Screening and prevention strategies and endoscopic management of early esophageal cancer
Epidemiology and incidence
Esophageal cancer is the 8th most common cancer worldwide, and the 6th most common cause of cancer-related death. The vast majority of cases of esophageal cancer globally occur in underdeveloped regions, and with a higher incidence in men compared to women. There is significant variability in disease incidence by world region, with the highest rates occurring in Eastern Asia (17.0 per 100,000 in men, 5.4 per 100,000 in women), Eastern Africa (11.9 per 100,000 in men, 7.8 per 100,000 in women) and Southern Africa (13.7 per 100,000 in men, 6.7 per 100,000 in women), and the lowest rates in Western Africa (0.8 per 100,000 in men, 0.4 per 100,000 in women) (1).
Esophageal cancer has two primary subtypes, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), and has a peak incidence in the 7th and 8th decades of life (2). ESCC tends to involve the proximal and middle esophagus, and is the predominant subtype worldwide comprising approximately 90% of all esophageal cancers, particularly in areas with the highest incidence of esophageal cancer, such as the “Asian Esophageal Cancer Belt” extending from North Iran to North-Central China, and into Russia (3). The incidence of ESCC has decreased in North America and Europe, largely due to concomitant decreases in tobacco and alcohol consumption (4,5). Conversely, the incidence of EAC is on the rise in Western countries, likely the result of increasing rates of obesity, gastro-esophageal reflux disease (GERD), and Barrett’s esophagus (BE) (6). EAC is now more prevalent than ESCC in the United States, and Western Europe.
Risk factors
Chronic GERD, obesity, and smoking are the main risk factors for EAC. Those with weekly GERD symptoms have 5 times the risk of EAC when compared to those without symptoms or infrequent symptoms. The risk increases to sevenfold in those with daily symptoms (7). Obesity, and particularly central adiposity, has been shown to approximately double the risk for EAC compared to those with a normal body habitus and body mass index (8,9). Men are 3 to 4 times as likely as women to develop EAC, with this gender-related difference possibly due to a greater prevalence of central adiposity in men (6). Helicobacter pylori infection is associated with decreased risk of EAC, with a meta-analysis of observational studies of both Eastern and Western populations showing a risk reduction of 41% in those infected with helicobacter pylori. It’s hypothesized that the protective effect of helicobacter pylori infection may be related to its propensity to cause gastric atrophy with resultant decrease in gastric acid secretion, which in turn may protect against GERD and development of BE (10,11).
The predominant risk factors for ESCC are tobacco and alcohol, with a synergistic effect seen in those who use both. Less common risk factors for ESCC include achalasia, a history of head and neck squamous cell carcinoma, and prior esophageal caustic injury. Achalasia is associated with a 16- to 33-fold increased risk of ESCC compared to the general population (12). The incidence of synchronous or metachronous ESCC ranges between 3% and 14% for those with prior head and neck squamous cell carcinoma (12,13). Esophageal caustic injury, most commonly due to ingested lye, significantly increases the risk of both ESCC and EAC, and has been found to be a contributing factor in up to 4% of all esophageal cancers (14).
The risk of EAC and ESCC is also impacted by a number of genetic factors. Tylosis is a rare autosomal-dominant disorder characterized by hyperkeratosis of the palms and soles, and oral leukokeratosis and is associated with an extremely high lifetime risk of ESCC (15-17). The pathogenesis of esophageal cancer in this condition is thought to be due to missense mutations in the RHBDF2 gene, which causes altered epidermal growth factor receptor (EGFR) signaling leading to cell hyperproliferation and dysregulated wound repair (15). Familial clustering has been seen in BE and EAC, and a few genome-wide studies have identified germline mutations that may be relevant to the pathogenesis of BE and EAC (18,19). These include mutations in the MSR1, ASCC1, and CTHRC1 genes, with MSR1 mutations occurring most frequently. It is unclear whether neoplastic progression can occur by virtue of these germline mutations alone or if it requires other oncogenic events (18). A study analyzing mutations from whole-exome sequencing of 149 EAC and normal tissue pairs identified significant mutations in 26 genes, 4 of which had been previously suggested to be associated with EAC in other studies (TP53, CDKN2A, SMAD4, PIK3CA), and with mutations in TP53 and CDKN2A occurring most commonly (20). With respect to ESCC, a study utilizing whole-genome and whole-exome sequencing of 158 patients with ESCC in a high-prevalence region of China identified significant mutations in six genes that have been implicated to be associated with ESCC (TP53, RB1, CDKN2A, PIK3CA, NOTCH1 and NFE2L2), and mutations in two genes that had not been previously described to be associated with ESCC (ADAM29, FAM135B). FAM135B, which was not previously linked to any malignancy, was found to be mutated in approximately 7% of cases in this study and associated with a poor prognosis in ESCC (21). Additional research is needed to clarify the role of these mutations on the pathogenesis of BE, EAC, ESCC, and how it may impact risk-stratification, prognosis, and management of these conditions.
Screening and surveillance
Various screening strategies for esophageal cancer have been studied. Screening is accomplished primarily through use of esophagogastroduodenoscopy (EGD) in at-risk patients with the goal of identifying and treating precursor lesions or early stage cancer.
In EAC, screening using EGD is focused on identifying individuals with BE. BE is a condition characterized histologically by specialized intestinal metaplasia in the tubular esophagus that develops as a response to chronic damage to esophageal squamous cells from reflux. BE can lead to EAC via progression through a metaplasia to low-grade dysplasia (LGD) to high-grade dysplasia (HGD) to carcinoma sequence (22). The annual incidence of EAC from non-dysplastic BE (NDBE) is between 0.2–0.5% (23). In comparison, patients with LGD have an annual incidence of EAC of 0.7%, and those with HGD have a risk of progression to cancer of approximately 7% per year (23). Risk factors for neoplastic progression in BE include central obesity, tobacco use, increased length of BE segment, advanced age, and lack of use of proton pump inhibitors (PPIs) (23). Although greater than 90% of patients diagnosed with EAC did not have BE previously, screening for BE is recommended in order to identify and treat esophageal dysplasia, thereby decreasing the risk of neoplastic progression (23).
The latest guidelines from the American College of Gastroenterology recommend screening for BE with EGD in men who have frequent (at least weekly) reflux symptoms and/or reflux symptoms for at least 5 years in addition to two or more risk factors for BE or EAC. These risk factors include age greater than 50, a waist circumference greater than 102 cm or a waist-to-hip ratio of greater than 0.9, Caucasian race, current or prior smoking, and a first degree relative with EAC or BE. Screening is not recommended for the general population, or in women with chronic reflux symptoms. Women with multiple risk factors for BE or EAC could be considered for screening on an individual basis. If a screening EGD does not reveal BE, a subsequent screening examination in the future is not recommended. An exception to this is in those who are found to have severe erosive esophagitis at the time of a screening EGD; they should undergo an additional endoscopy after an appropriate duration of antisecretory therapy in order to evaluate for underlying BE (23).
Screening for BE and subsequent surveillance has been found to be cost-effective in a number of studies (24-26). EGD is considered the gold standard modality for evaluation of BE. Viable screening alternatives have been evaluated, but are not widely available or used, including unsedated transnasal endoscopy (uTNE), and cytosponge. During uTNE, an ultrathin endoscope is introduced into the nasal cavity and advanced into the esophagus and stomach. It has similar sensitivity and specificity for detection of BE as EGD, but with lower cost given the absence of sedation (23,27). Cytosponge is a gelatin-coated sponge attached to a string that expands within the esophageal lumen after being swallowed and collects esophageal cytology specimens as it is removed. The sponge has a reported sensitivity and specificity of 73% and 94% respectively for detection of BE, and is cost-effective compared to EGD or no screening, but may be limited by poor patient participation (28,29).
Observational studies suggest that patients with BE who developed EAC while participating in an endoscopic surveillance program had earlier stage disease and improved survival compared to those who did not undergo endoscopic surveillance (22). Large population-based studies from the Netherlands and Northern Ireland showed improved survival in those diagnosed with EAC who had received endoscopic surveillance compared to those who did not participate in surveillance. This survival advantage was maintained, albeit blunted, when corrected for lead-time and length time bias (30,31). Conversely, a large, case-control study of patients with BE from Kaiser Permanente in California did not show any difference in survival from EAC for those participating in endoscopic surveillance (32). It is well known that adherence to published guidelines regarding endoscopic surveillance of BE is suboptimal, particularly in community practice settings, and decreases with longer segments of BE (33). Additionally, sampling error may limit even the most rigorous biopsy surveillance method given the patchy distribution of dysplasia in BE and variability of visual detection of dysplasia during endoscopy. The discordant results of these studies examining endoscopic surveillance of BE is likely due to these factors. The substantial survival benefit reported in the Dutch study was only seen in those who participated in adequate surveillance, as survival in those receiving inadequate surveillance was the same as those not participating in a surveillance program (30). It is strongly recommended that patients undergo counseling regarding the risks and benefits of endoscopic surveillance prior to commencing surveillance as the existing literature is mixed with respect to its benefits and prospective trials examining this question do not exist. If patients agree to endoscopic surveillance, it is recommended that surveillance be performed with high-definition, high-resolution white light endoscopy, with 4-quadrant biopsies of the affected esophagus collected at 2-cm intervals in those without a history of dysplasia, and at 1-cm intervals for those with prior dysplasia. Surveillance EGD is recommended every 3–5 years for NDBE. If indefinite for dysplasia, aggressive antisecretory therapy should be pursued followed by repeat endoscopy with biopsies. If this is again indefinite for dysplasia, surveillance endoscopy in 1 year is recommended. In cases of LGD, the diagnosis should be confirmed by a second expert pathologist prior to consideration of endoscopic eradication therapy. If endoscopic therapy is not performed, annual endoscopy is recommended until two consecutive endoscopies are negative for dysplasia, after which surveillance intervals for NDBE can be resumed. If HGD is found, confirmation of the diagnosis by second expert pathologist should be pursued prior to implementing endoscopic eradication therapy (23).
A number of advanced imaging techniques are available as a complement to high-definition white light endoscopy in the inspection of Barrett’s mucosa. Narrow band imaging (NBI) is a method of virtual chromoendoscopy that applies optical filters to restrict the white light to blue light, thereby enhancing the mucosal and vascular pattern of BE. A randomized controlled trial of NBI vs. high-definition white light endoscopy did not find any notable differences in the detection of dysplasia or neoplasia between the two modalities, but the use of NBI enabled targeted biopsies that resulted in fewer biopsies overall (34). Other advanced imaging techniques including chromoendoscopy, which uses dyes applied to the mucosa to improve visualization, and confocal laser endomicroscopy, which provides high magnification and resolution images of the esophageal mucosa in real-time, holds promise for increasing detection of dysplasia, but is not currently recommended for widespread implementation (35).
In those undergoing diagnostic EGD for GERD symptoms, BE is found in approximately 5–15% of patients, with those who have a longer duration of reflux symptoms, are over the age of 50, and Caucasian males having a higher likelihood of BE at the time of endoscopy (36). In patients with GERD, there is moderate evidence to suggest a screening EGD be considered for individuals at increased risk for BE, including those with prolonged GERD symptoms, and Caucasian males over the age of 50 (37). Despite these well-described epidemiologic risk factors for BE, approximately 25% of patients with BE are women younger than 50, thus highlighting the challenges with accurately risk-stratifying those who should undergo screening for BE (38,39).
Universal screening recommendations for ESCC do not exist given the substantial variation in disease incidence. In regions with the highest incidence of ESCC, such as China, screening for ESCC has been implemented and studied. A number of studies have examined screening using esophageal cytological techniques, but have been limited by poor sensitivity for the detection of both dysplasia and cancer (40). One study examined the impact of a one-time screening endoscopy compared to no screening amongst the general population in a region of China with a particularly high incidence of ESCC. Patients in the intervention group underwent a screening EGD with Lugol’s iodine staining, with subsequent endoscopic eradication therapy at a later date if dysplasia or early carcinoma was identified. Submucosal carcinoma or advanced disease was managed with standard therapies including esophagectomy and radiation. This study showed a reduced cumulative incidence (4.17% vs. 5.92%) and decreased mortality (3.35% vs. 5.05%) from ESCC in the group that underwent screening endoscopy (41). Another study estimated the cost-effectiveness of endoscopic screening for ESCC in high-risk regions of China and found a few different cost-effective strategies including one-time endoscopic screening beginning at age 50, as well as another strategy of three screening endoscopies at 10-year intervals beginning at age 40 (42).
Achalasia, tylosis, esophageal caustic injury, and a history of head and neck squamous cell carcinoma are less common risk factors for esophageal cancer, but warrant consideration for screening or surveillance. With respect to achalasia, endoscopy is recommended at the time of diagnosis, mainly to exclude the presence of esophageal cancer producing the symptoms of achalasia, a process that is termed pseudoachalasia. Despite the markedly increased risk of ESCC in achalasia, studies evaluating surveillance endoscopy in these patients have not demonstrated improved survival, and it is not currently recommended by the American gastroenterological societies. In those with tylosis, endoscopic screening is recommended beginning at age 30 or at the time of diagnosis, whichever is earlier, and repeated every 1 to 3 years (12). For those with a history of head and neck squamous cell carcinoma, studies to date have not demonstrated endoscopic screening in this population to be cost-effective or offer a mortality benefit (12,13). Caustic injury to the esophagus is associated with an increased risk of esophageal cancer compared to the general population, with fairly equal occurrences of EAC and ESCC. Current recommendations include screening endoscopy beginning 10 to 20 years after the ingestion, with surveillance examinations occurring every 2 to 3 years afterwards (12).
Advanced imaging techniques have also been examined in screening for squamous cell dysplasia and ESCC. NBI has shown promise for improving the detection of squamous cell dysplasia when compared to conventional white light endoscopy (43,44). However, it may be associated with a high false positive rate when used without endoscopic magnification, and additional studies are needed to clarify its appropriate use in screening. Lugol’s iodine staining is a method of chromoendoscopy by which an iodine solution is applied to the esophagus during EGD. The iodine stain adheres to normal esophageal mucosa causing it to appear brown, whereas neoplastic mucosa is unstained and appears light in color or pink. Detection of this color change as a sign of high-grade intraepithelial squamous neoplasia or ESCC has been reported to have a sensitivity and specificity of approximately 90% and 95%, respectively (45,46).
Prevention
Prevention of esophageal cancer is of paramount interest given the limitations of screening for ESCC and the small percentage of patients who develop EAC in the setting of BE. In addition to lifestyle interventions such as smoking cessation and alcohol abstinence, chemopreventive strategies involving PPIs, non-steroidal anti-inflammatory drugs (NSAIDs), and statins have been examined. In addition to its role in controlling GERD symptoms, a number of studies have suggested that PPIs decrease the risk of neoplasia in BE compared to those who use histamine receptor antagonists or who do not use any antisecretory therapy (47,48). As a result, it is currently recommended that patients with BE adhere to daily PPI therapy (23).
The cyclooxygenase-2 enzyme (COX-2) is an important mediator of inflammation and has been thought to contribute to the growth of malignant cells by multiple pathways including inhibition of apoptosis and stimulation of angiogenesis. As a result, it has been identified as an important potential target for chemoprevention of a number of cancers. With respect to EAC, COX-2 expression has been noted to be elevated in BE and expression levels have been observed to increase with neoplastic progression to EAC. Thus, COX-2 inhibition via use of aspirin and NSAIDs has been theorized to be beneficial in preventing the onset of BE or in decreasing the progression from BE to EAC. Aspirin and NSAID use has been examined in the chemoprevention of ESCC as well, but the role of COX-2 expression in the pathogenesis of ESCC is less clear (49,50). Data regarding the benefits of aspirin and NSAIDs in the chemoprevention of esophageal cancer is mixed. Results from observational studies and meta-analyses have suggested a protective effect of any use of aspirin or NSAIDs against development of EAC and ESCC (49-51). On the other hand, a randomized controlled trial of daily celecoxib vs. placebo did not show a reduction in neoplastic progression or cancer incidence among patients with BE and low or HGD (52). A similar randomized, placebo-controlled trial evaluated daily celecoxib and/or selenomethionine for prevention of ESCC in high-risk populations in China, and found neither to be effective in reducing neoplastic progression (53). Additionally, the significant potential adverse effects associated with NSAIDs and aspirin, including gastrointestinal bleeding, need to be weighed carefully against any potential chemoprotective benefit. This is particularly true in BE, where the low likelihood of EAC in NDBE likely makes chronic NSAID use for chemoprevention too risky, and in LGD or HGD, endoscopic eradication therapy offers a far more effective solution for management of dysplasia than chemoprevention. There is a large, multicenter randomized controlled trial (AspECT) examining the use of aspirin and esomeprazole for chemoprevention in BE that has reached its target recruitment of 2,500 patients and is expected to publish its results in 2018. This study should provide more definitive evidence regarding the preventive benefits of both PPIs and aspirin in BE (54).
Statins competitively inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the rate-limiting step in cholesterol biosynthesis. However, in addition to its benefits in cardiovascular disease via cholesterol reduction, animal and in vitro studies have suggested the impact of statins in other pathways including inducing anti-proliferative, pro-apoptotic, and anti-angiogenic effects. An in vitro study of Barrett’s EAC cell lines showed statins to inhibit proliferation and cause apoptosis in these cells by inhibiting activation of the extracellular signal-regulated kinase and protein kinase B pathways, and inhibiting Ras farnesylation (55). A meta-analysis of 13 studies examining the role of statins in the chemoprevention of EAC reported a 28% risk reduction in EAC amongst all patients taking statins compared to nonusers of statins. However, the results of this study were limited by significant inconsistency amongst the studies included in the analysis with respect to statin dose, and duration of use (56). Additional studies are needed to determine the benefit of statins in the prevention of esophageal cancer.
Endoscopic management of early disease
Patients found to have BE with dysplasia and esophageal squamous dysplasia are amenable to endoscopic eradication therapy. In patients with BE, nonnodular mucosa is generally managed with ablative therapy, which includes laser therapy, photodynamic therapy (PDT), radiofrequency ablation (RFA), argon plasma coagulation (APC), and cryoablation. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are utilized for therapy and staging of nodular mucosal irregularities. The National Comprehensive Cancer Network (NCCN) recommends a staging endoscopic ultrasound (EUS) to exclude lymph node metastasis or lymphovascular invasion prior to endoscopic resection of superficial carcinoma (57).
Laser therapy
Laser therapy involves application of a continuous-wave 940-nm diode laser or 1,064-nm neodymium yttrium aluminum garnet (Nd:YAG) laser to the esophageal mucosa for tissue destruction. It has been used for ablation of BE, with a complete ablation rate of approximately 65%, but has a limited treatment area, thus necessitating multiple sessions for ablation of larger regions of BE, as well as increased rates of complications, such as perforation. Laser therapy has largely fallen out of favor with the emergence of the other ablative techniques (58).
PDT
PDT involves multiple steps, the first of which is administration of a light-sensitizing agent that accumulates within the abnormal mucosa. Then, a light-diffusing fiber is placed in the esophagus and monochromatic laser light is applied, which subsequently induces free oxygen radical formation, tissue ischemia, and tissue destruction. PDT has been shown to be effective in eliminating BE with HGD and early EAC. However, it is limited by a high rate of stricture formation, skin photosensitivity, and inability to eradicate NDBE, and has given way to safer ablative techniques like RFA (12).
RFA
RFA utilizes a bipolar electrode to apply 465 kHz of energy directly to the esophageal mucosa, resulting in tissue destruction (Figure 1). In patients with BE, RFA has been demonstrated to be effective in eradicating both dysplasia and intestinal metaplasia and reducing the risk of progression to adenocarcinoma (59). As a result, ablation is recommended over esophagectomy or intense endoscopic surveillance for patients with HGD. In patients with nonnodular LGD, recent evidence from a randomized trial showed a 25% risk reduction in progression to HGD or EAC with ablation compared to endoscopic surveillance, as well as efficacy in eradicating dysplasia and intestinal metaplasia (60). In these patients, annual endoscopic surveillance is an acceptable alternative to endoscopic eradication (23). RFA is not recommended for patients with NDBE given the very low rate of progression to EAC in this population and the risk of potential complications of ablation including esophageal stricture and perforation. Patients with squamous cell dysplasia and superficial ESCC have been treated with RFA as well, but there is limited data regarding outcomes in this setting. A single center study of 29 patients with early esophagus squamous cell neoplasia treated with RFA showed 97% with complete eradication of disease at 12 months (61). However, a subsequent prospective cohort of patients with early esophagus squamous cell neoplasia in the United Kingdom treated with RFA revealed only 50% of patients with complete eradication of disease at 12 months (62). Additional studies are needed to determine the appropriate use of RFA in squamous dysplasia and ESCC.
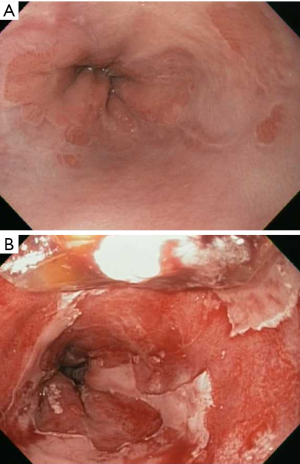
APC
APC uses a probe passed through the endoscope that delivers a constant flow of ionized argon gas that transmits high frequency current to the tissue causing superficial cautery and tissue destruction. When used in BE, APC has an eradication rate of 66% to 100% with relapse rates of 3% to 11% per year. Buried intestinal metaplasia has been reported in 20% to 30% of cases in the neosquamous epithelium following APC ablation. APC is limited by its narrow and non-uniform field of treatment when compared to RFA, and has been associated with various complications including perforation, esophageal stricture, and pleural effusions (63,64).
Cryoablation
Cryoablation involves application of liquid nitrogen to the abnormal esophageal mucosa that results in intense rapid cooling that ultimately causes tissue destruction via ischemia and apoptosis. There have been a few small studies that have looked at cryoablation for dysplastic BE, with complete eradication of dysplasia reported in 53%. Further research is needed before cryoablation can be considered for widespread use in esophageal dysplasia (63).
EMR and ESD
EMR is indicated for resection of short segments of nodular dysplasia, and superficial EAC and ESCC. It is performed by attaching a cap device to the tip of the endoscope, suctioning the desired tissue into the cap, and placing a snare around the base of the tissue by which the tissue can then be resected. Alternatively, the cap can be used to place rubber bands around the base of the desired tissue, thus creating a pseudo-polyp that can then be resected with a snare (Figure 2). ESD is a technique developed in Japan in the late 1990’s in order to allow for en bloc resection of superficial lesions. The technique begins by marking the perimeter of the lesion with cautery, then creating a circumferential mucosal incision around the lesion. The mucosa is then freed by carefully dissecting through the submucosa via endoscopic cautery. ESD has similar indications to EMR, but offers the advantage of deeper resection, thus leading to a higher probability of en bloc resection and curative removal of a lesion. A meta-analysis of 15 nonrandomized studies comparing ESD to EMR for superficial tumors of the gastrointestinal tract showed much higher en bloc and curative resection rates (odds ratio, 13.87 and 3.53, respectively) with ESD, regardless of lesion size, as well as decreased local recurrence rate (odds ratio, 0.09) (65). However, compared to EMR, ESD is associated with longer procedure times, and increased complications, most commonly in the form of bleeding (65). Studies have shown endoscopic resection to be effective in eradicating HGD or T1a EAC in 91% to 98% of cases (66). Retrospective data suggests cure and survival rates of endoscopic resection of T1a disease to be comparable to outcomes following surgery, but with substantially decreased procedure-related morbidity and mortality (67-69). Subsequent endoscopic resection or ablation of residual BE significantly reduces the risk of metachronous HGD or EAC (66). Oyama et al. performed ESD in 102 patients with superficial ESCC ranging in size from 4 to 64 mm, achieving en bloc resection in 95% and a recurrence rate of 0% at a mean 21 months follow-up. They did not report any bleeding complications or perforations, but had a few cases of mediastinal emphysema which were treated successfully with brief courses of antibiotics (70).
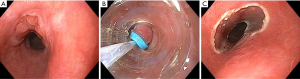
T1b EAC can be associated with a risk of lymph node metastasis as high as 15% to 25%. Some studies have suggested that the risk of lymph node metastasis varies significantly by depth of submucosal invasion, with minimal risk of lymphovascular invasion for disease limited to the upper third of the submucosa (sm1). Invasion beyond this is predictive of lymph node metastasis and thus may not be appropriate for curative endoscopic resection (71). This remains a point of controversy, however, as a subsequent study of esophagectomy patients did not show correlation between depth of submucosal invasion and likelihood of lymphovascular invasion (72). A study of 21 patients with BE who underwent endoscopic resection of “low-risk” submucosal esophageal cancer, defined as sm1 invasion and absence of lymphovascular invasion as determined by endosonography, showed complete remission in 95% of patients at 5 months, and a 5-year survival rate of 66% (73).
Summary
Esophageal cancer remains a prominent cause of cancer-related mortality worldwide. The prevalence of ESCC remains high in certain regions of the world, whereas in Western countries, the declining incidence of ESCC is countered by the rapidly rising incidence of EAC. Endoscopy plays a pivotal role in cancer screening, surveillance, and therapy of early stage disease. Advancements in endoscopic practice such as routine use of high definition white light endoscopy, RFA, EMR, and ESD have allowed for improved esophageal cancer screening, as well as safer and more effective treatments for dysplasia and superficial cancer. Our practice follows screening and surveillance guidelines put out by the American gastroenterological societies for BE and other conditions that predispose to the development of esophageal cancer, as well as the NCCN guidelines regarding use of staging EUS followed by endoscopic resection and ablation as the preferred treatment for patients with superficial esophageal cancer (74). Additional studies are needed to determine the capacity of advanced imaging techniques to improve detection of esophageal dysplasia, further applications of EMR and ESD, and cost-effective screening modalities for ESCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v50-7. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer 2009;101:855-9. [Crossref] [PubMed]
- Castro C, Bosetti C, Malvezzi M, et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980-2011) and predictions to 2015. Ann Oncol 2014;25:283-90. [Crossref] [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222-7. [Crossref] [PubMed]
- Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:872-8. [Crossref] [PubMed]
- Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1399-412.e7. [Crossref] [PubMed]
- Rokkas T, Pistiolas D, Sechopoulos P, et al. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol 2007;5:1413-7, 1417.e1-2.
- Rubenstein JH, Inadomi JM, Scheiman J, et al. Association between Helicobacter pylori and Barrett's esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol 2014;12:239-45. [Crossref] [PubMed]
- Committee ASoP, Evans JA, Early DS, et al. The role of endoscopy in Barrett's esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc 2012;76:1087-94. [Crossref] [PubMed]
- Petit T, Georges C, Jung GM, et al. Systematic esophageal endoscopy screening in patients previously treated for head and neck squamous-cell carcinoma. Ann Oncol 2001;12:643-6. [Crossref] [PubMed]
- Kochhar R, Sethy PK, Kochhar S, et al. Corrosive induced carcinoma of esophagus: report of three patients and review of literature. J Gastroenterol Hepatol 2006;21:777-80. [Crossref] [PubMed]
- Blaydon DC, Etheridge SL, Risk JM, et al. RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am J Hum Genet 2012;90:340-6. [Crossref] [PubMed]
- Ellis A, Field JK, Field EA, et al. Tylosis associated with carcinoma of the oesophagus and oral leukoplakia in a large Liverpool family--a review of six generations. Eur J Cancer B Oral Oncol 1994;30B:102-12. [Crossref] [PubMed]
- Risk JM, Evans KE, Jones J, et al. Characterization of a 500 kb region on 17q25 and the exclusion of candidate genes as the familial Tylosis Oesophageal Cancer (TOC) locus. Oncogene 2002;21:6395-402. [Crossref] [PubMed]
- Orloff M, Peterson C, He X, et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011;306:410-9. [Crossref] [PubMed]
- Robertson EV, Jankowski JA. Genetics of gastroesophageal cancer: paradigms, paradoxes, and prognostic utility. Am J Gastroenterol 2008;103:443-9. [Crossref] [PubMed]
- Dulak AM, Stojanov P, Peng S, et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013;45:478-86. [Crossref] [PubMed]
- Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 2014;509:91-5. [Crossref] [PubMed]
- Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med 2014;371:836-45. [Crossref] [PubMed]
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett's Esophagus. Am J Gastroenterol 2016;111:30-50. [Crossref] [PubMed]
- Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2004;2:868-79. [Crossref] [PubMed]
- Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003;138:176-86. [Crossref] [PubMed]
- Gerson LB. Cost-Analyses Studies in Barrett's Esophagus: What Is Their Utility? Gastroenterol Clin North Am 2015;44:425-38. [Crossref] [PubMed]
- Saeian K, Staff DM, Vasilopoulos S, et al. Unsedated transnasal endoscopy accurately detects Barrett's metaplasia and dysplasia. Gastrointest Endosc 2002;56:472-8. [Crossref] [PubMed]
- Kadri SR, Lao-Sirieix P, O'Donovan M, et al. Acceptability and accuracy of a non-endoscopic screening test for Barrett's oesophagus in primary care: cohort study. BMJ 2010;341:c4372. [Crossref] [PubMed]
- Benaglia T, Sharples LD, Fitzgerald RC, et al. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett's esophagus. Gastroenterology 2013;144:62-73.e6. [Crossref] [PubMed]
- Verbeek RE, Leenders M, Ten Kate FJ, et al. Surveillance of Barrett's esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. Am J Gastroenterol 2014;109:1215-22. [Crossref] [PubMed]
- Bhat SK, McManus DT, Coleman HG, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett's oesophagus: a population-based study. Gut 2015;64:20-5. [Crossref] [PubMed]
- Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology 2013;145:312-9.e1. [Crossref] [PubMed]
- Abrams JA, Kapel RC, Lindberg GM, et al. Adherence to biopsy guidelines for Barrett's esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol 2009;7:736-42. [Crossref] [PubMed]
- Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett's oesophagus: a prospective, international, randomised controlled trial. Gut 2013;62:15-21. [Crossref] [PubMed]
- Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett's esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562-70.e1-2.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett's esophagus in high-risk patients with chronic GERD. Gastrointest Endosc 2005;61:226-31. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28. [Crossref] [PubMed]
- Falk GW, Thota PN, Richter JE, et al. Barrett's esophagus in women: demographic features and progression to high-grade dysplasia and cancer. Clin Gastroenterol Hepatol 2005;3:1089-94. [Crossref] [PubMed]
- Guardino JM, Khandwala F, Lopez R, et al. Barrett's esophagus at a tertiary care center: association of age on incidence and prevalence of dysplasia and adenocarcinoma. Am J Gastroenterol 2006;101:2187-93. [Crossref] [PubMed]
- Dawsey SM, Shen Q, Nieberg RK, et al. Studies of esophageal balloon cytology in Linxian, China. Cancer Epidemiol Biomarkers Prev 1997;6:121-30. [PubMed]
- Wei WQ, Chen ZF, He YT, et al. Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China. J Clin Oncol 2015;33:1951-7. [Crossref] [PubMed]
- Yang J, Wei WQ, Niu J, et al. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol 2012;18:2493-501. [Crossref] [PubMed]
- Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol 2010;28:1566-72. [Crossref] [PubMed]
- Takenaka R, Kawahara Y, Okada H, et al. Narrow-band imaging provides reliable screening for esophageal malignancy in patients with head and neck cancers. Am J Gastroenterol 2009;104:2942-8. [Crossref] [PubMed]
- Shimizu Y, Omori T, Yokoyama A, et al. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: high-grade intra-epithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol 2008;23:546-50. [Crossref] [PubMed]
- Ishihara R, Yamada T, Iishi H, et al. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest Endosc 2009;69:213-8. [Crossref] [PubMed]
- Singh S, Garg SK, Singh PP, et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut 2014;63:1229-37. [Crossref] [PubMed]
- Kastelein F, Spaander MC, Steyerberg EW, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2013;11:382-8. [Crossref] [PubMed]
- Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 2012;142:442-52.e5; quiz e22-3.
- Corley DA, Kerlikowske K, Verma R, et al. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003;124:47-56. [Crossref] [PubMed]
- Chan AT, Detering E. An emerging role for anti-inflammatory agents for chemoprevention. Recent Results Cancer Res 2013;191:1-5. [Crossref] [PubMed]
- Heath EI, Canto MI, Piantadosi S, et al. Secondary chemoprevention of Barrett's esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst 2007;99:545-57. [Crossref] [PubMed]
- Limburg PJ, Wei W, Ahnen DJ, et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology 2005;129:863-73. [Crossref] [PubMed]
- Das D, Chilton AP, Jankowski JA. Chemoprevention of oesophageal cancer and the AspECT trial. Recent Results Cancer Res 2009;181:161-9. [Crossref] [PubMed]
- Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett's esophageal adenocarcinoma cells. Am J Gastroenterol 2008;103:825-37. [Crossref] [PubMed]
- Singh S, Singh AG, Singh PP, et al. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett's esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:620-9. [Crossref] [PubMed]
- Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw 2011;9:830-87. [Crossref] [PubMed]
- Nealis TB, Washington K, Keswani RN. Endoscopic therapy of esophageal premalignancy and early malignancy. J Natl Compr Canc Netw 2011;9:890-9. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Bergman JJ, Zhang YM, He S, et al. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc 2011;74:1181-90. [Crossref] [PubMed]
- Haidry RJ, Butt MA, Dunn J, et al. Radiofrequency ablation for early oesophageal squamous neoplasia: outcomes form United Kingdom registry. World J Gastroenterol 2013;19:6011-9. [Crossref] [PubMed]
- Leggett CL, Gorospe EC, Wang KK. Endoscopic therapy for Barrett's esophagus and early esophageal adenocarcinoma. Gastroenterol Clin North Am 2013;42:175-85. [Crossref] [PubMed]
- Ragunath K, Krasner N, Raman VS, et al. Endoscopic ablation of dysplastic Barrett's oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol 2005;40:750-8. [Crossref] [PubMed]
- Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Bennett C, Green S, Decaestecker J, et al. Surgery versus radical endotherapies for early cancer and high-grade dysplasia in Barrett's oesophagus. Cochrane Database Syst Rev 2012;11:CD007334. [PubMed]
- Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424-9.e2; quiz e81.
- Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg 2011;254:67-72. [Crossref] [PubMed]
- Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [Crossref] [PubMed]
- Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004;60:703-10. [Crossref] [PubMed]
- Badreddine RJ, Prasad GA, Lewis JT, et al. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol 2010;8:248-53. [Crossref] [PubMed]
- Manner H, May A, Pech O, et al. Early Barrett's carcinoma with "low-risk" submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol 2008;103:2589-97. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]


