Stereotactic radiosurgery of the brain: a review of common indications
Introduction
It has been over 100 years since Horsley and Clarke’s 1908 invention of the term “stereotaxis” to describe the lesioning of targets in a monkey brain using skull-based landmarks and a Cartesian coordinate system (1). These first steps utilized probes to reach a predetermined point in the cranium without a significant surgical dissection (2). Since these early beginnings, the use of “stereotaxis” in the treatment of central nervous system (CNS) disorders has ebbed and flowed with changes in medicine. Its first use in humans—almost 40 years after Horsley and Clarke—was to improve surgery for patients with movement disorders (3). In the early 1950s, the first attempt to use ionizing radiation in place of electrodes, for what he termed “radiosurgery,” was pioneered by Lars Leksell, culminating in the creation of the Gamma Knife apparatus (4). During this time he also experimented with proton beam therapy and X-ray sources but the technology was medically impractical at that time (5). Linear accelerator (LINAC) use began to gain momentum in the 1980s after LINAC advancements and modifications allowed for submillimetrically precise treatment (6,7). With continued growth in the field, a new name was proposed in 1973 with “stereotactic” replacing “stereotaxis” as it was felt that the purpose of the surgery was to “touch” the desired area with a probe or electrode (3). Advances in imaging helped to further localize radiosurgical targets with the adoption of CT and MR image guidance in the 1970s and 1980s (8). From these early pioneers, and through the dedication and work of thousands of practitioners and physicists, the field and indications for stereotactic radiosurgery (SRS) have grown markedly for a wide spectrum of diseases. In this review, we will examine common indications and areas of interest for the application of SRS as well as discuss potential future directions.
Malignant
Brain metastasis
Brain metastases are the most common intracranial malignancy, occurring at a frequency of 10 times that of primary brain tumors. Approximately 200,000 patients are diagnosed with brain metastases each year in the United States alone, and the incidence has been increasing due to better control of systemic disease. Of patients diagnosed with cancer, 20–40% will develop brain metastases. The most common primary tumors from which brain metastases arise are lung, breast, melanoma, and genitourinary cancers (9).
In the era before routine use of MR imaging for staging and surveillance, patients were often diagnosed with brain metastases after presenting with headaches, nausea and vomiting, focal neurologic deficits, cognitive changes, ataxia, and/or seizures. With widespread availability of imaging, patients are now more often diagnosed when their tumors are small and asymptomatic. Metastases are classically contrast-enhancing on T1-weighted MR imaging, often with larger lesions showing peripheral “ring-enhancement” with central necrosis. At presentation, more than half of patients present with 3 or more metastases, with the majority arising within the cerebral hemispheres (10,11). The development of brain metastases portends a poor prognosis for patients, with a multi-institutional analysis of nearly 4,000 patients who had largely been treated aggressively with combinations of regional therapies such as whole brain radiation therapy (WBRT), systemic therapies, and local therapies such as surgery or radiosurgery showing an overall median survival (MS) of 7.2 months and MS ranging from 2.8–25.3 months depending on such factors as age, performance status, extracranial disease burden, histology, etc. (12).
In patients with a history of metastatic cancer and imaging suggestive of intracranial metastases, definitive treatment may be pursued without pathologic confirmation; however, in patients without this prior diagnosis or an unclear etiology of their intracranial disease, a systemic workup and biopsy may be required before proceeding with definitive treatment. Patients with symptomatic disease should be started on steroids once a diagnosis is established, using the lowest dose that allows for control of neurologic symptoms. Surgery is often considered first line for patients with a large (usually defined as >3 cm in diameter) or symptomatic brain metastasis; however, many patients are not optimal candidates for resection because of other medical comorbidities, extensive extracranial burden of disease, or multiple intracranial metastases. In these cases, radiation, either as WBRT or SRS, are considered. Although WBRT has been used for decades for the treatment of brain metastases, its benefits have been questioned given its lack of data showing a benefit to overall survival or quality of life compared to supportive care alone (13). The addition of an SRS boost to WBRT was hypothesized to improve local control, and was found to improve survival in patients with a single brain metastasis compared to treatment with WBRT alone. In patients with multiple metastases, an SRS boost was found to improve local control, but had no added benefit on overall survival (14,15).
In the post-operative setting, SRS also appears to be a viable alternative to WBRT for those with limited numbers of metastases, avoiding WBRT in over 70% of cases (16). In these cases, target delineation adds another level of complexity. Allowing for a shallower dose fall-off around the resection cavity may be beneficial: Choi et al. discovered that less conformal plans had better control and subsequently reported that adding a 2 mm margin to the resection cavity significantly decreased local failure (3% vs. 16%) (17). Further, the relationship of SRS to surgery is an active area of investigation. Patel et al. reported early results of their retrospective review of preoperative vs. postoperative SRS and found a significant decrease in leptomeningeal disease and symptomatic radionecrosis for preoperatively treated patients (18). A clinical trial may be forthcoming by the NRG to formally assess this issue.
In patients with a limited number of brain metastases, the use of SRS without WBRT has been increasingly used to avoid the toxicities associated with WBRT (19). The concern with omitting WBRT has always been the increased risk of developing distant brain metastases from microscopic tumor deposits, which could impair neurologic function and survival. Several randomized trials have evaluated the use of SRS with or without WBRT, and while improved local and distant brain control were seen with the addition of WBRT, survival was unaffected (20,21). A randomized trial conducted by the EORTC also evaluated quality of life and found improved global health, physical functioning, cognitive functioning, and fatigue scores in patients who underwent focal treatment (either surgery or SRS) without additional WBRT (22). Further, another randomized study of SRS with or without additional WBRT evaluated neurocognitive preservation as a primary endpoint, and was ended early because significantly improved neurocognitive outcomes were seen in the group not receiving additional WBRT (23). Given the class I evidence demonstrating the equivalent survival of SRS alone in patients with ≤3 brain metastases, the omission of WBRT in this group of patients has been widely adopted (24).
An area of controversy is the definition of a “limited” number of metastases when considering what is safe and practical for treating with SRS. Currently, the best evidence for treating patients with ≥4 metastases with SRS alone comes from the Japanese Leksell Gamma Knife Society, where patients with ≤10 brain metastases were treated with SRS alone. In this study, patients with a single brain metastasis had the best survival; however, patients with 5–10 metastases had an equivalent survival to those with 2–4 with similar rates of toxicities (25). Reports from the University of Pittsburgh suggest that treatment volume may be a more useful predictor of outcome after SRS than number of metastases alone (26,27). Given these studies, SRS treatment for >3 brain metastases has been adopted by the NCCN (28). An example of one such treatment utilizing Gamma Knife SRS is illustrated in Figure 1.
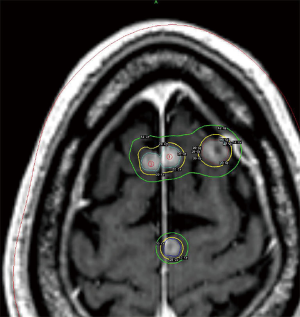
Regarding dose, the optimal dose-fraction scheme (i.e., single fraction vs. multiple fractions) for radiosurgery with brain metastases is unclear and there are no good comparisons between regimens. The doses most often used with single-fraction SRS are based on the findings of RTOG 90-05, which utilized maximum tumor diameter as a dose determinant (29). Tumor control appears correlated with the dose delivered, with local control rates for larger tumors typically lower than for smaller tumors. In these cases, a fractionated approach may be advantageous. In one retrospective analysis, patients with tumors >2 cm in diameter received either 27 Gy in 3 fractions or 15–18 Gy in 1 fraction, with the fractionated approach showing better local control (90% vs. 77% at 1 year) (30). Other fractionation schemes (35 Gy in 5 fractions, 36 Gy in 6 fractions, 40 Gy in 10 fractions) have been used with similar outcomes (31,32). In the post-operative setting, similar dosing schedules are used. The Stanford group recently reported on their phase I/II dose-escalation trial involving 3-fraction SRS and found that the maximum tolerated dose for 2–4 cm cavities was 27–30 Gy (33). The optimal approach utilizing SRS in the treatment of brain metastases continues to be an area of active investigation.
Glioblastoma multiforme
Glioblastomas (GBM) account for approximately 15% of all primary brain tumors and 46% of primary malignant brain tumors and tend to be more common in older white males. Despite aggressive therapy, expected overall survival at 5 years is 5%, with better outcomes for those diagnosed at young ages (34). The only factors that have been conclusively shown to increase the risk of having a GBM have so far been prior exposure to high doses of ionizing radiation and inherited mutations associated with rare syndromes (35). Patients typically present with headaches or seizures, along with focal neurologic deficits and symptoms of increased intracranial pressure. The most useful imaging for diagnosis is a T1-weighted MRI with contrast along with T2-weighted and fluid-attenuated inversion recovery sequences (FLAIR), which classically demonstrate an irregular ring-enhancing lesion with surrounding edema.
The typical treatment for GBM is a combination of surgery followed by external beam radiation therapy (EBRT) to 60 Gy directed to the brain infiltrated by tumor and resection site with concurrent and adjuvant temozolomide (36), though shorter radiotherapy courses in the elderly do not appear to provide inferior survival in phase III trials (37,38). Despite aggressive locoregional irradiation, most recurrences will appear within 2 cm of the primary site of disease (39,40). Though primary treatment approaches with SRS seem perhaps counterintuitive given the widely infiltrative nature of this disease, this treatment has been examined as part of a boost therapy. In RTOG 93-05, 203 patients were randomized to size-dependent SRS doses from 15–24 Gy followed by EBRT to 60 Gy with BCNU chemotherapy vs. EBRT 60 Gy with BCNU alone (41). No difference in survival (14.7 vs. 14.2 months), quality of life, or failure patterns between the two arms were observed. Critics note the non-standard use of a “pre-EBRT” SRS boost in this trial as differing from standard clinical practice (42,43). Nevertheless, the trial remains the only level I evidence available with which to measure this intervention.
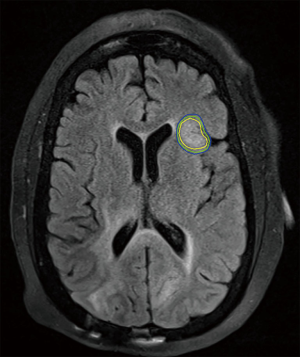
Otherwise, SRS for recurrent GBM is supported by a series of small prospective and retrospective series with either hypofractionated stereotactic radiotherapy or SRS, often with concurrent bevacizumab, with an expected 10–14 months MS following therapy following dosing schemes similar to RTOG 90-05 and is supported by society guidelines (29,44-49). Larger trials, such as the ongoing RTOG 12-05, are needed to formalize this treatment approach, but it remains a viable option for patients without extensive progression when recurrent GBM is diagnosed. An example of such a treatment is illustrated in Figure 2.
Benign
Meningiomas
Meningiomas are the most common benign tumors of the CNS, originating from the arachnoid layers of the brain, constituting an estimated 36% of all primary brain tumors, and over 50% of benign CNS tumors (50). They occur twice as often in women than men, which is attributed to hormonal stimulation of their growth. The most common presenting symptom for these lesions is a headache, but other localizing symptoms may manifest depending on the location of the tumor (34,51,52). Diagnosis is typically made radiographically on CT or MRI based upon the appearance of a homogeneously and intensely enhancing extra-axial mass with or without the presence of a dural tail (53).
The typical management of meningiomas involves either observation, EBRT, SRS, or surgical resection, the extent of which is graded on the Simpson scale (which relates to the risk of postoperative recurrence) (54). Histopathologic grading is classified by into three grades by the World Health Organization with Grade I considered benign, Grade II considered atypical, and Grade III considered malignant (55). If treated surgically, indications for post-operative radiotherapy typically include incompletely resected disease or WHO Grade II/III disease with the expectation of improved local control and, for high grades, a possible increase in overall survival (52,56-58). RTOG 05-39 placed patients with meningiomas into risk groups depending on degree of resection, grade, and status of the tumor as a recurrence or de novo disease. The low risk group was observed and the intermediate/high risk groups received radiotherapy to either 54 or 60 Gy, respectively. The first analyses report equivalence between local control within the low and intermediate risk groups following this treatment strategy (59).
For the past several decades, SRS has been used as an alternative to resection or EBRT as definitive treatment for benign-appearing meningiomas. Pollock et al. examined 416 patients treated with single-fraction SRS for either imaging defined or histologically confirmed WHO grade I tumors treated to a median marginal dose of 16 Gy. The disease-specific survival rate was excellent at 97% at 5 years and 94% at 10 years with an 11% rate of permanent radiation-related complications at 5 years (60). Santacroce et al. retrospectively examined 5,300 benign meningiomas receiving Gamma Knife SRS at least 5 years prior to analysis. The median marginal dose was 14 Gy and led to 5- and 10-year progression free survival rates of 95.2% and 88.6%, respectively. Permanent morbidity was 6.6% in this series (61). Kollová et al. examined 368 patients treated with Gamma Knife SRS with actuarial local control of 97.9% at 5 years with a permanent morbidity rate of 5.7% (62). It is important to note, in these studies and others, that the histopathologic grade of the meningioma may not be confirmed and these series include a significant proportion of postoperative tumor remnants or recurrences of previously resected tumors.
Radiosurgery for WHO Grade II and III meningiomas is less well elucidated. Retrospective studies suggest a dose response and authors advocate a marginal dose above 12 Gy, perhaps in the range of 16–20 Gy, understanding that there is a higher chance for local toxicity (63,64). Unlike the benign variant, fewer studies and patient numbers guide treatment for these locally aggressive tumors and it is an ongoing area of investigation.
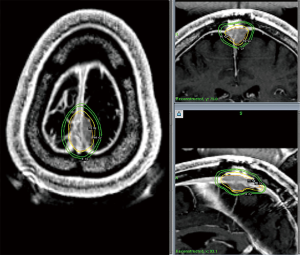
In summary, the role of SRS for meningioma is varied and may include definitive management for small or unresectable tumors, adjuvant treatment to address the remnant of a planned subtotal resection or high grade tumors, or as a salvage for late recurrences. An example of an SRS treatment following subtotal resection for a WHO Grade I meningioma is illustrated in Figure 3. Clinical decision making should be shared between the patient, radiation oncologist, and neurosurgeon and take into consideration tumor size, location, grade, prior surgery, and patient preference in order to achieve optimal care (60).
Arteriovenous malformations (AVMs)
AVMs are complex lesions of abnormal arteries and veins that lack a capillary bed and are distinct from other intracranial vascular malformations such as strictly venous malformations, dural arteriovenous fistulas, Galen malformations, etc. (65,66). An estimated 0.1% of the population is postulated to have an AVM (67). The origins of AVMs remain unclear, as does the proposed triggering mechanism. The commonly held hypothesis is that these lesions arise from a disturbance during the embryonic stage of vessel formation, while others contend that that they may arise from an initial lesion in the border regions served by the distal cerebral arteries during late fetal or immediate postpartum life (65).
Regardless, AVMs will typically be found incidentally on imaging performed for other indications, though patients may also present with symptomatic hemorrhage or seizure (67). Diagnostically, AVMs are identified via high resolution CT or MR imaging supplemented by cerebral angiography (66). Most AVMs will have deep venous drainage and approximately 40% will be small (<2.5–3 cm). AVMs present patients with the risk of intracranial hemorrhage and associated long-term neurologic consequences. The annual rate of hemorrhage is suggested to be 2.2% per year, 4.5% per year for re-rupture in AVMs that have a history of rupture, or 3% overall with an associated risk of death of approximately 1%. Factors that increase the risk for bleeding include a deep brain location, exclusively deep venous drainage, and associated aneurysms (68). The grading system for AVMs proposed by Spetzler and Martin is based upon the size, pattern of venous drainage, and neurologic eloquence of the adjacent brain (69).
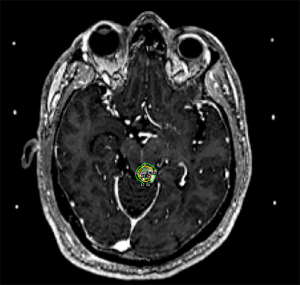
Typical management options include observation, surgical excision, SRS, or endovascular embolization. The goal of treatment is complete obliteration of the vascular nidus to eliminate the risk of hemorrhage. Observation may be appropriate for large volume AVMs (4–5 cm diameter) in patients without prior bleeding and whose AVMs lack other high-risk features (66). SRS is considered for patients with unresectable AVMs, with the best outcomes for small volume AVMs or otherwise located in non-critical locations where single doses >18 Gy to the AVM margin are feasible. There is a protracted response time following SRS with the earliest obliterations noted within 2–3 months, approximately 50% after one year and up to 90% within 3 years. If, at the end of three years there is still residual AVM, repeat SRS or another management strategy may be considered. A threshold of radiation dose is required to achieve AVM obliteration, with >80% obliteration rates above 18 Gy, and the chance of cure based upon volume and location (70). The side effects of SRS for AVMs will also differ based upon location in the brain. For AVMs with treatment volumes expected to be larger than 15 cc, it is recommended to perform volume-staged or dose-staged SRS as increased rates of complications or lower rates of obliteration efficacy are reported at these higher volumes (71-75). An example of Gamma Knife SRS treatment for a small non-resectable AVM of the left tectum is illustrated in Figure 4.
Of important note is the recent publication of the ARUBA trial—“A Randomized Trial of Unruptured Brain AVMs”—by Mohr et al. This multicenter, non-blinded, randomized trial compared adult patients with unruptured brain AVMs to either interventional therapy (i.e., neurosurgery, embolization, and/or SRS alone or in combination) vs. medical management (pharmacological therapy for neurological symptoms as needed). The primary outcome was a composite endpoint of death or symptomatic stroke with a secondary endpoint of clinical impairment. The trial was stopped early when a clear early superiority was found within the medical management arm. For the primary endpoint there were 12 vs. 45 strokes as well as 1 vs. 14 neurologic deficits unrelated to strokes (76). Despite these seemingly impressive results, critics point out several flaws in the study, including a large segment of treated but unanalyzed patients, an uncontrolled treatment arm with low utilization of surgical intervention alone, scant details on the radiosurgery provided, and a higher number of patients managed with embolization alone, which is known to have low obliteration rates and which may adversely affect AVM hemodynamics (77). The ARUBA trial will continue to follow patients for 5 years, which may be too short to demonstrate the benefit of AVM eradication (78). Ultimately, this trial should be interpreted with caution and the treatment of AVMs should be undertaken with the involvement of an experienced multidisciplinary team.
Vestibular schwannomas
Arising from Schwann cells surrounding the nerves, vestibular schwannomas, also known as “acoustic neuromas”, are benign, non-invasive, slow growing tumors that surround the vestibulocochlear nerve (CN VIII). Typically arising sporadically and unilaterally, these lesions may occur bilaterally as well, as in type 2 neurofibromatosis (NF-2), where they act as a hallmark of that disease. Schwannomas may also occur around other cranial nerves or elsewhere in the neuraxis, with CN V as the 2nd most common intracranial site. Vestibular schwannomas arise in the internal auditory canal (IAC), and eventually extend medially into the cerebellar pontine angle (CPA) (53). This extension, and subsequent mass effect upon other cranial nerves in the area of the brainstem/cerebellum, explains the varied signs of this disease beyond CN VIII. The most common symptoms will typically be hearing loss (95%) and tinnitus (60%) followed by disequilibrium (40%), dizziness (22%) and vertigo (28%) (79). Facial hypesthesia or paresthesias may arise from CN V being compressed by a large vestibular schwannoma. On MRI, schwannomas are well-demarcated contrast-enhancing lesions that arise from an often widened IAC.
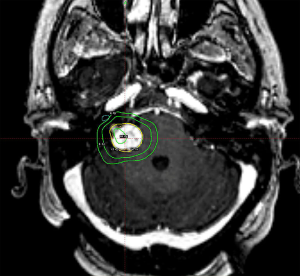
Treatment options for vestibular schwannomas include observation, SRS, fractionated radiotherapy, and surgery. To date, there is no high quality evidence comparing these treatment modalities (80). The best evidence comes from various meta-analyses published over the years. Expected local control with SRS is ≥90% with only 4% requiring additional treatment (79). Post-SRS rates of facial and trigeminal neuropathy have declined in concert with decreasing radiation dose, without an unacceptably high recurrence rate at 5–10 years’ time (81). Currently, single doses of 12–13 Gy appear to confer high rates of local control with greater sparing of nearby nerve function. An example of one such treatment is illustrated in Figure 5. Useful hearing preservation appears to be associated with a ≤4 Gy cochlear dose, but this has not been confirmed in a prospective study (82). At doses of 12–13 Gy, 5-year actuarial hearing-level preservation rate is approximately 70% with most changes occurring within the first 4–5 years and stabilizing thereafter (83). Surgery remains a reasonable choice of therapy, with similar rates of control but slightly higher rates of associated operative morbidities and a substantially more protracted post-procedural recovery period (79).
Pituitary adenomas
The pituitary gland is an endocrine organ directly connected to the brain via the infundibulum and rests upon the hypophyseal fossa within the sella turcica, anterior and, for the most part, inferior to the optic chiasm. The gland is constituted, embryologically, of the adenohypophysis and neurohypophysis. Pituitary adenomas, arising from cells in the adenohypophysis, comprise approximately 10–15% of all intracranial tumors. They can be classified by their secretory status, cell of origin, and size. Functional pituitary adenomas (FPAs) or “secretory” adenomas make up approximately 75% of all adenomas and produce hormones related to their cell of origin (i.e., prolactinomas produce prolactin). The remainder are non-functioning pituitary adenomas (NFPAs) or “non-secretory” tumors that cause symptoms related to their mass effect on surrounding tissues. Picoadenomas, microadenomas, and macroadenomas comprise lesions of <0.3, <1, and >1 cm, respectively (53).
Clinically, presentations involve hypersecretion of hormones, evidence of hypopituitarism, visual field deficits caused by compression of the anterior visual pathway, and headache (51). Though neurologic changes are usually gradual, acute changes may occur in cases of pituitary apoplexy (53). The most common hypersecretion syndrome is the overproduction of prolactin, causing amenorrhea and galactorrhea in women and loss of libido in men. MRI is classically the imaging method of choice as it allows for the evaluation of the tumor and its relation to the chiasm, hypothalamus, cavernous sinus, and can otherwise distinguish the tumor from an aneurysm (51).
Traditionally, surgery is the first line treatment for pituitary adenomas, as it can relieve mass effect, establish a pathologic diagnosis, and when necessary, physically separate the optic apparatus from underlying tumor. There is a role for primary medical treatment for some FPAs, such as prolactinomas, with targeted therapies. Following transsphenoidal surgery, expected recurrence rates range between 50–80%, depending on secretory status, cell type, and perhaps local invasion (84-86). EBRT has long been used to treat residual surgical disease or the medically inoperable patient. Common indications for EBRT include macroadenomas, recurrent tumors, and secretory tumors that do not return to baseline hormone levels with surgery or medical therapy (87). Inconsistent standards for hormone levels have hindered interpretation of the literature regarding local control and “cure” rates of FPAs and NFPAs, however, EBRT is expected to provide 80-98% local growth control as well as 67–89% hormone control for secretory tumors with adequate doses above 45 Gy (87-89).
In a similar vein, SRS has been used as an effective treatment since Dr. Leksell began targeting intracranial pathology in the 1950s and is an attractive alternative to EBRT (8). Though most available reports involve small series from single centers, Kim et al. published on an analysis of 16 contemporary studies of NFPAs and found that local control ranged from 83–100% with most papers reporting >90% for a median/mean marginal dose of 14–20 Gy (90). In the largest of the studies by Sheehan et al., 418 patients received SRS to a marginal dose of 12–18 Gy for NFPAs and 18–30 for FPAs. They reported 90% control of tumor growth for both tumor types (91).
For FPAs, the outcomes are not as clear, mostly owing to differences in reporting and increasingly stringent definitions of hormonal normalization. Prolactinomas treated with a median dose of 25 Gy have complete endocrine normalization anywhere from 11–80% in 2–8 years. Long-term data suggests a roughly 50% remission rate with differences between FPA cell types (90,92). It appears there may be a dose response relationship with both the rate of hormonal normalization and with time to remission (91,93).
The most common complication of therapy includes new anterior pituitary deficits, occurring in 20–40%. Half of these occur within 48–96 months after treatment with the risk of hypopituitarism decreasing markedly after 120 months (92). Retrospective analyses suggest that dose to the normal gland and dose to the distal infundibulum affect the rate of this complication, but the role of an enlarging pituitary adenoma or of any pre-radiosurgical neurosurgical interventions on reducing functional pituitary reserve is rarely acknowledged (90). Cranial nerve deficits and brain injury may occur in <1% of cases, along with rare vascular injury (94), with a higher likelihood of these side-effects occurring in previously irradiated patients. It has been recommended that the target be at least 2–3 mm from the optic chiasm to provide adequate protection for that critical structure (86,91,95), but point doses of 10–12 Gy to the chiasm with frame-based radiosurgery appear reasonably safe in the hands of experienced radiosurgeons (96). Given the significant hormonal component to this disease, all patients should also be followed by an endocrinologist.
Functional disorders
The root of all radiosurgery may well lie in functional radiosurgery, stemming from the desire to create small, precisely defined, focal lesions deep in the brain where surgery was unsuitable, and forming the foundation upon which all else has been built (4,8). Functional neurosurgical and radiosurgical procedures have waxed and waned with the development of medications that obviated the need for direct intervention. However, there remains disease that is intractable to medical management and which is not easily addressed by surgery that may benefit from a radiosurgical intervention. We will briefly review these indications.
Trigeminal neuralgia (TN) and other pain disorders
Perhaps the best described and studied use of SRS is for relief of pain related to TN. Often, these procedures are undertaken after previous medical or surgical management. Typically, the prepontine trigeminal nerve or the trigeminal root entrance zone is targeted with maximum radiosurgical doses of 70–90 Gy, which is largely based upon a multi-institutional study involving 50 patients that showed a dose response relationship for complete pain relief at >70 Gy (72% vs. 9%). In this study, 54% of patients experienced 100% pain relief, 88% had between 50–100% pain relief, and 6% had treatment failure. The median time to pain relief was 1 month. Out of 50 patients, 3 developed increased facial paresthesia and decreased sensation after SRS with one experiencing complete resolution, and one with subtotal improvement (97).
A recent review of 19 contemporary studies from 2006–2011 somewhat tempers these expectations (98). There is heterogeneity in how pain outcomes are reported, but the majority of the studies made use of the Barrow Neurological Institute pain intensity scale wherein a score of I = no pain, II = occasional pain, not requiring medication, III = some pain, controlled with medication, IV = some pain, not controlled with medication; V = severe pain/no pain relief (99). For institutions utilizing this scale, the reported range of BNI scores I-III was 45–94% (98). It should be recognized that TN refractory to prior neurosurgical interventions is less likely to respond to SRS, and that patients who have components of pain consistent with atypical facial pain are also less likely to achieve satisfactory pain relief. Should patients not experience pain relief following their initial treatment, additional small series suggest radiosurgical retreatment is a viable option with a salvage success of approximately 70%. In addition, surgical options continue to be available (e.g., microvascular decompression or trans-foramen ovale procedures such as radiofrequency thermorhizotomy, glycerol rhizolysis, or balloon rhizotomy).
SRS for chronic and thalamic pain syndrome as well as cancer-related pain syndrome are less well elucidated and are based upon too few patients to draw strong conclusions (100,101).
Tremors
Another functional disorder in which SRS may be well utilized is in the treatment of movement disorders such as essential tremor (ET) or Parkinson’s disease (PD) (102-105). The largest report is from Young et al., (105) which describes 158 patients receiving a Gamma Knife ventralis intermedius (VIM) thalamotomy for PD (102 patients), ET (52 patients), and other tremor causes (4 patients). Utilizing a single 4 mm shot with maximum doses of 120 to 160 Gy, 88% of PD patients became fully or nearly tremor free with 4 years follow-up or more, with a similar rate of relief for ET patients. In terms of complications, only two patients had permanent side effects including mild contralateral paresthesias of the face/upper extremity for one and mild weakness of the contralateral arm/leg and minimal dysphasia for the other. Niranjan et al. (103) reported on the Pittsburgh 19-year experience and demonstrated that Gamma Knife SRS was able to control 50% of ET at 6 months and 90% at one year with 90% of patients maintaining this control at 10 years. Like Young et al., the VIM was targeted with a median maximal dose of 140 Gy. Given the absence of eletrophysiologic information and the latency of the clinical response with a median time of 2 months, many prefer SRS for those patients with advanced age or with medical disorders in whom electrode placement for deep brain stimulation would be high risk (105).
Epilepsy
In the treatment of AVMs, it was observed that seizures associated with these lesions would resolve following radiosurgical treatment, independent of the angiographic response. It was postulated that ionizing radiation itself may be modulating the neurologic function of brain tissue adjacent to the AVM (106,107). In the early 1980s and 1990s Barcia-Salorio et al. (108) treated 11 patients with idiopathic focal epilepsy with doses of 10–20 Gy. There was complete cessation of seizures in 4 cases and reduction of seizures in an additional 5 cases. Since that time, experiments in animal models have shown a clear relationship between increasing dose and decreasing seizure frequency measured by EEG, with a maximum dose of 40–60 Gy found to be sufficient for seizure control (109,110). Régis and colleagues (111) in Marseille performed selective amygdalohippocampal radiosurgery for mesial temporal lobe epilepsy and delivered a dose of 25 Gy to the 50% isodose line to a 7 mL volume, the largest functional target up to that time. Of the two patients that underwent this procedure, 1 was seizure free immediately and the other became seizure free after a latency of 1 year. In 2009, Barbaro et al. (112) reported on a multicenter pilot study of 17 patients using SRS for mesial temporal lobe epilepsy as an alternative to open surgery using marginal doses of 20 or 24 Gy targeting the amygdala, hippocampus, and parahippocampal gyrus. There was a 67% seizure free rate of at least 1 year at 3-year follow-up.
The recently completed ROSE trial (Radiosurgery or Open Surgery for Epilepsy) (113) led by Drs. Quigg and Barbaro attempted to take their pilot study a step farther by comparing a marginal dose of 24 Gy SRS against anterior temporal lobectomy. Pre-publication results show that the trial only accrued 58 patients and did not have the statistical power to properly assess the primary endpoint of seizure freedom. At 3 years, 78% vs. 52% of patients were seizure free for 12 prior months, favoring the surgery arm. Similar to what was seen previously, there was a latent period of approximately one year, with initially more seizures (mostly auras), in the SRS arm. Given the low numbers involved in the study, it is difficult to draw conclusions regarding the inferiority of one treatment versus the other. The use of SRS to treat epilepsy is appealing from a minimally invasive approach but suffers from similar roadblocks as other focal therapies, namely the difficulty in localizing non-lesional epilepsy and in defining the target volume. The risks associated with continued seizures during the latency period merits consideration when choosing therapy for this condition. In properly selected patients, SRS remains a viable treatment option.
Future directions
Immunotherapy and radiosurgery
The CNS has long been viewed as an immunologically privileged site. For example, tissues that are rejected by the immune system when grafted in other sites, e.g., the skin, exhibit survival within the CNS. The blood brain barrier, lack of clear lymphatics, and proposed immunoincompetence of the microglia, drove forward this line of thinking. Yet for the past several decades it has become apparent that the CNS has far greater interaction with the immune system than previously believed. Indeed, peripheral immune cells can cross an intact blood brain barrier, neurons and glia actively regulate lymphocyte and macrophage responses, and microglia cells are competent but differ from the traditional macrophage/dendritic cell in terms of their ability to direct responses (114). Traditionally, radiotherapy has generally been viewed as an immunosuppressive entity, but a greater understanding of radiation’s impact on the release of tumor antigens to the immune system and its effects on the tumor microenvironment have shed light on this complex relationship, opening a new and exciting avenue for treatment (115-118).
Immunoradiotherapy is still in its infancy, but several institutions have published retrospective results combining various immunomodulators. At Yale, 77 patients with melanoma brain metastases who were treated with SRS and ipilimumab versus SRS alone had MS of 21.3 vs. 4.9 months and 2-year survival rates of 47.2% vs. 19.7%, far beyond the commonly predicted MS of 4–6 months (119). Ahmed et al. analyzed 26 patients with metastatic melanoma treated with SRS and nivolumab and also showed results above the historical norms, with unresected patients having a MS of approximately 12 months and resected patients not yet having reached MS with a median follow-up of 15 months (120).
At this stage, the optimal dose, fractionation, and timing of radiosurgery with immunotherapy is unknown and may depend not only on the disease being targeted but also the drug being used (121-124). An excellent review of current clinical trials by Kang et al. published in late 2016 lists over 90 phase I and phase II clinical trials evaluating the interplay of radiation and the current immunotherapy armamentarium (125). Yet, while there are many single and multifraction stereotactic body radiation therapy trials, there are few intracranial SRS trials examining this relationship. Currently, MD Anderson is examining the treatment of non-small cell lung cancer brain metastases in a phase I/II trial with WBRT, hypofractionated stereotactic radiotherapy, and SRS, to find an appropriate dose of nivolumab (PD-1) and ipilimumab (CTLA-4) (126). The University of Michigan has started a phase II trial examining ipilimumab and SRS for metastatic melanoma to the brain (127), and the Sidney Kimmel Comprehensive Cancer Center is examining a similar question but with nivolumab (128). For patients with recurrent glioblastoma, the University of Virginia is examining SRS with nivolumab in a phase I study (129). These trials and numerous others represent the possibility of a powerful union which may shift the paradigm of how we use radiation in the approach of metastatic and regional disease. We await with anticipation the results of these studies.
Conclusions
SRS is a powerful tool in the treatment of intracranial disease spanning the spectrum of malignant, benign, and functional disorders of the brain. With careful selection and judicious use, it may act as both a definitive treatment and in an adjunctive fashion to other procedures. Proper utilization of this treatment modality is a multidisciplinary enterprise between numerous specialties, starting with both neurosurgery and radiation oncology. The future of this therapy is bright as medicine and patients exhibit a growing preference for minimally invasive procedures and developments in immunotherapy open new avenues of treatment in the fight against cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rahman M, Murad GJ, Mocco J. Early history of the stereotactic apparatus in neurosurgery. Neurosurg Focus 2009;27:E12. [Crossref] [PubMed]
- Oliver L, Bennett AM. Stereotaxis in the treatment of parkinsonism. Postgrad Med J 1961;37:423-6. [Crossref] [PubMed]
- Gildenberg PL, Krauss JK. History of Stereotactic Surgery. In: Lozano AM, Gildenberg PL, Tasker RR. editors. Textbook of Stereotactic and Functional Neurosurgery. Berlin, Heidelberg: Springer Berlin Heidelberg, 2009:1-33.
- Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 1951;102:316-9. [PubMed]
- Larsson B, Leksell L, Rexed B, et al. The high-energy proton beam as a neurosurgical tool. Nature 1958;182:1222-3. [Crossref] [PubMed]
- Kushnirsky M, Patil V, Schulder M. The History of Stereotactic Radiosurgery. In: Chin LS, Regine WF, editors. Principles and Practice of Stereotactic Radiosurgery. New York, NY: Springer New York, 2015:3-10.
- Colombo F, Benedetti A, Pozza F, et al. External stereotactic irradiation by linear accelerator. Neurosurgery 1985;16:154-60. [Crossref] [PubMed]
- Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry 1983;46:797-803. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Delattre JY, Krol G, Thaler HT, et al. Distribution of brain metastases. Arch Neurol 1988;45:741-4. [Crossref] [PubMed]
- Arbit E, Wroński M, Burt M, et al. The treatment of patients with recurrent brain metastases. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer 1995;76:765-73. [Crossref] [PubMed]
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012;30:419-25. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427-34. [Crossref] [PubMed]
- Quigley MR, Fuhrer R, Karlovits S, et al. Single session stereotactic radiosurgery boost to the post-operative site in lieu of whole brain radiation in metastatic brain disease. J Neurooncol 2008;87:327-32. [Crossref] [PubMed]
- Choi CY, Chang SD, Gibbs IC, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: prospective evaluation of target margin on tumor control. Int J Radiat Oncol Biol Phys 2012;84:336-42. [Crossref] [PubMed]
- Patel K, Asher A, Burri SH, et al. Comparing preoperative stereotactic radiosurgery (SRS) to postoperative srs for resectable brain metastases. Int J Radiat Oncol Biol Phys 2015;93:S38-S39. [Crossref]
- Knisely JP, Yamamoto M, Gross CP, et al. Radiosurgery alone for 5 or more brain metastases: expert opinion survey. J Neurosurg 2010;113 Suppl:84-9. [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2015;91:710-7. [Crossref] [PubMed]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [Crossref] [PubMed]
- Bhatnagar AK, Flickinger JC, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys 2006;64:898-903. [Crossref] [PubMed]
- Bhatnagar AK, Kondziolka D, Lunsford LD, et al. Recursive partitioning analysis of prognostic factors for patients with four or more intracranial metastases treated with radiosurgery. Technol Cancer Res Treat 2007;6:153-60. [Crossref] [PubMed]
- Lo SS, Sloan AE, Machtay M. Stereotactic radiosurgery for more than four brain metastases. The Lancet Oncology 2014;15:362-3. [Crossref] [PubMed]
- Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000;47:291-8. [Crossref] [PubMed]
- Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 2016;95:1142-8. [Crossref] [PubMed]
- Kim YJ, Cho KH, Kim JY, et al. Single-dose versus fractionated stereotactic radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys 2011;81:483-9. [Crossref] [PubMed]
- Fokas E, Henzel M, Surber G, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol 2012;109:91-8. [Crossref] [PubMed]
- Soltys SG, Seiger K, Modlin LA, et al. A phase I/II dose-escalation trial of 3-fraction stereotactic radiosurgery (SRS) for large resection cavities of brain metastases. Int J Radiat Oncol Biol Phys 2015;93:S38. [Crossref]
- Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the united states in 2008-2012. Neuro Oncol 2015;17 Suppl 4:iv1-iv62. [Crossref] [PubMed]
- Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2006;2:494-503; quiz 1 p following 16.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Perry JR, Laperriere N, O'Callaghan CJ, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 2017;376:1027-37. [Crossref] [PubMed]
- Roa W, Kepka L, Kumar N, et al. International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 2015;33:4145-50. [Crossref] [PubMed]
- Wallner KE, Galicich JH, Krol G, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 1989;16:1405-9. [Crossref] [PubMed]
- Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology 1980;30:907-11. [Crossref] [PubMed]
- Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys 2004;60:853-60. [Crossref] [PubMed]
- Vordermark D, Kölbl O. Lack of survival benefit after stereotactic radiosurgery boost for glioblastoma multiforme: randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of radiation therapy oncology group 93-05 protocol: in regard to Souhami et al. (Int J Radiat Oncol Biol Phys 2004;60:853-860). Int J Radiat Oncol Biol Phys 2005;62:296-7; author reply 7. [Crossref] [PubMed]
- Kondziolka D, Lunsford LD, Flickinger JC. In regard to Dr. Souhami et al. (Int J Radiat Oncol Biol Phys 2004;60:853-860). Int J Radiat Oncol Biol Phys 2005;62:614-5; author reply 5-6. [Crossref] [PubMed]
- Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2009;75:156-63. [Crossref] [PubMed]
- Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2012;82:2018-24. [Crossref] [PubMed]
- Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 2010;28:3048-53. [Crossref] [PubMed]
- Cabrera AR, Cuneo KC, Desjardins A, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys 2013;86:873-9. [Crossref] [PubMed]
- Redmond KJ, Mehta M. Stereotactic radiosurgery for glioblastoma. Cureus 2015;7:e413. [PubMed]
- Cabrera AR, Kirkpatrick JP, Fiveash JB, et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol 2016;6:217-25. [Crossref] [PubMed]
- Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol 2012;14 Suppl 5:v1-49. [Crossref] [PubMed]
- Black PM. Brain tumors. New England Journal of Medicine 1991;324:1471-6. [Crossref] [PubMed]
- Yang SY, Park CK, Park SH, et al. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry 2008;79:574-80. [Crossref] [PubMed]
- Winkfield KM, Bazan JG, Gibbs IC, et al. Nonmalignant Diseases. Principles and Practice of Radiation Oncology. Sixth ed: Lippincott Williams & Wilkins.
- Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22-39. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathologica 2016;131:803-20. [Crossref] [PubMed]
- Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 2009;64:56-60; discussion 60. [Crossref] [PubMed]
- Soyuer S, Chang EL, Selek U, et al. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol 2004;71:85-90. [Crossref] [PubMed]
- Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol 2000;48:151-60. [Crossref] [PubMed]
- Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: Initial outcomes from NRG Oncology/RTOG-0539. Int J Radiat Oncol Biol Phys 2015;93:S139-S140. [Crossref]
- Pollock BE, Stafford SL, Link MJ, et al. Single-fraction radiosurgery of benign intracranial meningiomas. Neurosurgery 2012;71:604-12; discussion 613. [Crossref] [PubMed]
- Santacroce A, Walier M, Régis J, et al. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 2012;70:32-9; discussion 39. [Crossref] [PubMed]
- Kollová A, Liscák R, Novotný J, et al. Gamma Knife surgery for benign meningioma. J Neurosurg 2007;107:325-36. [Crossref] [PubMed]
- Sethi RA, Rush SC, Liu S, et al. Dose-Response Relationships for Meningioma Radiosurgery. Am J Clin Oncol 2015;38:600-4. [Crossref] [PubMed]
- Valery CA, Faillot M, Lamproglou I, et al. Grade II meningiomas and Gamma Knife radiosurgery: analysis of success and failure to improve treatment paradigm. J Neurosurg 2016;125:89-96. [PubMed]
- Stapf C, Mohr JP, Pile-Spellman J, et al. Epidemiology and natural history of arteriovenous malformations. Neurosurg Focus 2001;11:e1. [Crossref] [PubMed]
- Lunsford LD, Kondziolka D, Niranjan A, et al. Stereotactic Radiosurgery for Patients with Intracranial Arteriovenous Malformations (AVM). Radiosurgery Practice Guideline Report #2-03: International Radiosurgery Association, 2009. Available online: http://www.irsa.org/avms.html
- AVM Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med 1999;340:1812-8. [Crossref] [PubMed]
- Gross BA, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg 2013;118:437-43. [Crossref] [PubMed]
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986;65:476-83. [Crossref] [PubMed]
- Flickinger JC, Pollock BE, Kondziolka D, et al. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys 1996;36:873-9. [Crossref] [PubMed]
- Pan DH, Guo WY, Chung WY, et al. Gamma Knife radiosurgery as a single treatment modality for large cerebral arteriovenous malformations. J Neurosurg 2000;93 Suppl 3:113-9. [PubMed]
- Miyawaki L, Dowd C, Wara W, et al. Five year results of LINAC radiosurgery for arteriovenous malformations: outcome for large AVMs. Int J Radiat Oncol Biol Phys 1999;44:1089-106. [Crossref] [PubMed]
- Kim HY, Chang WS, Kim DJ, et al. Gamma Knife surgery for large cerebral arteriovenous malformations. J Neurosurg 2010;113 Suppl:2-8. [PubMed]
- Pollock BE, Kline RW, Stafford SL, et al. The rationale and technique of staged-volume arteriovenous malformation radiosurgery. Int J Radiat Oncol Biol Phys 2000;48:817-24. [Crossref] [PubMed]
- Moosa S, Chen CJ, Ding D, et al. Volume-staged versus dose-staged radiosurgery outcomes for large intracranial arteriovenous malformations. Neurosurg Focus 2014;37:E18. [Crossref] [PubMed]
- Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 2014;383:614-21. [Crossref] [PubMed]
- van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 2011;306:2011-9. [Crossref] [PubMed]
- Russin J, Spetzler R. Commentary: The ARUBA trial. Neurosurgery 2014;75:E96-7. [Crossref] [PubMed]
- Arthurs BJ, Fairbanks RK, Demakas JJ, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev 2011;34:265-77; discussion 77-9. [Crossref] [PubMed]
- Muzevic D, Legcevic J, Splavski B, et al. Stereotactic radiotherapy for vestibular schwannoma. Cochrane Database Syst Rev 2014.CD009897. [PubMed]
- Miller RC, Foote RL, Coffey RJ, et al. Decrease in cranial nerve complications after radiosurgery for acoustic neuromas: a prospective study of dose and volume. Int J Radiat Oncol Biol Phys 1999;43:305-11. [Crossref] [PubMed]
- Kano H, Kondziolka D, Khan A, et al. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma: clinical article. J Neurosurg 2013;119 Suppl:863-73. [PubMed]
- Flickinger JC, Kondziolka D, Niranjan A, et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2004;60:225-30. [Crossref] [PubMed]
- Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 2012;15:71-83. [Crossref] [PubMed]
- Brochier S, Galland F, Kujas M, et al. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol 2010;163:193-200. [Crossref] [PubMed]
- Laws ER, Sheehan JP, Sheehan JM, et al. Stereotactic Radiosurgery for Pituitary Adenomas: A Review of the Literature. Journal of Neuro-Oncology 2004;69:257-72. [Crossref] [PubMed]
- Becker G, Kocher M, Kortmann RD, et al. Radiation therapy in the multimodal treatment approach of pituitary adenoma. Strahlenther Onkol 2002;178:173-86. [Crossref] [PubMed]
- Zierhut D, Flentje M, Adolph J, et al. External radiotherapy of pituitary adenomas. Int J Radiat Oncol Biol Phys 1995;33:307-14. [Crossref] [PubMed]
- McCollough WM, Marcus RB, Rhoton AL, et al. Long-term follow-up of radiotherapy for pituitary adenoma: the absence of late recurrence after greater than or equal to 4500 cGy. Int J Radiat Oncol Biol Phys 1991;21:607-14. [Crossref] [PubMed]
- Kim W, Clelland C, Yang I, et al. Comprehensive review of stereotactic radiosurgery for medically and surgically refractory pituitary adenomas. Surg Neurol Int 2012;3:S79-89. [Crossref] [PubMed]
- Sheehan JP, Pouratian N, Steiner L, et al. Gamma Knife surgery for pituitary adenomas: factors related to radiological and endocrine outcomes. J Neurosurg 2011;114:303-9. [Crossref] [PubMed]
- Castinetti F, Régis J, Dufour H, et al. Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat Rev Endocrinol 2010;6:214-23. [Crossref] [PubMed]
- Grant RA, Whicker M, Lleva R, et al. Efficacy and safety of higher dose stereotactic radiosurgery for functional pituitary adenomas: a preliminary report. World Neurosurg 2014;82:195-201. [Crossref] [PubMed]
- Witt TC. Stereotactic radiosurgery for pituitary tumors. Neurosurg Focus 2003;14:e10. [Crossref] [PubMed]
- Sheehan JP, Jagannathan J, Pouratian N, et al. Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res 2006;34:185-205. [Crossref] [PubMed]
- Pollock BE, Link MJ, Leavitt JA, et al. Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery 2014;75:456-60; discussion 60. [Crossref] [PubMed]
- Kondziolka D, Lunsford LD, Flickinger JC, et al. Stereotactic radiosurgery for trigeminal neuralgia: a multiinstitutional study using the gamma unit. J Neurosurg 1996;84:940-5. [Crossref] [PubMed]
- Elaimy AL, Hanson PW, Lamoreaux WT, et al. Clinical outcomes of Gamma Knife radiosurgery in the treatment of patients with trigeminal neuralgia. Int J Otolaryngol 2012;2012:919186. [Crossref] [PubMed]
- Rogers CL, Shetter AG, Fiedler JA, et al. Gamma Knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys 2000;47:1013-9. [Crossref] [PubMed]
- Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for epilepsy and functional disorders. Neurosurg Clin N Am 2013;24:623-32. [Crossref] [PubMed]
- Friehs GM, Park MC, Goldman MA, et al. Stereotactic radiosurgery for functional disorders. Neurosurg Focus 2007;23:E3. [Crossref] [PubMed]
- Young RF, Jacques S, Mark R, et al. Gamma Knife thalamotomy for treatment of tremor: long-term results. J Neurosurg 2000;93 Suppl 3:128-35. [PubMed]
- Niranjan A, Kondziolka D, Baser S, et al. Functional outcomes after Gamma Knife thalamotomy for essential tremor and MS-related tremor. Neurology 2000;55:443-6. [Crossref] [PubMed]
- Ohye C, Shibazaki T, Hirato M, et al. Gamma thalamotomy for parkinsonian and other kinds of tremor. Stereotact Funct Neurosurg 1996;66 Suppl 1:333-42. [Crossref] [PubMed]
- Young RF, Shumway-Cook A, Vermeulen SS, et al. Gamma Knife radiosurgery as a lesioning technique in movement disorder surgery. J Neurosurg 1998;89:183-93. [Crossref] [PubMed]
- Heikkinen ER, Konnov B, Melnikov L, et al. Relief of epilepsy by radiosurgery of cerebral arteriovenous malformations. Stereotact Funct Neurosurg 1989;53:157-66. [Crossref] [PubMed]
- Régis J, Carron R, Park M. Is radiosurgery a neuromodulation therapy? Journal of Neuro-Oncology 2010;98:155-62. [Crossref] [PubMed]
- Barcia-Salorio JL, Barcia JA, Hernández G, et al. Radiosurgery of epilepsy. Long-term results. Acta Neurochir Suppl 1994;62:111-3. [Crossref] [PubMed]
- Régis J, Kerkerian-Legoff L, Rey M, et al. First biochemical evidence of differential functional effects following Gamma Knife surgery. Stereotact Funct Neurosurg 1996;66 Suppl 1:29-38. [Crossref] [PubMed]
- Mori Y, Kondziolka D, Balzer J, et al. Effects of stereotactic radiosurgery on an animal model of hippocampal epilepsy. Neurosurgery 2000;46:157-65; discussion 65-8. [Crossref] [PubMed]
- Régis J, Peragui JC, Rey M, et al. First selective amygdalohippocampal radiosurgery for 'mesial temporal lobe epilepsy'. Stereotact Funct Neurosurg 1995;64 Suppl 1:193-201. [Crossref] [PubMed]
- Barbaro NM, Quigg M, Broshek DK, et al. A multicenter, prospective pilot study of Gamma Knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol 2009;65:167-75. [Crossref] [PubMed]
- Oakes K. Radiosurgery Found Not Superior to Open Surgery for Temporal Lobe Epilepsy2017 [cited 2017 April 19th]. Available online: http://www.mdedge.com/clinicalneurologynews/article/129561/epilepsy-seizures/radiosurgery-found-not-superior-open-surgery
- Carson MJ, Doose JM, Melchior B, et al. CNS immune privilege: hiding in plain sight. Immunol Rev 2006;213:48-65. [Crossref] [PubMed]
- Hong JH, Chiang CS, Campbell IL, et al. Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys 1995;33:619-26. [Crossref] [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
- Demaria S, Bhardwaj N, McBride WH, et al. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys 2005;63:655-66. [Crossref] [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [Crossref] [PubMed]
- Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012;117:227-33. [Crossref] [PubMed]
- Ahmed KA, Stallworth DG, Kim Y, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 2016;27:434-41. [Crossref] [PubMed]
- Young KH, Baird JR, Savage T, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One 2016;11:e0157164. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009;114:589-95. [Crossref] [PubMed]
- Schaue D, Ratikan JA, Iwamoto KS, et al. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306-10. [Crossref] [PubMed]
- Kang J, Demaria S, Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer 2016;4:51. [Crossref] [PubMed]
- ClinicalTrials.gov[Internet]. Trial of nivolumab with radiation or nivolumab and ipilimumab with radiation for the treatment of intracranial metastases from non-small cell lung cancer. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02696993
- ClinicalTrials.Gov[Internet]. Ipilimumab inducation in patients with melanoma brain metastases receiving stereotactic radiosurgery. Available online: https://clinicaltrials.gov/ct2/show/NCT02097732
- ClinicalTrials.gov[Internet]. SRS and nivolumab in treating patients with newly diagnosed melanoma metastases in brain or spine. Available online: https://clinicaltrials.gov/ct2/show/NCT02716948
- ClinicalTrials.gov[Internet]. Stereotactic radiosurgery with nivolumab and valproate in patients with recurrent glioblastoma. Available online: https://clinicaltrials.gov/ct2/show/NCT02648633


