Chemotherapy induced oral mucositis: prevention is possible
Introduction
Oral mucositis (OM) is the inflammation of the oral mucosa induced by oncological treatments. Radiotherapy (RT) seem to produce it in 80% of the patients, chemotherapy (CM) in 20–80% depending on the regimen and >75% of patients undergoing bone marrow transplantation will suffer from this side-effect (1-5). This complication usually starts 5–10 days after the treatment and lasts for 7–14 days.
It is well known that chemotherapeutic drugs target rapidly multiplying healthy cells such as the oral mucosa. This damages the mucosal lining of the mouth leading to atrophy and ulcers.
Recent studies have suggested that submucosal areas are damaged first (1,6). Inflammatory cytokines and reactive oxygen species are released in the mucosa. These will activate transcription factors (nuclear factor kappa B) and over-regulate genes (tumor necrosis factor, IL-6, and IL-1) which will activate apoptosis (4,7,8).
It has also been described a loss of epithelial growth factors (keratinocyte growth factor), leading to apoptosis of fibroblasts and vascular endothelial cells which results in submucosal injury. All these changes will disturb the normal epithelial growth resulting in ulcers (4,6).
Other authors have pointed that bacterial colonization may extend the healing period (4), but this is not a key factor in the OM (8).
OM is dependent on the kind of tumor (3-5), patient age (more frequent in young), grade of oral hygiene and health (5), nutritional status, renal and hepatic functions, CM agent (antimetabolites such as 5-fluorouracil (5-FU) or purine analogs as cytarabine) or combination CM, concurrent administration with RT in head and neck tumors etc. (4-8). It is well known that drugs such as methotrexate and etoposide are secreted in saliva, which increases the chances of OM.
OM appears as erythema, swelling or ulceration and it is described as sensations of mild burning to painful ulcers. These deteriorate patients’ quality of life and could affect the speech, swallowing of saliva or eating (6). In cases receiving conventional CM, this occurs in 20–40%. OM appears sooner after CM than after RT, and more often affects the non-keratinized mucosa (4).
This mucosal damage gradually recovers without any scars over a period of 2–3 weeks after the CM cycle (6). During the period of active OM, there is a high risk of infections, mainly caused by herpes simplex virus or Candida (generally albicans). This is more frequent in patients with prolonged neutropenia (4).
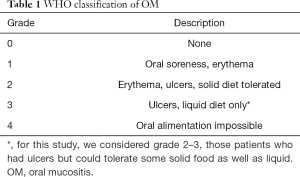
Although there are some tools to quantify the damage in the oral mucosa, the most common system is the World Health Organization (WHO) (9) which combines clinical features with the patient’s capacity to eat.
This scale grades the OM as shown in Table 1 and depending on the grade, a decision about CM dosing for next cycles is made. It also helps decide the best supportive treatment.
Full table
The management of OM is a challenge and many strategies are used to minimize the damage caused by antineoplastics (1,3,5), including CM dose reduction (DR) which could impact on final results (10). It has been described that the soreness in mouth and throat of OM intensifies with successive cycles, with amplification of as much as 44% (11).
Although there are several treatments to alleviate the pain and improve patient’s nutrition, the ideal aim would be prevention. So far, no definite measure has shown to be able to prevent it effectively and recurrent episodes of OM, will double the likelihood of DR and unplanned treatment breaks (1,2,12).
We decided to put in practice a combination mouthwash to prevent OM after the first episode had occurred. Several combination rinses have been put in practice but none of them has shown clear benefits. We checked with our pharmacists the safety and stability of this combination and after a positive reply, we designed a prospective study to examine whether the mixture of soluble prednisolone, nystatin and salt water applied before the expected OM appears, would reduce the incidence of OM grade 2–3 after a first episode had appeared without CM DR.
CM DR occurs if OM is grade 3 or above and if OM is grade 2 at the time of the following cycle which forces to delay the cycle (10).
Methods
Eligible patients
We included breast cancer patients undergoing neoadjuvant or adjuvant treatment with FEC (5 fluorouracil, epirubicin, cyclophosphamide) or docetaxel who developed OM grade 2 or 2–3 with the previous cycle treated in our institution. All these patients had good dental health and all were educated to continue oral hygiene. All of them had uses benzidamine rinses to treat the previous episode of OM.
Special mouthwash
The special mouthwash consisted of a combination of 100 mL of water, 5 mg of soluble prednisolone, 2 drops of nystatin and 2.300 mg of salt (1 teaspoon).
The recommendation was to use it three times daily, starting 3 days before the expected episode of OM appearance (depending on chronology of first episode of OM after first cycle of chemo). At the time of using it, it needs to be kept in the mouth for more than 30 seconds to ensure enough time of contact with mucosa. Patients carried on using it for 3 days after the expected duration of OM based again on previous cycle.
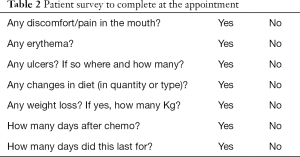
Patient’s interview as part of the routine clinical assessment
Before each cycle the patient is assessed by the medical oncologist and to complete the survey included on Table 2.
Full table
Efficacy end-points
The primary end-point was the incidence of OM grade 2–3 with the following cycle of CM. Secondary end-points included the rate of CM DR and the incidence of OM grade 0, 1 and 2 with the following treatment after having used this special mouthwash.
We hypothesized that less than 50% of patients would develop OM grade 2–3 or higher with the especial mouthwash compared with a historical rate of 80%. Using 80% power and a two-sided significance level of 0.05 it was determined that 44 valuable patients would be needed. The comparison was performed using an exact test for the binomial distribution.
Results
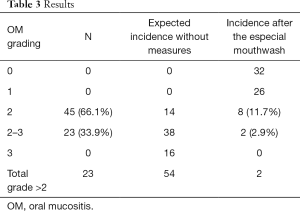
We included 68 patients (29 FEC and 39 docetaxel), all women. Twenty-three cases developed OM grade 2–3 after the previous cycle (erythema, ulcers, mainly liquid diet but tolerated some solids too), recovered to grade 0 when attending for pre-CM before next cycle. Forty-five patients developed OM grade 2. Those patients started to use the mouthwash mixture with the following cycle.
After this, only 2 cases developed grade 2–3, 8 cases grade 2, 26 cases grade 1 and 32 grade 0. Only four cases (5.8%) needed a CM DR based on OM grading (two who continued to present OM grade 2–3 after second cycle and two who developed grade 2 but long-lasting and persistent grade 1 at the time of next pre-CM). These results could be seen on the Table 3.
Full table
A binomial test indicated that the probability of grade 2–3 OM after using this mouthwash as instructed, was lower than the expected P=0.000087 (1-sided).
And the probability of grade 2 OM was P=0.000015 (1-sided).
Discussion
The management of OM continues to be a challenging matter in Oncology. Many strategies are used to minimize the adverse effects of cancer treatment, including CM DR and the prescription of other therapeutic and preventive agents (4,8). Unfortunately for OM, no definite measure has shown to be able to prevent it effectively and recurrent episodes of OM will double the likelihood of DR and unplanned treatment breaks which might negatively impact on survival (1,2).
Oral hygiene has been recommended by many, however, its role in prevention is controversial. It seems to have some benefits but mainly by reducing the risk of infections (1,3,7). In any case, all patients included on this study had good oral hygiene as this was one of the inclusion criteria and developed OM.
Agents with anti-inflammatory properties have been used such as benzidamine. This drug has also got analgesic, anesthetic and antimicrobial properties. It is used as prevention or treatment but with conflicting results (4,13,14). Our patients had used this drug once the previous episode had happened to help with recovery.
With the standard measures to treat OM, we could only expect that those patients developing OM grade 2 or 2–3 after the first cycle, would develop either same grade or more likely higher with subsequent cycles and eventually the dose of CM should be reduced.
In fact, many patients with OM grade 2–3 would have had the CM reduced for second cycle depending on other associated factors (active smoking, poor dental hygiene, poor oral health, weight loss associated to this and the grade and speed of recovery, etc).
Our study showed a very low CM DR rate (5.8%) after this mouthwash was used which is relevant as these patients were receiving neoadjuvant or adjuvant chemotherapies, no palliative treatments.
We used steroids as part of the combination. Other studies using other anti-inflammatory drugs or even steroids have shown conflicting results.
Misoprostol (15) has shown a higher incidence and severity of OM versus placebo.
Topical application of prostaglandins E2 showed conflicting results (4,13,16).
There is a “magic mouthwash” with diphenhydramine, viscous lidocaine, bismuth subsalicylate and steroids. Its aim is to control the pain and reduce the inflammation. However, it has not shown a great pain reduction (7,17).
Antiseptics have also been used such as saline or chlorhexidine. The latter is another product with contradictory results (18) and it has been reported that rinses with saline solution or bicarbonate may be equally effective (7,8). Potting et al. (19) did not find any benefits with chlorhexidine in comparison with mouthwashes with sterile water or saline solution.
Povidone iodine is another antiseptic used to reduce the severity of OM. When compared with sterile water, it has shown to reduce it by 30% (19).
Our mixture included saline which may help increase the oral hygiene and reduce inflammation in the oral mouth which seems to be the most relevant mechanism to produce OM. In fact, it has been described that the high osmolality of saline reduces inflammation and can be microbicidal (20). Despite the general thought that antimicrobials would help, Rubenstein et al. (8) published a review where they indicated that antimicrobial agents for the prevention of OM are not recommended. They concluded that the benefit could only be expected in patients with late stage ulcerative mucositis, when the risk of bacterial infection is higher. However, as mentioned before in this article, saline is considered as microbicidal due to its high osmolality and our study resulted in positive outcome.
The role of other agents in OM is again conflicting. Cytoprotective agents such as amifostine which suppresses reactive oxygen species, have not found any benefit on duration or severity of OM (4,7). Sucralfate has shown conflicting results (1), although Ala et al. (21) have reported positive results with sucralfate mouthwash compared to placebo. Sucralfate showed a reduction in incidence and severity of 5-FU induced OM.
Rubenstein et al. (8) evaluated iv glutamine in patients treated with 5FU and showed a significant reduction in OM, although others did not find good results (18) and moreover they found that OM could worsen. Peterson et al. carried out a phase III, double-blind, multicenter clinical trial (22) in breast cancer patients, assessing oral glutamine (2.5 g/5 mL) three times/daily versus placebo and found a reduction in the incidence and severity of OM but once again more studies are needed.
Nystatin rinse has not shown to be effective in reducing the severity of OM (23). We used it as part of the combination mouthwash due to the higher risk of oral candidiasis in our patients (CM, oral steroids for a few days after CM, the steroids in contact with the oral mucosa as part of the mouthwash). We cannot know how much this product helped but none of the patients included, developed oral thrush.
Our especial mouthwash was easy to elaborate and clearly helped to our patients as it has been reported here.
The significant reduction in OM grade 2–3 with the following cycle of CM would have only been expected with an active product. We could also see a significant reduction in other grades of OM and only 4 patients needed a DR based on OM grading.
Other trials have been trying other different approaches. Cryotherapy or the application of ice to the oral mucosa which produces local vasoconstriction, has prevented OM in some patients (4). This cannot be used with oxaliplatin, as cold related dysesthesia could be triggered (maxillar stiffness and laryngopharyngeal spasm) (7).
However, 5FU seems to benefit from cryotherapy. Several studies have shown that the rate of OM decreases after its use (24-28). This treatment modality has shown inconclusive findings with cisplatin, etoposide, mitomycin or vinca alkaloids (28,29).
Rinses with granulocyte colony-stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) have been described as significantly beneficial in reducing the duration and severity of OM (29-32). However, Cartee et al. (33) did not find any benefit.
Others have shown that the subcutaneous (sc) use of these drugs are also beneficial to reduce the rate of OM (29). However, the randomized, controlled trial by Patte et al. (34) did not find a benefit in prevention.
Palifermin is a truncated human recombinant keratinocyte growth factor (KGF) produced in Escherichia coli. It causes proliferation of the oral mucosa and protects against mucosal damage. It is an option for patients at high risk of OM, especially in patients with hematological malignancies receiving stem cell transplantation (4,13).
Palifermin is given intravenously at a dose of 60 µg/kg/day for three consecutive days before and after myelosuppressive treatment, for 6 doses. The third dose is administered 24–48 hours before bone marrow suppression (4,13,35). It has been described to reduce the incidence and severity of OM (35-38).
Caphosol is an electrolyte solution, designed to replace the normal pH in the oral cavity and it has been considered useful in prevention and treatment of OM in cancer patients (39,40).
When the calcium and phosphate solution are mixed, those form a stable supersaturated solution resembling natural saliva. It is thought that its high ionic content mediates the inflammatory process, coagulation cascade and helps repair the tissue (41).
Waśko-Grabowska et al. (42) found the administration of Caphosol® rinses to reduce the incidence, severity and duration of OM in patients treated with BEAM regimens (carmustine, cytarabine, etoposide and melphalan), in contrast to the group treated with melphalan 200.
Arbabi-kalati et al. (43) administered 220 mg of zinc sulfate daily in capsule form to patients receiving CM, and observed a decrease in the intensity of mucositis. However, the incidence in the control group was similar.
Honey has also been assessed in the management of OM as it has got antibacterial and regenerative benefits. It may improve the symptoms and shorten the duration of OM but further studies are needed. A trial carried out by Jayalekshmi et al. assessed the effects of applying honey on OM during radiation therapy. Patients included in the active arm received 15 mL natural honey for applying on oral mucosa. Authors found a statistically significant reduction in degree of OM (P<0.01) (44). A meta-analysis of the efficacy of honey in the management of OM during RT in patients with head and neck cancer showed that oral administration of honey after RT could prevent moderate to severe OM and associated weight loss. However, those results were based on a small number of trials (45).
Conclusions
The management of OM is still a challenge. Several strategies have been used to try prevention but especially to treat this complication. Unfortunately, the available results are heterogeneous and inconclusive (28).
Worthington et al. (13), in their review have concluded that only some interventions (cryotherapy, G-CSF, iv glutamine, honey, KGF, sucralfate among others) offer some benefit in terms of prevention. The most relevant ones are cryotherapy, palifermin and sucralfate as those showed statistically significant benefit in preventing or reducing the severity of OM.
Several anti-inflammatory agents have been used with conflicting results. Our study used a combination mouthwash with anti-inflammatories, antifungal and saline water to prevent OM or reduce the intensity of OM. We achieved the end-points and although this is only a small study, taken into account its good results, further studies would help in confirming its real role.
Acknowledgements
To our Nursing Staff in Oncology (Royal Bournemouth Hospital and Poole Hospital), our helpful Secretaries, Heath Care Assistants and the helpful colleagues at the patient’s reception desk for their valuable comments, their cooperation with patient education tasks and care and for their support to our patients.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453-61. [Crossref] [PubMed]
- Thomson M, Quinn B, Horn J, et al. Mouth care guidance and support in cancer and palliative care. 2nd edition, UKOMIC, May 2015.
- Chaveli López B, Gavaldá Esteve C, Sarrión Pérez MG. Dental treatment considerations in the chemotherapy patient. J Clin Exp Dent 2011;3:e31-42. [Crossref]
- Scully C, Sonis S, Diz PD. Oral mucositis. Oral Dis 2006;12:229-41. [Crossref] [PubMed]
- Chan CW, Chang AM, Molassiotis A, et al. Oral complications in Chinese cancer patients undergoing chemotherapy. Support Care Cancer 2003;11:48-55. [PubMed]
- Chaveli-López B. Oral toxicity produced by chemotherapy: A systematic review. J Clin Exp Dent 2014;6:e81-90. [Crossref] [PubMed]
- Harris DJ, Eilers J, Harriman A, et al. Putting evidence into practice: evidence-based interventions for the management of oral mucositis. Clin J Oncol Nurs 2008;12:141-52. [Crossref] [PubMed]
- Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004;100:2026-46. [Crossref] [PubMed]
- WHO toxiticy criteria. Available online: http://www.icssc.org/Documents/Resources/AEManual2003AppendicesFebruary_06_2003%20final.pdf
- Chaveli-López B, Bagán-Sebastián JV. Treatment of oral mucositis due to chemotherapy. J Clin Exp Dent 2016;8:e201-9. [PubMed]
- Chi KH, Chen CH, Chow KC, et al. Effect of granuclocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients after cisplatin, fluorouracil and leucovorin chemotherapy. J Clin Oncol 1995;13:2620-8. [Crossref] [PubMed]
- Elting LS, Cooksley CD, Chambers MS, et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003;98:1531-9. [Crossref] [PubMed]
- Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2011.CD000978. [PubMed]
- Lalla RV, Gordon GB, Schubert M, et al. A randomized, double-blind, placebo-controlled trial of misoprostol for oral mucositis secondary to high-dose CM. Support Care Cancer 2012;20:1797-804. [Crossref] [PubMed]
- Dueñas-Gonzalez A, Sobrevilla-Calvo P, Frias-Mendivil M, et al. Misoprostol prophylaxis for high-dose CM-induced mucositis: a rando¬mized double-blind study. Bone Marrow Transplant 1996;17:809-12. [PubMed]
- Sung L, Tomlinson GA, Greenberg ML, et al. Serial controlled N-of-1 trials of topical vitamin E as prophylaxis for CM-induced oral mucositis in paediatric patients. Eur J Cancer 2007;43:1269-75. [Crossref] [PubMed]
- Clarkson JE, Worthington HV, Furness S, et al. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2010.CD001973. [PubMed]
- Nashwan AJ. Use of chlorhexidine mouthwash in children receiving CM: a review of literature. J Pediatr Oncol Nurs 2011;28:295-9. [Crossref] [PubMed]
- Potting CM, Uitterhoeve R, Op Reimer WS, et al. The effectiveness of commonly used mouthwashes for the prevention of CM-induced oral mucositis: a systematic review. Eur J Cancer Care (Engl) 2006;15:431-9. [Crossref] [PubMed]
- Math MV, Balasubramaniam P. Oral health and water. Indian J Nutr Diet 2008;45:388-91.
- Ala S, Saeedi M, Janbabai G, et al. Efficacy of Sucralfate Mouth Wash in Prevention of 5-fluorouracil Induced Oral Mucositis: A Prospective, Randomized, Double-Blind, Controlled Trial. Nutr Cancer 2016;68:456-63. [Crossref] [PubMed]
- Peterson DE, Jones JB, Petit RG 2nd. Randomized, placebo-controlled trial of Saforis for prevention and treatment of oral mucositis in breast cancer patients receiving anthracycline-based CM. Cancer 2007;109:322-31. [Crossref] [PubMed]
- Epstein JB, Vickars L, Spinelli J, et al. Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg Oral Med Oral Pathol 1992;73:682-9. [Crossref] [PubMed]
- Nikoletti S, Hyde S, Shaw T, et al. Comparison of plain ice and flavoured ice for preventing oral mucositis associated with the use of 5 fluorouracil. J Clin Nurs 2005;14:750-3. [Crossref] [PubMed]
- Sorensen JB, Skovsgaard T, Bork E, et al. Double-blind, placebo-controlled, randomized study of chlorhexidine prophylaxis for 5-fluorouracil-based CM-induced oral mu¬cositis with nonblinded randomized comparison to oral cooling (cryotherapy) in gastrointestinal malignancies. Cancer 2008;112:1600-6. [Crossref] [PubMed]
- Papadeas E, Naxakis S, Riga M, et al. Prevention of 5-fluorouracil-related stomatitis by oral cryotherapy: A randomized controlled study. Eur J Oncol Nurs 2007;11:60-5. [Crossref] [PubMed]
- Vokurka S, Bystricka E, Scudlova J, et al. The risk factors for oral mucositis and the effect of cryotherapy in patients after the BEAM and HD-l-PAM 200 mg/m(2) autologous hematopoietic stem cell transplantation. Eur J Oncol Nurs 2011;15:508-12. [Crossref] [PubMed]
- Katrancı N, Ovayolu N, Ovayolu O, et al. Evaluation of the effect of cryotherapy in preventing oral mucositis associated with CM - a randomized controlled trial. Eur J Oncol Nurs 2012;16:339-44. [Crossref] [PubMed]
- Crawford J, Tomita DK, Mazanet R, et al. Reduction of oral mucositis by filgrastim (r-metHuG-CSF) in patients receiving CM. Cytokines Cell Mol Ther 1999;5:187-93. [PubMed]
- Karthaus M, Rosenthal C, Huebner G, et al. Effect of topical oral G-CSF on oral mucositis: a randomised placebo-controlled trial. Bone Marrow Transplant 1998;22:781-5. [Crossref] [PubMed]
- Ibrahim EM, al-Mulhim FA. Effect of granulocyte-macrophage colony-stimulating factor on CM-induced oral mucositis in non-neutropenic cancer patients. Med Oncol 1997;14:47-51. [Crossref] [PubMed]
- Hejna M, Köstler WJ, Raderer M, et al. Decrease of duration and symptoms in CM-induced oral mucositis by topical GM-CSF: results of a prospective randomised trial. Eur J Cancer 2001;37:1994-2002. [Crossref] [PubMed]
- Cartee L, Petros WP, Rosner GL, et al. Evaluation of GM-CSF mouthwash for prevention of CM-induced mucositis: a randomized, double-blind, dose-ranging study. Cytokine 1995;7:471-7. [Crossref] [PubMed]
- Patte C, Laplanche A, Bertozzi AI, et al. Granulocyte colony-stimulating factor in induction treatment of children with non-Hodgkin’s lymphoma: a randomized study of the French Society of Pediatric Oncology. J Clin Oncol 2002;20:441-8. [PubMed]
- Vadhan-Raj S, Trent J, Patel S, et al. Single-dose palifermin prevents severe oral mucositis during multicycle CM in patients with cancer: A randomized trial. Ann Intern Med 2010;153:358-67. [Crossref] [PubMed]
- Horsley P, Bauer JD, Mazkowiack R, et al. Pali¬fermin improves severe mucositis, swallowing problems, nutrition im¬pact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation. Support Care Cancer 2007;15:105-9. [Crossref] [PubMed]
- Ayago Flores D, Ferriols Lisart R. Effectiveness of palifermin in the prevention of oral mucositis in patients with haematological cancers. Farm Hosp 2010;34:163-9. [Crossref] [PubMed]
- Schmidt E, Thoennissen NH, Rudat A, et al. Use of palifermin for the prevention of high-dose methotrexate-induced oral mucositis. Ann Oncol 2008;19:1644-9. [Crossref] [PubMed]
- Papas AJE, Sobel S, Olsen TO. Effects of preventive regimen on oral mucositis. J Dent Res IADR 1981;544:940.
- Waśko-Grabowska A, Rzepecki P, Oborska S, et al. Efficiency of supersaturated calcium phosphate mouth rinse treatment in patients receiving high-dose melphalan or BEAM prior to autologous blood stem cell transplantation: a single-center experience. Transplant Proc 2011;43:3111-3. [Crossref] [PubMed]
- Papas AS, Clark RE, Martuscelli G, et al. A prospective, randomized trial for the prevention of mucositis in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2003;31:705-12. [Crossref] [PubMed]
- Waśko-Grabowska A, Rzepecki P, Oborska S, et al. Efficiency of supersaturated calcium phosphate mouth rinse treatment in patients receiving high-dose melphalan or BEAM prior to autologous blood stem cell transplantation: a single-center experience. Transplant Proc 2011;43:3111-3. [Crossref] [PubMed]
- Arbabi-kalati F, Arbabi-kalati F, Deghatipour M, et al. Evaluation of the efficacy of zinc sulfate in the prevention of CM-induced mucositis: A double-blind randomized clinical trial. Arch Iran Med 2012;15:413-7. [PubMed]
- Jayalekshmi JL, Lakshmi R, Mukerji A. Honey on oral mucositis: A Randomized controlled trial. Gulf J Oncolog 2016;1:30-7. [PubMed]
- Cho HK, Jeong YM, Lee HS, et al. Effects of honey on oral mucositis in patients with head and neck cancer: A meta-analysis. Laryngoscope 2015;125:2085-92. [Crossref] [PubMed]


