Glioblastoma in the elderly: initial management
Introduction
Glioblastoma is associated with a dismal prognosis and remains one of the greatest challenges in neuro-oncology (1). Despite the rapid growth in knowledge and publications (2,3), only a few additional months have been added to median survival in the last 30 years (4-6). Glioblastoma continues to present as a fatal illness associated with a median survival of less than 18 months for both young and elderly patients (7,8).
Glioblastoma is the most common primary central nervous system (CNS) malignancy in adults. Data from the Central Brain Tumor Registry of the United States (CBTRUS) statistical report encompassing the years 2008–2012 documented 112,458 malignant primary CNS from tumor of which almost half were glioblastoma (45.2%) (1). The current median age is 64 years and glioblastoma incidence increases with age with a peak incidence of 14.93 cases per 100,000 population in the 75–84 year age range (1).
Histologically glioblastoma is a neuroglial tumor with cellular polymorphism, nuclear atypia, a high mitotic index, microvascular proliferation, and necrosis (9). The molecular assessment of glioblastoma in elderly patients is not significantly different when compared to younger patients. Isocitrate dehydrogenase (IDH) mutations confers better prognosis however they are virtually absent in the elderly population and occurs in approximately 5% of all glioblastoma, typically in younger patients in their third or fourth decade (10,11). The frequency of the O-6-methylguanine-DNA methyltransferase (MGMT) promoter methylation (an important positive predictive marker) does not alter with age (12).
The published report from the CBTRUS indicates a 1-year survival rate of 28.7% and 12.2%, for patient with glioblastoma and aged with 65–74 years and 75 years or older, respectively (1). In most countries worldwide, life expectancy is rising and as result the number of glioblastoma cases in elderly patients will significantly increase. Consequently, there is a considerable need to improve treatment outcomes and to better define standards of care for elderly patients with glioblastoma.
Surgical approach: biopsy or resection?
A tissue diagnosis is very helpful in the majority of cases for confirmation of diagnosis and molecular characterization of individual cases. Maximal safe resection aiming to preserve neurological function and provide maximal tumour resection is associated with improved survival rates (13-15). Secondary analysis of approximately 350 newly diagnosed glioblastoma patients from the German Glioma Network (GGN) demonstrated gross tumor resection, in the era of combined modality therapy (radiation and temozolomide, CMT), was associated with superior overall survival (OS) (median 17.1 months; P=0.0001) compared with incomplete resection and biopsy alone. However, incomplete resection was not statistically significantly better than biopsy only (median: 11.7 vs. 8.7 months; P=0.1) although there was definitely a trend supporting sub-total resection over biopsy only (15).
A small-randomized controlled trial in elderly patients of type of surgical intervention in newly diagnosed glioblastoma was performed (16). Patients age 65 years or older with Karnofsky Performance Status (KPS) >60 were randomized to undergo stereotactic biopsy (16 patients with median age 72, range 67–79) or open craniotomy and resection (14 patients with median age 70, range 66–80). The median OS was 171 days after craniotomy compared to 85 days after biopsy (P=0.035) supporting the use of resection in elderly patients.
More recently, a retrospective cohort study of the National Cancer Database (NCDB) reported outcomes on 16,717 elderly patients with glioblastoma in the temozolomide era (16). On subgroup analysis, the authors reported a consistent OS advantage of CMT over chemotherapy (CT) alone and radiotherapy (RT) alone with a greater OS benefit on multivariate analysis when tumor was resected when compared to biopsy alone (HR, 0.58; 95% CI, 0.56–0.60; P≤0.001). Additionally, a case control study reported a subgroup analysis of 52 patients (70 years or older). Median OS was 4.5 months and 3 months for surgical resection and needle biopsy, respectively (P=0.03) (17). A multivariate analysis of the Nordic trial (n=342; intervention: standard 6 weeks of RT vs. hypofractionated 2 weeks of RT (HRT) vs. CT alone in newly diagnosed glioblastoma patients aged 65 or older) showed a survival benefit favoring surgery over biopsy alone (HR, 1.50; 95% CI, 1.17–1.92; P=0.001) (18). As well, the Neuro-oncology Working Group of the German Cancer Society NOA-08 trial, reported on the extent of surgery as being an independent prognostic factor for OS among glioblastoma patients aged 65 and older (19).
These data taken together strongly support that tumor resection is beneficial and prolongs survival in elderly glioblastoma patients. Subgroup analysis from the recently published Canadian Cancer Trials Group CE.6/European Organization for Research and Treatment of Cancer 26062-22061 (CCTG CE.6/EORTC 26062-22061) randomized phase III trial will help to better understand outcomes after surgery or biopsy only in the temozolomide era (8).
The role of radiotherapy
As a result of the poor prognosis of elderly glioblastoma patients, there has been concern whether there was any significant benefit of any therapy in these patients. This concern is even more important in frail patients with multiples comorbidities who may not be eligible or fit to tolerate RT or chemotherapy or both without significant side effects or significant adverse impacts on quality of life.
A randomized trial investigated best supportive care (n=42) vs. RT with 50 Gy in fractions of 1.8 Gy in addition to supportive care (n=39) in elderly patients (aged 70 or older) with newly diagnosed glioblastoma (20). The combination of RT and supportive care resulted in prolonged OS (29.1 vs. 16.9 weeks; HR, 0.47; P=0.002) and an extension of PFS (14.9 vs. 5.4 weeks; HR, 0.28; P<0.001). Regarding toxicity, RT was safe, well tolerated with no severe adverse events reported. Furthermore, evaluation of QoL and cognitive function was similar in both populations.
The NCDB was recently reviewed glioblastoma patients aged 65 and older (16). A total of 16,717 patients with a median age of 73 years (range, 65–90 years) were identified. Patients were stratified by treatment received; 8,345 received radiation and temozolomide, 1,693 RT alone, 1,018 CT alone and 5,571 no therapy. The median OS by treatment was 9.0 months (95% CI, 8.8–9.3 months) for RT + TMZ, 4.7 months (95% CI, 4.5–5.0 months) with RT alone vs. 4.3 months (95%CI, 4.0–4.7 months) with CT alone vs. 2.8 months (95% CI, 2.8–2.9 months) with no therapy (P<0.001). Clearly there was patient selection bias in this retrospective review, but it does support the view that therapy does prolong survival in elderly glioblastoma patients. The Surveillance, Epidemiology, and End Results (SEER) registry database was reviewed for elderly glioblastoma patients (21). These investigators reported on 2,836 patients with median age of 76.9 years (range, 71–98 years). Median cancer-specific survivals were 8 months for patients undergoing both surgery and RT, 4 months for RT alone, 3 months for surgery alone and 2 months for neither surgery nor radiotherapy (log rank P<001).
With data supporting RT to be safe and effective in elderly patients with glioblastoma, other investigators investigated the possible value of shorter course RT in this patient population. Elderly patients are more often frail and possibly more susceptible to complications with higher dose RT. A randomized study investigating 40 Gy in 15 fractions (HRT) vs. 60 Gy in 30 fractions (standard RT) showed no difference in survival in 100 patients with glioblastoma age 60 or older (22). Overall survival was 5.1 months for standard RT vs. 5.6 months for the HRT arm (P=0.57) showing that HRT was a reasonable treatment option for older patients with glioblastoma. In a trial performed by the International Atomic Energy Agency, HRT with 40 Gy in 15 fractions was compared to a very short course of RT with 25 Gy in 5 fractions (23). The trial included newly diagnosed glioblastoma aged 65 years or older and patients aged 50 years or older with a Karnofsky performance score (KPS) of 50–70. With 98 patients enrolled, there were no reported differences in OS between the two groups: the 25 Gy cohort had a median OS of 7.9 months and the 40 Gy cohort had a median survival of 6.4 months (P=0.988). The median PFS was 4.2 months for both groups and QoL was similar at 4 and 8 weeks. One would need to be very careful with the use of 25 Gy in 5 fractions, as there would be a concern that it would not be well tolerated in patients receiving these large fractions in situations where the treatment volumes would be larger than average or where there was a large residual tumour because of possible acute swelling in either situation with the first few fractions of radiotherapy.
The Nordic Trial in elderly glioblastoma patients randomized individuals to one of three arms: HRT 34 Gy in 10 fractions, RT 60 Gy in 30 fractions, or temozolomide alone. The median survival with the HRT was similar to standard 6 week RT in the age group 60–70, but was statistically superior to the 6 week RT cohort in patients older than age 70, strongly supporting the possible toxicity of 60 Gy in 30 in these older patients.
Data from multiple studies supports the use of hypofractionated radiotherapy in the elderly. As a result, hypofractionated RT has become an acceptable option for elderly patients.
The role of temozolomide
Temozolomide in addition to radiotherapy has become the standard care for young adults with glioblastoma treatment since the groundbreaking publications of Stupp et al. in 2005 (7). Temozolomide is an alkylating agent active in glioblastoma and can be safely added in a concomitant and adjuvant fashion to RT. The drug mechanism is believed to occur with methylation of DNA at the O-6 position of guanine by a non-enzymatic chemical degradation product of TMZ (24). O-6-methylguanine-DNA methyltransferase is a DNA repair protein that repairs alkylating agent DNA damage (25). It is well established that methylation of the MGMT gene promoter (suppression of the MGMT gene expression) leads to an increased likelihood of better clinical outcomes with TMZ, so that the use of TMZ in elderly patients could potentially be a safe and active treatment option (8,23,26,27).
Temozolomide alone was investigated in a non-randomized phase II clinical trial (28). Patients with glioblastoma, aged 70 or older and with a KPS of 70% or less were treated with TMZ alone (consisted of 150 to 200 mg/m2/d temozolomide for 5 days every 4 weeks until disease progression). Seventy patients with a median age of 77 years were enrolled. Median PFS and OS of 16 weeks (95% CI, 10–20 weeks), and 25 weeks (95% CI, 19–28 weeks), respectively were reported. Quality of life and KPS were reported to improve over time. Temozolomide was well tolerated with grades 3 or 4 neutropenia and thrombocytopenia in less than 15% of the patients. In addition, methylation of the MGMT promoter was associated with longer PFS and OS.
The therapeutic benefit of TMZ alone was compared to RT in elderly (aged 60 or older) patients in two RCT. The NOA-08 trial investigated 373 elderly with newly diagnosed glioblastoma (89%) or anaplastic astrocytoma (11%) and KPS of 60 or greater (19). Patients were randomized to one of 2 groups: (I) temozolomide alone administered in a dose-intensified regimen of 100 mg/m2 given on days 1–7 every other week and (II) to standard radiotherapy consisting of 60 Gy given in 30 fractions. The MGMT promoter methylation status was assessed and primary study endpoint was OS. The study reported that TMZ alone was not inferior to RT alone (P for non-inferiority =0.03). Median survival was 8.6 vs. 9.6 months in the TMZ and RT alone groups respectively. The MGMT-methylated tumors responded better (event free survival) to temozolomide than to radiotherapy.
The Nordic trial randomized 291 patients aged 60 years or older with newly diagnosed glioblastoma to one of three different treatment regimens: (I) temozolomide only 200 mg/m2 on days 1–5 of every 28 days for up to six cycles; (II) hypofractionated radiotherapy 34.0 Gy administered in 3.4 Gy fractions over 2 weeks; or (III) standard radiotherapy 60.0 Gy administered in 2.0 Gy fractions over 6 weeks (18). The MGMT promoter methylation and IDH1 mutation status were assessed and primary study endpoint was OS. Median OS was significantly longer (P=0.02) in patients aged 70 or older treated with TMZ alone (8.3 months) or hypofractionated RT (7.5 months) compared to standard RT (6.0 months). Patients with MGMT promoter methylation assigned to TMZ alone had significantly longer survival than those without MGMT promoter methylation (9.7 vs. 6.8 months; P=0.02). Treatment with either TMZ only or RT only were found to be safe and MGMT promoter methylation status was described as a useful predictive marker for benefit from temozolomide.
A secondary analysis of 233 elderly (aged >70 years) patients with glioblastoma identified from nonrandomized prospective trial from in the GGN was published on the predictive value of MGMT methylation promoter status (26). When compared with the unmethylated MGMT promoter patients (OS 6.4 months), MGMT promoter methylation was associated with improved OS (8.4 months, P=0.031). Patients with MGMT methylated tumors had longer PFS when treated with CMT or CT alone compared to patients treated with RT alone.
Combined temozolomide and radiotherapy
Previously, the EORTC 26981-22981/NCIC CE.3 RCT reported OS benefit with the addition of TMZ to RT (60 Gy in 30 fractions) in the concomitant and sequential adjuvant setting in glioblastoma patients aged 18 to 70 years (7,21). The addition of TMZ resulted in a median survival of 14.6 months compared with 12.1 months for radiotherapy alone (P<0.0001). However, in an elderly subgroup analysis the combined treatment OS benefit became less pronounced with increasing age [(60 to 65 years; HR, 0.64; 0.43–0.94; P=0.02); (65 to 70 years; HR, 0.78; 0.50–1.24; P=0.29)].
The recently completed and reported CCTG CE.6/EORTC 26062-22061 phase III trial randomized newly diagnosed glioblastoma patients aged 65 or older to receive either HRT alone (n=281; RT 40 Gy in 15 fractions) or HRT with concomitant and adjuvant temozolomide (TMZ) (n=281). The median age was 73 years (range, 65–90 years) and eligible patients required an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2, no prior chemotherapy or radiotherapy and surgery or biopsy <4 weeks from randomization. The median OS was longer with the addition of TMZ to HRT (9.3 months) than with HRT alone (7.6 months) with a HR of 0.67 (95% CI, 0.56–0.80; P<0.001). Progression free survival (PFS) rates were longer with the addition of TMZ (5.3 months) when compared to HRT alone (3.9 months) with a HR of 0.50 (95% CI, 0.41–0.60; P<0.001). Overall survival benefit was reported in patients with methylated MGMT status (13.5 vs. 7.7 months; for CMT and HRT alone, respectively P<0.001). For unmethylated patients, OS survival was 10 months for HRT and TMZ vs. 7.9 months with HRT alone (P=0.055). Toxicity and quality of life (QoL) were remarkably similar between the two treatment arms.
The CCTG CE.6/EORTC 26062–22061 study provides robust results to support the combined approach in the elderly, particularly in patients with methylated MGMT status. Table 1 summarizes the findings the effect of combined treatment modality (NCIC CE.6/EORTC and EORTC/NCIC CE.3) and MGMT Promoter Methylation Status on OS and PFS (7,8,27,29,30).
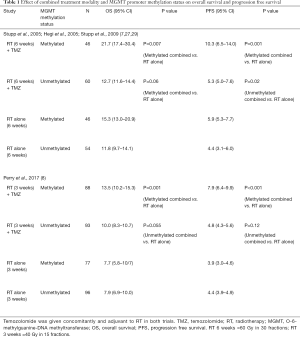
Full table
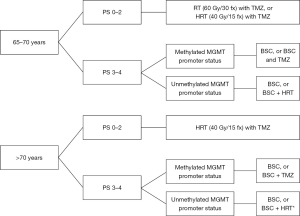
Based, on the above sections, elderly patients are likely to benefit from combined treatment with hypofractionated RT and TMZ. For patients with methylated tumours, management options could include TMZ alone or hypofractionated RT with concurrent and adjuvant TMZ. For patients with unmethylated tumours, the possible benefit of TMZ is likely to be less (Figure 1).
Beyond radiotherapy and temozolomide
There continue to be many studies in glioblastoma of additional possible treatment options. Data for early uncontrolled studies suggested a possible benefit of adding bevacizumab, a monoclonal antibody that targets vascular endothelial growth factor (VEGF) in frail or elderly patients (31,32). Two RCT (AVAglio and RTOG 0825) assessing the addition of bevacizumab to TMZ and RT in patients with newly diagnosed glioblastoma did not demonstrate OS benefit (33,34). The AVAglio trial reported a trend for better survival with bevacizumab in elderly patients (older than age 65) with MGMT promoter-unmethylated tumors.
Currently, a randomized phase II trial is assessing outcomes of hypofractionated radiotherapy alone or radiotherapy plus bevacizumab in elderly glioblastoma patients (ARTE, NCT01443676). Further studies are ongoing and immunotherapeutic approaches may be a promising approach (35).
Conclusions
Glioblastoma is the most common primary CNS malignancy and it is becoming more frequently diagnosed in the elderly population. Post-operative treatment options include: (I) HRT with concurrent and adjuvant temozolomide (II) hypofractionated RT alone (unmethylated patients) or (III) TMZ alone (methylated patients) when combined modality is not feasible due to patient poor performance status or multiple comorbidities. MGMT promoter methylation status may be a useful indicator of whether single modality (RT or TMZ alone) or combined modality treatment may be indicated. Most centres either do not have access to this test, or the test result may not be available at the time of making a treatment decision, in which case we would recommend CMT with hypofractionated RT with concurrent temozolomide as the initial approach in patients well enough to have treatment. Future trials addressing new approaches are needed to improve outcomes in this fatal disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Moraes has no conflicts of interest to declare. Dr. Laperriere has received honoraria and travel funding from Merck.
References
- Ostrom QT, Gittleman H, Xu J, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro Oncol 2016;18:v1-v75. [Crossref] [PubMed]
- Marosi C, Preusser M. Milestones of the last 10 years: CNS cancer. Memo 2017;10:18-21. [Crossref] [PubMed]
- Moraes FY, Bonifacio LA, Marta GN, et al. Hierarchy of evidence referring to the central nervous system in a high-impact radiation oncology journal: a 10-year assessment. Descriptive critical appraisal study. Sao Paulo Med J 2015;133:307-13. [Crossref] [PubMed]
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA 2013;310:1842-50. [Crossref] [PubMed]
- Mason M, Laperriere N, Wick W, et al. Glioblastoma in the elderly: making sense of the evidence. Neurooncol Pract 2016;3:77-86.
- Taunk NK, Moraes FY, Escorcia FE, et al. External beam re-irradiation, combination chemoradiotherapy, and particle therapy for the treatment of recurrent glioblastoma. Expert Rev Anticancer Ther 2016;16:347-58. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med 2017;376:1027-37. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 2010;120:707-18. [Crossref] [PubMed]
- van den Bent MJ, Weller M, Wen PY, et al. A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro Oncol 2017;19:614-24. [Crossref] [PubMed]
- Wiestler B, Claus R, Hartlieb SA, et al. Malignant astrocytomas of elderly patients lack favorable molecular markers: an analysis of the NOA-08 study collective. Neuro Oncol 2013;15:1017-26. [Crossref] [PubMed]
- Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 1993;26:239-44. [Crossref] [PubMed]
- Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95:190-8. [Crossref] [PubMed]
- Kreth F-W, Thon N, Simon M, et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol 2013;24:3117-23. [Crossref] [PubMed]
- Rusthoven CG, Koshy M, Sher DJ, et al. Combined-Modality Therapy With Radiation and Chemotherapy for Elderly Patients With Glioblastoma in the Temozolomide Era: A National Cancer Database Analysis. JAMA Neurol 2016;73:821-8. [Crossref] [PubMed]
- Chaichana KL, Garzon-Muvdi T, Parker S, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol 2011;18:239-45. [Crossref] [PubMed]
- Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916-26. [Crossref] [PubMed]
- Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13:707-15. [Crossref] [PubMed]
- Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med 2007;356:1527-35. [Crossref] [PubMed]
- Scott J, Tsai YY, Chinnaiyan P, et al. Effectiveness of radiotherapy for elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys 2011;81:206-10. [Crossref] [PubMed]
- Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 2004;22:1583-8. [Crossref] [PubMed]
- Roa W, Kepka L, Kumar N, et al. International Atomic Energy Agency Randomized Phase III Study of Radiation Therapy in Elderly and/or Frail Patients With Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol 2015;33:4145-50. [Crossref] [PubMed]
- Baker SD, Wirth M, Statkevich P, et al. Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res 1999;5:309-17. [PubMed]
- Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 2010;6:39-51. [Crossref] [PubMed]
- Reifenberger G, Hentschel B, Felsberg J, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer 2012;131:1342-50. [Crossref] [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [Crossref] [PubMed]
- Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol 2011;29:3050-5. [Crossref] [PubMed]
- Hegi ME, Diserens A-C, Gorlia T, et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med 2005;352:997-1003. [Crossref] [PubMed]
- Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol 2008;9:29-38. [Crossref] [PubMed]
- Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740-5. [Crossref] [PubMed]
- Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2011;29:142-8. [Crossref] [PubMed]
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus Radiotherapy-Temozolomide for Newly Diagnosed Glioblastoma. N Engl J Med 2014;370:709-22. [Crossref] [PubMed]
- Gilbert MR, Dignam JJ, Armstrong TS, et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N Engl J Med 2014;370:699-708. [Crossref] [PubMed]
- Seystahl K, Gramatzki D, Roth P, et al. Pharmacotherapies for the treatment of glioblastoma - current evidence and perspectives. Expert Opin Pharmacother 2016;17:1259-70. [Crossref] [PubMed]


