuvant gemcitabine, with overall survival as the primary outcome. The SWOG S1505 trial (NCT02562716) is testing the perioperative approach, comparing FOLFIRINOX versus gemcitabine plus nab-paclitaxel, with overall survival as the primary outcome. The NEONAX study (NCT02047513) is comparing perioperative gemcitabine plus nab-paclitaxel with adjuvant use of the same regimen; disease-free survival is the primary outcome. Studies of IMRT and SBRT in pancreatic cancer encompass the spectrum from resectable to unresectable cancer (NCT02839343, NCT02208024, NCT02643498); lessons learned from such trials will inform us further about safety and efficacy of these approaches. Similar to the adjuvant setting, a key question will be identifying which regimen—based on efficacy and safety profiles—may be the best choice to take forward in future studies.
Summary
Resectable pancreatic adenocarcinoma presents the best opportunity for cure of this highly lethal disease. Use of geometry-based objective definitions for resectability can help reduce controversy and complexity. Upfront surgical resection followed by adjuvant gemcitabine-based chemotherapy remains the standard of care. The use of adjuvant radiation is controversial—ongoing clinical trials will help answer the question with more clarity. The neoadjuvant approach is based on scientific and pragmatic reasons and holds the promise of improved outcomes. Multiple studies testing this approach are ongoing and will help us understand the best way to move the field forward. Finally, innovative therapies are sorely needed in this arena—novel systemic therapy targets being tested in metastatic disease may hopefully improve outcomes and can lend themselves to the curative setting as well.
Acknowledgements
None.
Footnote
Conflict of Interest: Research funding (to institution) from Celgene, Bayer, OncoMed. Consulting fees from Perthera.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. [Crossref] [PubMed]
- Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol 2009;16:1725-6. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014;12:1083-93. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg 2014;18:269-78; discussion 78. [Crossref] [PubMed]
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014;270:248-60. [Crossref] [PubMed]
- Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20:2787-95. [Crossref] [PubMed]
- Kamarajah SK, Burns WR, Frankel TL, et al. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol 2017;24:2023-30.
- Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol 2012;19:169-75. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 82-4. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005;23:6957-65. [Crossref] [PubMed]
- Lee JL, Kang HJ, Kang YK, et al. Phase I/II study of 3-week combination of S-1 and cisplatin chemotherapy for metastatic or recurrent gastric cancer. Cancer Chemother Pharmacol 2008;61:837-45. [Crossref] [PubMed]
- Chuah B, Goh BC, Lee SC, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci 2011;102:478-83. [Crossref] [PubMed]
- Liao WC, Chien KL, Lin YL, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol 2013;14:1095-103. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Sohal DP, Walsh RM, Ramanathan RK, et al. Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst 2014;106:dju011. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol 2016. [Epub ahead of print]. [PubMed]
- Pisters PW, Wolff RA, Janjan NA, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol 2002;20:2537-44. [Crossref] [PubMed]
- Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol 2007;14:2088-96. [Crossref] [PubMed]
- Heinrich S, Pestalozzi BC, Schafer M, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:2526-31. [Crossref] [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [Crossref] [PubMed]
- Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. [Crossref] [PubMed]
- Turrini O, Ychou M, Moureau-Zabotto L, et al. Neoadjuvant docetaxel-based chemoradiation for resectable adenocarcinoma of the pancreas: New neoadjuvant regimen was safe and provided an interesting pathologic response. Eur J Surg Oncol 2010;36:987-92. [Crossref] [PubMed]
- Van Buren G 2nd, Ramanathan RK, Krasinskas AM, et al. Phase II study of induction fixed-dose rate gemcitabine and bevacizumab followed by 30 Gy radiotherapy as preoperative treatment for potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol 2013;20:3787-93. [Crossref] [PubMed]
- O'Reilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg 2014;260:142-8. [Crossref] [PubMed]
- Andriulli A, Festa V, Botteri E, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol 2012;19:1644-62. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Boyle J, Czito B, Willett C, et al. Adjuvant radiation therapy for pancreatic cancer: a review of the old and the new. J Gastrointest Oncol 2015;6:436-44. [PubMed]
- Yovino S, Maidment BW 3rd, Herman JM, et al. Analysis of local control in patients receiving IMRT for resected pancreatic cancers. Int J Radiat Oncol Biol Phys 2012;83:916-20. [Crossref] [PubMed]
- Abelson JA, Murphy JD, Minn AY, et al. Intensity-modulated radiotherapy for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2012;82:e595-601. [Crossref] [PubMed]


