Current management of primary central nervous system lymphoma
Introduction
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin’s lymphoma, usually diffuse large B-cell lymphoma (DLBCL), confined to the brain or spinal cord parenchyma, leptomeninges or intraocular contents without evidence of systemic disease.
In the USA, its incidence is 0.47 per 100,000 people per year. It accounts for approximately 1% of all lymphomas and 2% to 5% of all primary brain tumors. The incidence is higher in patients with congenital or acquired immunosuppression, and may be seen up to 7% of patients with human immunodeficiency virus (HIV) infection.
In immunocompetent patients, the median age at diagnosis is 56 and there is a male predominance. Its incidence is increasing in the elderly population. The age of onset is younger in patients with immunosuppression (1).
In patients with immunosuppression, PCNSL is associated with Epstein Barr virus, whereas an association is rare in immunocompetent patients, though it may be seen in the elderly (2).
Pathology and pathogenesis
PCNSL is characterized pathologically by an angiocentric appearance, with lymphoma and inflammatory cells surrounding small blood vessels. The vast majority, approximately 95%, of PCNSLs are DLBCL. However, T cell lymphomas constitute approximately 2%, with the remainder reported as being of other histologies, including lymphoblastic and small cell lymphomas (3).
DLBCL PCNSL contains unique genetic aberrations that may serve as targets for therapy. These support a late germinal origin and are most consistent with a late germinal cell exit B-cell. BCL6 is seen in 60% of tumors, and has been associated with a worse prognosis. Interferon regulatory factor 4 (IRF4/MUM1) is positive in 95% of tumors. BCL2 expression is likewise detectable in 95% of tumors. Increased MYC expression is also common (4).
Most mutations are common in the DLBCL and are not unique to primary CNS lymphoma The B cell receptor (BCR) is frequently activated and may serve as a therapeutic target. Activation of other pathways including toll-like receptor and nuclear factor κB are also found. These promote tumor cell proliferation and survival, and are related to mutations in one or more genes including BCL2, BLNK, CARD11, CBL, CD79B, MALT1, MYD88 PAX5, PIM1, SHIP and TTF genes (4,5).
However, PCNSL is unique given its central nervous system trophism. The mechanism by which PCNSL cells are attracted exclusively to the central nervous system has not been fully elucidated. These cells are known to express a variety of adhesion molecules, and a number of chemokines, in particular CXCL-12 and CXCL-13 may be expressed by endothelium and microglia resulting in interactions with tumor cells that lead to extravasation and local infiltration. Additional interactions with the microenvironments may also have a role in tumor proliferation. However, the mechanism of CNS homing and tumor proliferation remains under investigation (4).
Diagnosis and approach to patient
Typically, PCNSL presents with progressive neurological signs and symptoms. The onset is usually rapid and progressive. One or more focal neurological deficits representing location of the neoplasm is the most common presentation, seen in approximately 60% of patients. Other common presentations are changes in mental status, signs of increased intracranial pressure, seizures and visual changes (that may reflect intra-ocular involvement). Seizures are less common. Primary presentation in the spinal cord is uncommon.
Typically, patients who presented with a focal neurological deficit for an encephalopathy will undergo a head computed tomography (CT) scan, which will often show a mass-like area and vasogenic edema. Magnetic resonance imaging (MRI) without and with contrast will provide further information into the etiology of any abnormality seen on MRI. In immunocompetent patients, MRI typically shows diffuse homogeneous contrast enhancement with T2 hypointensity and surrounding vasogenic edema. Most tumors are single (approximately 60%) and supratentorial. Tumors are typically found at the gray white junction or in the periventricular region. Frank meningeal involvement is seen in a small percentage of patients, though it is typically asymptomatic.
If PCNSL is suspected based on radiographic findings, glucocorticoids should be avoided for symptom control unless required for management of severe focal deficits or encephalopathy. These have a cytotoxic effect on primary CNS lymphoma, particularly at the time of presentation, which may lead to a false-negative biopsy.
A CT scan of the chest, abdomen and pelvis and a bone marrow biopsy should be performed to evaluate for systemic lymphoma. Positron emission tomography (PET) may be performed if necessary to clarify findings on CT scan (6). A scrotal ultrasound should be performed in older males as there is an association between testicular lymphoma and brain lymphomas (6).
If feasible, all patients should undergo a lumbar puncture and a slit-lamp examination to determine the presence of meningeal and intra-ocular disease. A diagnosis made by cerebrospinal fluid (CSF) or vitreous cytology or flow cytometry may eliminate the need for brain biopsy. At the time of lumbar puncture, interleukin 10 (IL10) level should also be measured and followed in subsequent studies as the presence of a detectable IL10 level at the completion of therapy portends an early recurrence (7).
If central nervous system tissue is needed, a stereotactic or open biopsy may be performed for diagnosis. There is no need for tumor resection unless it is for symptom management. The amount of resection will not affect the outcome. In patients who have a significant response to corticosteroids prior to obtaining diagnostic tissue, steroids should be tapered and discontinued and biopsy deferred until the time of tumor regrowth. Likewise, a negative biopsy in this setting should be repeated on tumor recurrence. Pretreatment diagnostic evaluations should include testing for HIV, hepatitis B virus and hepatitis C virus (2,8).
Prognostic factors
In determining prognosis, age and performance status are highly relevant. Additional prognostic factors include the presence of an elevated LDH, elevated CSF protein and involvement of the brain structures. Ferreri et al. performed a retrospective review of 378 patients, scoring each of the following with one point: age greater than 60 years, performance status 2–4, elevated LDH, elevated CSF protein and involvement of the brain structures. Patients with a score of 0–1 had a two-year overall survival (OS) of 80%±8% those with a score of 2–3 had a 2-year OS of 48%±7%, and those with a score of 4–5 had a 2-year OS of 15%±7% (P=0.00001) (9).
The G-PCNSL-SG-1 trial (10) identified age (<60 vs. over 60 years), Karnofsky Performance Score (70 or above vs. less than 70), and body mass index over 25 were strongly associated with improved OS.
Treatment
Until the late 1980s, PCNSL were routinely treated with whole-brain radiation therapy (WBRT). A prospective clinical trial from the radiation therapy oncology group (RTOG) treated patients with 40 Gy of WBRT with a 20 Gy boost to the tumor. The median OS was 12.2 months (11). A follow-up trial from RTOG treated patients with cyclophosphamide, doxorubicin, vincristine and dexamethasone (CHOD) prior to radiation therapy with a similar regimen. While there were objective radiographic responses to treatment, the median survival was not much improved (16.1 months) (12).
Anecdotal reports in the 1970s led to high-dose intravenous methotrexate (HDMTX) being identified as highly active in obtaining objective radiographic responses in patients with newly diagnosed PCNSL (13). DeAngelis and colleagues at Memorial Sloan Kettering Medical Center treated a series of patients with HDMTX and intrathecal (IT) methotrexate followed by WBRT, with a resulting improvement in progression free survival (PFS) and OS (14). These findings were subsequent reproduced (15), and a prospective clinical trial (RTOG 93–10) confirmed the efficacy of HDMTX followed by radiation therapy (16). Methotrexate based chemotherapy is now the basis and standard of care for the treatment of PCNSL.
Since that time, there have been many single institutions, multi institution and cooperative group trials that have sought to define optimal treatment for PCNSL. Strategies have included the addition of different antineoplastic agents to methotrexate, the escalation of chemotherapy dosing (high dose chemotherapy with stem cell support), the addition of a targeted agent [rituximab (RTX)] to treatment and evaluation of strategies to eliminate radiation therapy (17). A sampling of these trials is detailed in Table 1. There has, thus far, been no clear evidence that the intensification of chemotherapy with the use of additional cytotoxic agents provides any survival advantage, though objective response rates and PFS may be improved (31).
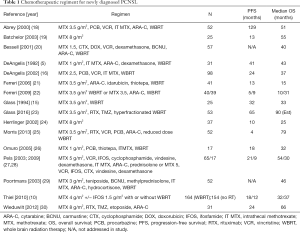
Full table
The role of radiation therapy in the treatment of PCNSL is pertinent, as improved survival allows for the emergence of late neurological effects of radiation therapy, in particular leukoencephalopathy, resulting in cognitive decline and dementia (32).
Neurotoxicity as a result of whole brain radiation therapy has been a driving factor in the reduction in radiation dose or in the elimination of radiation altogether. Late neurotoxicity, in most cases leukoencephalopathy, were seen in 20% to 50% of patients, especially in those older than 60 (33,34).
In 1991, Neuwelt et al. demonstrated that intensive chemotherapy with osmotic blood brain barrier opening and no initial WBRT resulted in prolonged median OS without cognitive decline (35).
Batchelor et al. published results of a clinical trial in which patients received methotrexate-based chemotherapy with deferred radiation therapy. While PFS was not affected, OS was similar to that of other clinical trials (19).
The German PCNSL study group performed a randomized clinical trial in which patients were randomized to receive whole brain radiotherapy immediately after initial methotrexate based chemotherapy or deferred radiotherapy (at relapse). Patients randomized to deferred therapy received no treatment with a complete response and cytarabine without a complete response after completion of initial chemotherapy. Of the 208 patients in the deferred radiation arm, 112 did not have a complete response. Of these, 68 were treated with cytarabine per protocol, 33 received radiation therapy and 11 no treatment. There was no statistically significant difference in median OS or PFS between the two groups (10).
Reductions in WBRT dosing have also been investigated. In RTOG 93-10, patients were initially treated with WBRT to a dose of 45 Gy. Evidence of late radiation effect (leukoencephalopathy) prompted a change in dosing during this study to 36 Gy in twice daily fractions (16). There was an apparent reduction in leukoencephalopathy in patients receiving this dose. The subsequent RTOG trial, 02–27, used this protocol in all patients and had a low rate of delayed neurotoxicity (23).
Rubenstein et al. provided patients with high-dose methotrexate, temozolomide and RTX followed by consolidation chemotherapy with intravenous etoposide and cytarabine (CALGB/Alliance 50202). The time to tumor progression was 4.0 years with median OS not reached at the time of provocation (36).
RTOG 11–14 randomized patients between low-dose radiation therapy (23.4 Gy) and no initial radiation therapy (immediate consolidation with cytarabine). The results of this clinical trial are still pending.
The inclusion of a targeted therapy, RTX, has become standard in initial treatment regimens. While there is evidence that RTX improves radiographic response rates (37) there is no evidence that it improves PFS or OS (38).
High-dose chemotherapy with stem cell support can provide effective treatment at relapse. It has been used in a limited fashion as initial therapy in a number of academic centers, though it is overall improving outcomes has not been fully elucidated in this population (39-43).
Treatment at relapse
The optimal treatment of recurrent disease has not been fully elucidated. Patients not previously receiving radiation therapy may be rechallenged with methotrexate. The use of methotrexate in patients previously treated with radiation therapy increases the risk of leukoencephalopathy. Options for treatment at relapse are detailed in Table 2. Systemic treatment at relapses includes temozolomide monotherapy, RTX, RTX combined with temozolomide and other forms of chemotherapy.
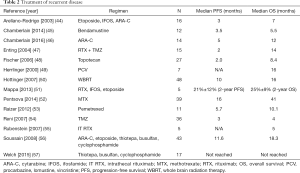
Full table
Intrathecal therapy
While meningeal dissemination of disease is frequency PCNSL at presentation, there is no evidence that its addition to initial treatment improves outcomes (58). Methotrexate enters the CSF in amounts sufficient to have a cytotoxic effect on meningeal lymphoma. Patient’s to have refractory or relapsed disease may benefit from intrathecal chemotherapy. Intrathecal therapy can include methotrexate, cytarabine or liposomal cytarabine (59).
Primary vitreoretinal lymphoma (PVRL)
PVRL is a DLBCL involving the contents of the eyes in the absence of evidence of systemic disease. It will typically present as visual loss including blurred vision, loss of visual acuity and floaters. Disease is most often present in both eyes, though may be symptomatic in only one eye. Diagnosis may be made by vitrectomy and cytologic examinations. Measurement of IL10 is also helpful (60,61). All patients with PVRL should be screened at the time of presentation and subsequently for central nervous system disease. Intra-ocular involvement is present in approximately 15 percent of all patients with PCNSL at initial presentation. In patients presenting with PVRL without central nervous system involvement, there is a 65% to 90% chance of progression to PCNSL (60). The optimum treatment for PVRL has not been determined. Treatment options include ocular radiation with or without systemic chemotherapy or intravitreal chemotherapy (methotrexate, RTX). Systemic chemotherapy produces temporary responses with a high recurrence rate.
Other targeted therapies
The use of targeted therapies has attracted attention in the treatment of PCNSL. RTX, and anti CD20 monoclonal antibody, is the only targeted therapy that is currently use. However, large molecules such as RTX have limitations owing to their inability to cross an attack blood brain barrier. While objective responses can be obtained with RTX, this occurs in the setting of a blood brain barrier breakdown and the ability of RTX to enter the brain parenchyma and effectively treat the lymphoma. However, treatment including corticosteroids and chemotherapy may result in reconstitution of the blood-brain barrier, eliminating its efficacy. Smaller molecules that are able to cross the blood brain barrier. The BCR transduction cascade has a potential target in Bruton’s tyrosine kinase, which is a target for a commercially available agent, ibrutinib. Another driver in CNS lymphoma is BCL6, which a number of targeted agents are undergoing clinical trials. Another commercially available targeted therapy, lenalidomide, targets IRF4/MUM1. The JAK/STAT signaling pathway is also implicated in tumor proliferation and targeted agents are under investigation in other cancers (62).
Conclusions
PCNSL is a lymphoma isolated to the central nervous system and intra-ocular contents that is sensitive to cytotoxic chemotherapies. The optimum treatment has not yet been determined, but treatment is methotrexate based, often in association with other forms of chemotherapy or with RTX. The early use of radiation therapy does not ultimately affect outcomes, and may be deferred until tumor recurrence. Investigations are ongoing into the optimum treatment for this disorder, including optimum chemotherapeutic and radial therapeutic approaches and targeted therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 2011;105:1414-8. [Crossref] [PubMed]
- Giannini C, Dogan A, Salomão DR. CNS lymphoma:a practical diagnostic approach. J Neuropathol Exp Neurol 2014;73:478-94. [Crossref] [PubMed]
- Sugita Y, Muta H, Ohshima K, et al. Primary central nervous system lymphomas and related diseases:Pathological characteristics and discussion of the differential diagnosis. Neuropathology 2016;36:313-24. [Crossref] [PubMed]
- Deckert M, Montesinos-Rongen M, Brunn A, et al. Systems biology of primary CNS lymphoma:from genetic aberrations to modeling in mice. Acta Neuropathol 2014;127:175-88. [Crossref] [PubMed]
- DeAngelis LM, Yahalom J, Thaler HT, et al. Combined modality treatment for primary central nervous system lymphoma. J Clin Oncol 1992;10:635-43. [Crossref] [PubMed]
- Brandão LA, Castillo M. Lymphomas-Part 1. Neuroimaging Clin N Am 2016;26:511-36. [Crossref] [PubMed]
- Song Y, Zhang W, Zhang L, et al. Cerebrospinal Fluid IL-10 and IL-10/IL-6 as Accurate Diagnostic Biomarkers for Primary Central Nervous System Large B-cell Lymphoma. Sci Rep 2016;6:38671. [Crossref] [PubMed]
- Wang CC, Carnevale J, Rubenstein JL. Progress in central nervous system lymphomas. Br J Haematol 2014;166:311-25. [Crossref] [PubMed]
- Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas:the International Extranodal Lymphoma Study Group experience. J Clin Oncol 2003;21:266-72. [Crossref] [PubMed]
- Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1):a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036-47. [Crossref] [PubMed]
- Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain:can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys 1992;23:9-17. [Crossref] [PubMed]
- Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas:initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol 1996;14:556-64. [Crossref] [PubMed]
- Ervin T., Canellos GR. Successful treatment of recurrent primary central nervous system lymphoma with high-dose methotrexate. Cancer 1980;45:1556-7. [Crossref] [PubMed]
- DeAngelis LM, Yahalom J, Heinemann MH, et al. Primary CNS lymphoma:combined treatment with chemotherapy and radiotherapy. Neurology 1990;40:80-6. [Crossref] [PubMed]
- Glass J, Gruber ML, Cher L, et al. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma:long-term outcome. J Neurosurg 1994;81:188-95. [Crossref] [PubMed]
- DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma:Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643-48. [Crossref] [PubMed]
- Carnevale J, Rubenstein JL. The Challenge of Primary Central Nervous System Lymphoma. Hematol Oncol Clin North Am 2016;30:1293-316. [Crossref] [PubMed]
- Abrey LE, Yahalom J, De Angelis L. Treatment of primary CNS lymphoma:the next step. J Clin Oncol 2000;18:3144-50. [Crossref] [PubMed]
- Batchelor T, Carson K, O’Neill A, et al. Treatment of primary cerebral lymphoma with methotrexate and deferred radiotherapy:report of NABTT 96-07. J Clin Oncol 2003;21:1044-9. [Crossref] [PubMed]
- Bessell EM, Graus F, Lopez-Guillermo A, et al. CHOD/BVAM regimen plus radiotherapy in patients with primary CNS non-Hodgkins lymphoma. Int J Radiat Oncol Biol Phys 2001;50:457-64. [Crossref] [PubMed]
- Ferreri AJ, Dell’Oro S, Foppoli M, et al. MATILDE regimen followed by radiotherapy is an active strategy against primary CNS lymphomas. Neurology 2006;66:1435-8. [Crossref] [PubMed]
- Ferreri AJ, Reni M, Foppoli M, et al. International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma:a randomised phase 2 trial. Lancet 2009;374:1512-20. [Crossref] [PubMed]
- Glass J, Won M, Schultz CJ, et al. Phase I and II Study of Induction Chemotherapy With Methotrexate, Rituximab, and Temozolomide, Followed By Whole-Brain Radiotherapy and Postirradiation Temozolomide for Primary CNS Lymphoma:NRG Oncology RTOG 0227. J Clin Oncol 2016;34:1620-5. [Crossref] [PubMed]
- Herrlinger U, Schabet M, Brugger W, et al. German Cancer Society Neuro-Oncology Working Group NOA-03 multicentre trial of single agent high dose methotrexate for primary CNS lymphoma. Ann Neurol 2002;51:247-52. [Crossref] [PubMed]
- Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma:fi nal results and long-term outcome. J Clin Oncol 2013;31:3971-9. [Crossref] [PubMed]
- Omuro AM, DeAngelis LM, Yahalom J, et al. Chemoradiotherapy for primary CNS lymphoma:an intent-to-treat analysis with complete follow-up. Neurology 2005;64:69-74. [Crossref] [PubMed]
- Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma:results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol 2003;21:4489-95. [Crossref] [PubMed]
- Pels H, Juergens A, Glasmacher A, et al. Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment:results of a phase II study. J Neurooncol 2009;91:299-305. [Crossref] [PubMed]
- Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma:European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol 2003;21:4483-8. [Crossref] [PubMed]
- Wieduwilt MJ, Valles F, Issa S, et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma:a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res 2012;18:1146-55. [Crossref] [PubMed]
- Ferreri AJ, Marturano E. Primary CNS lymphoma. Best Pract Res Clin Haematol 2012;25:119-30. [Crossref] [PubMed]
- Kasenda B, Loeffler J, Illerhaus G, et al. The role of whole brain radiation in primary CNS lymphoma. Blood 2016;128:32-6. [Crossref] [PubMed]
- Correa DD, Shi W, Abrey LE, et al. Cognitive functions in primary CNS lymphoma after single or combined modality regimens. Neuro Oncol 2012;14:101-8. [Crossref] [PubMed]
- Durand T, Bernier MO, Léger I, et al. Cognitive outcome after radiotherapy in brain tumor. Curr Opin Oncol 2015;27:510-5. [Crossref] [PubMed]
- Neuwelt EA, Goldman DL, Dahlborg SA, et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption:prolonged survival and preservation of cognitive function. J Clin Oncol 1991;9:1580-90. [Crossref] [PubMed]
- Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive Chemotherapy and Immunotherapy in Patients With Newly Diagnosed Primary CNS Lymphoma:CALGB 50202 (Alliance 50202). J Clin Oncol 2013;31:3061-8. [Crossref] [PubMed]
- Birnbaum T, Stadler EA, von Baumgarten L, et al. Rituximab significantly improves complete response rate in patients with primary CNS lymphoma. J Neurooncol 2012;109:285-91. [Crossref] [PubMed]
- Kansara R, Shenkier TN, Connors JM, et al. Rituximab with high-dose methotrexate in primary central nervous system lymphoma. Am J Hematol 2015;90:1149-54. [Crossref] [PubMed]
- Alimohamed N, Daly A, Owen C, et al. Upfront thiotepa, busulfan, cyclophosphamide, and autologous stem cell transplantation for primary CNS lymphoma:a single centre experience. Leuk Lymphoma 2012;53:862-7. [Crossref] [PubMed]
- Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as fi rst-line therapy for primary CNS lymphoma in patients younger than 60 years:a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant 2006;38:417-20. [Crossref] [PubMed]
- Illerhaus G, Müller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as fi rst-line treatment for primary lymphoma of the central nervous system. Haematologica 2008;93:147-8. [Crossref] [PubMed]
- Kiefer T, Hirt C, Späth C, et al. Long-term follow-up of high-dose chemotherapy with autologous stem-cell transplantation and response-adapted whole-brain radiotherapy for newly diagnosed primary CNS lymphoma: results of the multicenter Ostdeutsche Studiengruppe Hamatologie und Onkologie OSHO-53 phase II study. Ann Oncol 2012;23:1809-12. [Crossref] [PubMed]
- Schorb E, Kasenda B, Atta J, et al. Prognosis of patients with primary central nervous system lymphoma after high-dose chemotherapy followed by autologous stem cell transplantation. Haematologica 2013;98:765-70. [Crossref] [PubMed]
- Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol 2003;70:219-24. [Crossref] [PubMed]
- Chamberlain MC. Salvage therapy with bendamustine for methotrexate refractory recurrent primary CNS lymphoma:a retrospective case series. J Neurooncol 2014;118:155-62. [Crossref] [PubMed]
- Chamberlain MC. High-dose cytarabine salvage therapy for recurrent primary CNS lymphoma. J Neurooncol 2016;126:545-50. [Crossref] [PubMed]
- Enting RH, Demopoulos A, DeAngelis LM, et al. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology 2004;63:901-3. [Crossref] [PubMed]
- Fischer L, Thiel E, Klasen HA, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol 2006;17:1141-5. [Crossref] [PubMed]
- Herrlinger U, Brugger W, Bamberg M, et al. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology 2000;54:1707-8. [Crossref] [PubMed]
- Hottinger AF, DeAngelis LM, Yahalom J, et al. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology 2007;69:1178-82. [Crossref] [PubMed]
- Mappa S, Marturano E, Licata G, et al. Salvage chemoimmunotherapy with rituximab, ifosfamide and etoposide (R-IE regimen) in patients with primary CNS lymphoma relapsed or refractory to high-dose methotrexate-based chemotherapy. Hematol Oncol 2013;31:143-50. [Crossref] [PubMed]
- Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol 2014;117:161-5. [Crossref] [PubMed]
- Raizer JJ, Rademaker A, Evens AM, et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer 2012;118:3743-8. [Crossref] [PubMed]
- Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer 2007;96:864-7. [Crossref] [PubMed]
- Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25:1350-6. [Crossref] [PubMed]
- Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol 2008;26:2512-8. [Crossref] [PubMed]
- Welch MR, Sauter CS, Matasar MJ, et al. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma 2015;56:361-7. [Crossref] [PubMed]
- del Rio MS, Choquet S, Hoang-Xuan K, et al. Platine and cytarabine-based salvage treatment for primary central nervous system lymphoma. J Neurooncol 2011;105:409-14. [Crossref] [PubMed]
- Murthy H, Anasetti C, Ayala E. Diagnosis and Management of Leukemic and Lymphomatous Meningitis. Cancer Control 2017;24:33-41. [PubMed]
- Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma:a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist 2011;16:1589-99. [Crossref] [PubMed]
- Fend F, Ferreri AJ, Coupland SE. How we diagnose and treat vitreoretinal lymphoma. Br J Haematol 2016;173:680-92. [Crossref] [PubMed]
- Ponzoni M, Issa S, Batchelor TT, et al. Beyond high-dose methotrexate and brain radiotherapy:novel targets and agents for primary CNS lymphoma. Ann Oncol 2014;25:316-22. [Crossref] [PubMed]


