
Immunotherapy: a glimmer of hope for metastatic prostate cancer
Introduction
Metastatic prostate cancer is the second leading cause of cancer deaths worldwide. It was the first cancer in which vaccine immunotherapy was approved by FDA thus an immune responsive disease. However, the immunotherapeutic advances in prostate cancers are quite restricted, as no other drugs have shown clinical results in phase 3 trials. Still there is glimmer of hope, as active research is ongoing on combination of different forms of immunotherapy for prostate cancer. Recently new targets have been identified for prostate cancer like VISTA and PARP inhibition. So there are chances that immunotherapy will evolve as effective therapy in advanced prostate cancer.
Vaccine therapy
Sipuleucel-T (Provenge)
Sipuleucel-T is FDA approved cancer vaccine for prostate cancer. It is an autologous cellular immunotherapy, in which immune cells from host are in vitro activated against specific cancer antigens, to mount an immune response. In this vaccine, antigen presenting cell (APC) or dendritic cells are harvested with leukopheresis, these immune cells are incubated with fusion protein (PA2024) incorporating the antigens associated with prostate cancer, prostate acid phosphatase (PAP) and granulocyte-macrophage colony stimulating factor (GM-CSF) (1). After processing for 48 hours these activated immune cells are transfused back into patients to mount an immune response. Total 3 cycles are given to patients every 2 weekly and course was completed in 1 month (2).
It was approved by FDA in 2010 after phase 3 trial results of Immunotherapy for Prostate Adenocarcinoma treatment (IMPACT) (3). In this study 512 patients with mCRPC (metastatic castration-resistant prostate cancer) were enrolled. The patients who received Sipuleucel-T had a median overall survival (OS) of 25.8 months as compared to 21.7 months with placebo. It was statistically significant and there was 22% reduction in relative risk for mortality. However, there was no significant improvement in progression free survival (PFS). IMPACT also revealed that the greatest benefit occurs in patients with lower disease burden. As in this trial there is no improvement in PFS, short-term outcome and level of prostate specific antigen (PSA) didn’t decrease, concern was raised regarding efficacy of Sipuleucel-T. At that time, we were unfamiliar with immunotherapy, but later on multiple trials on immunotherapy have showed similar findings of improved long term outcome but no short term improvement (4-6). It has been suggested that Sipuleucel-T slows the progression of disease over time, as it has to enhance the immune system and cause proliferation and activation of anticancer cytotoxic T cells. Therefore, this antitumor effect may not be apparent initially and may be considered as disease progression in short term.
To check the immune response after treatment with Sipuleucel-T, a study was conducted by Fong et al. In this study 37 men with localized surgically resectable prostate cancer were treated with Sipuleucel-T as neoadjuvant therapy prior to radical prostatectomy (7). Surgical prostatectomy samples were evaluated for immune response. There was threefold increase in infiltration of CD4 and CD8 T cells as compared to pretreatment biopsy and twelve patients who didn’t get Sipuleucel-T as neo-adjuvant chemotherapy did not have similar immune response. This suggest sipuleucel-T mobilize T cells against the prostate cancer.
Given its OS benefit, enhanced immune response and favorable toxicity profile it should be a good option for patients with asymptomatic or minimally symptomatic mCRPC. However full three dose treatment is not cost effective (8), require lot of coordinated effort for leukopheresis and sample transportation, with no benefit in PFS and short term outcome. Alone Sipuleucel-T doesn’t seem to be have bright future, but in combination with chemotherapy, hormonal therapy or radium 223 it may have better outcomes (9-12). Currently lot of early phase studies are going on individual and combination therapies of Sipuleucel-T, which have been summarized in Table 1.
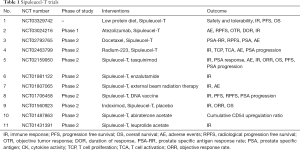
Full table
PROSTVAC (PSA-TRICOM)
PROSTVAC is a prostate specific viral recombinant antigen targeted vaccine. Basically it has combination of two viral factors—immunologic priming agent “Vaccinia” and immunologic boosting agent “Fowlpox” (13). Each vector carries for PSA and three immunologic molecules- B7.1 (costimulatory molecule for T cells), ICAM-1 (intracellular adhesion molecule 1) and LFA-3 (lymphocyte function associated antigen 3) (14). Virus infects the APCs and induces presentation of tumor associated antigens. It promotes cell surface protein expression on APC and lead to activation and proliferation of T cells. Enhanced T cell function lead to aggravated immune response and promote degradation of cancer cells (15). PROSTVAC enhances the avidity of T cells 100 folds (16), this is significant as higher avidity lead to rapid activation of T cells with even minimal amount of antigen presentation. As cancers suppress the immune system, this property is more helpful to have tumor lysis effect. In this patient receive priming dose on day 1 and then subsequently booster vaccine on day 14, 28 and then monthly until disease progression.
Various studies have shown the immune response of PROSTVAC. One trial has shown the twofold increase in PSA specific T cell response in 57% of patients (17). In a phase 2 trial (14) of PROSTVAC vs. control vector, it has been shown the improvement in OS. In PROSTVAC arm survival improved to 25.1 vs. 16.6 months in placebo control vector but the decline in PSA was minimal. Phase 3 multicentric study (NCT01322490) of PROSTVAC with or without GM-CSF vs. placebo in patients with mCRPC has recently been concluded and results are eagerly awaited.
Some of the early phase studies of PROSTVAC is going on which are summarized in Table 2.

Full table
GVAX
It is a cancer vaccine consists of two allogeneic irradiated prostate cancer cell lines LNCap and PC-3 which express GM-CSF (18). Phase 1 and phase 2 studies showed promising results. In one study done to evaluate immunogenicity of GVAX on chemotherapy naïve mCRPC patients (19). Patients were distributed in three groups, radiotherapy alone, GVAX high dose boost and GVAX low dose boost. The OS was 26.2, 34.9 and 24 months respectively. This vaccine in early studies was well tolerated with minimum autoimmune toxicities and improved patient survival. After that two phase 3 trials were conducted VITAL-1 and VITAL-2 which were prematurely terminated due to no therapeutic effect and increase in mortality (20,21).
DCVAC/PCa
In this autologous vaccine, dendritic cells are obtained by leukopheresis and exposed to killed prostate cancer cells which stimulates patient’s lymphocytes when they are reinjected into his body. In a phase 1/2 trial 25 patients with mCRPC received DCVAC vaccine with docetaxel. Patients also received Toll like receptor agonist to enhance immune system and cyclophosphamide to reduce regulatory T cells. The median OS was 19 months in patient who got DCVAC vaccine, whereas predicted OS was 11.8 months with Halabi nomogram and 13 months with MSKCC nomogram (22). After success of phase 1/2 trial, a global Phase 3 trial was started named as VIABLE trial. In this trial DCVAC/PCa with first line chemotherapy for mCRPC is studied. The results of study are awaited (23).
ProstAtak
It is based on gene transfer technology, via adenoviral vector, herpes simplex virus thymidine kinase is transferred to prostate cancer cells. Along with ProstAtak, radiation therapy and valacyclovir are also given. Radiation therapy will destroy the tumor and due to ProstAtak there will be enhanced immune response against cancer cells. If given in newly diagnosed and localized prostate cancer, it may prevent recurrence also. In a phase 1/2 trial (24), 9 men with newly diagnosed localized prostate cancer got ProstAtak before radiotherapy, and after follow up of 11.3 years, 6 patients were alive and 3 patients passed away due to issues unrelated to prostate cancer. Now Phase 3 trial is going on to determine the immune response due to combination of ProstAtak and radiation therapy in intermediate to high risk localized prostate cancer. In this study patient will receive transrectal ultrasound guided injection of ProstAtak after 15 days to 8 weeks of radiation therapy. The primary end point of study is PFS and secondary end point will be OS and PSA progression (NCT01436968).
Immune checkpoint inhibitors
Ipilimumab is a monoclonal antibody which acts against CTLA-4 (cytotoxic T-lymphocyte associated protein 4) and up regulates the activity of cytotoxic T cells. It was approved by FDA for melanoma as it improved the survival and increased antitumor efficacy. CTLA-4 is a protein receptor that is present on surface of T lymphocytes. When APC activate T cells to fight against foreign antigens, there are costimulatory molecules like CD28 and B-7 which enhances the immune response. But along with that there are immune check points like CTLA-4 which binds B7, counteract the costimulatory effect of CD28 and negatively regulate the immune response (25,26). Cancer cells exploit this property of CTLA-4, enhances their action and subsides the immune response. Ipilimumab acts against CTLA-4 and suppress the immune checkpoints therefore causes activation and proliferation of cytotoxic T cells which helps in degradation and lysis of tumor cells (27,28).
In patients with prostate cancer some of early phase 1 trials were conducted investigating effect of CTLA-4 blockage (29-31). Until now two phase 3 trials have studied the effect of ipilimumab on OS of patients with mCRPC. The first trial was done to assess the use ipilimumab after radiotherapy in patients with mCRPC who have progressed after docetaxel chemotherapy (32). Total 799 patients were enrolled, in which at least one bone metastasis from CRPC should be there. Patients were assigned into 1:1 ratio to receive ipilimumab 10 mg/kg or placebo, every 3 weeks for up to four doses with maintenance ipilimumab or placebo every 3 monthly until progression of disease. The trial failed to show improvement in OS as it was 11.2 months in ipilimumab arm and 10 months in placebo arm. But there was improvement in PFS which was statistically significant (ipilimumab 4 months vs. placebo 3 months). The most common grade 3–4 adverse events were immune related, occurring in 26% of ipilimumab group and 3% of placebo group. In this study, subgroup analysis was also conducted which showed higher chances of benefit from ipilimumab therapy in patients with absence of visceral metastasis, low Alkaline phosphatase and hemoglobin more than 11 g/dL.
Second study was done to evaluate treatment with ipilimumab in chemotherapy naïve asymptomatic or minimally symptomatic mCRPC patients, without visceral metastasis (33). Patients were assigned in 2:1 ratio to ipilimumab and placebo therapy. Medial OS was statistically comparable in both the groups (ipilimumab 28.7 months and placebo 29.7 months). But the PFS was better in ipilimumab group (ipilimumab 5.6 months and placebo 3.8 months). Immune related adverse events occurred in 31% of ipilimumab arm and 2% of placebo. Above mentioned two studies have shown that ipilimumab has no benefit in OS, but it improves PFS and PSA response rate suggest antitumor activity. So if ipilimumab used in combination with other therapies it may still benefit mCRPC patients (Table 3).
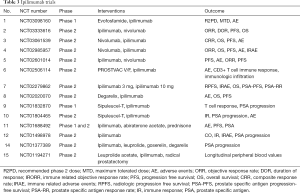
Full table
PD-1/PD-L1 inhibitors
PD-1 is a transmembrane glycoprotein (34), which is expressed on surface of activated cytotoxic T cells, B cells, dendritic cells, natural killer (NK) cells and macrophages (35). PD-1 regulates immune response and programmed cell death. It is basically an immune checkpoint receptor which controls the inflammatory reaction and reduce T cell activity in peripheral tissue therefore prevents autoimmunity (36,37). There are two ligands PD-L1 (programmed death ligand-1) and PD-L2 (programmed death ligand 2), they are present on cancer cells, and when these ligands bind with PD-1 they suppress the immune reaction (38). It is one of the mechanisms of evasion of immunity by cancer cells. PD-1/PD-L1 inhibitors block these receptors so there is no signal to suppress immune response (39). It leads to activation and proliferation of cytotoxic T cells which lead to degradation and lysis of tumor cells. Recent studies have shown that PD-1/PD-L1 inhibitors are efficacious in improving OS in various cancers. FDA approved Nivolumab (PD-1 inhibitor) in metastatic melanoma, non-small cell lung cancer and renal cancer and Pembrolizumab (PD-L1 inhibitor) in treatment of advanced melanoma (40-42).
In phase 1 trial of nivolumab, there was no objective clinical response in patients with mCRPC. Three phase 1 dose escalation studies were conducted to study the response of nivolumab in mCRPC, total 27 patients were enrolled but there was no measurable clinical response (43-45). Thus targeting PD-1 as monotherapy for mCRPC was curtailed. The most likely reason for failure of monotherapy of PD-1 inhibitor is, paucity of PD-L1 expression in tumor microenvironment (46), as expression of PD-L1 in tumor tissue is associated with immune response (47). Expression of immune check points is a dynamic process, with more infiltration of T cells there is upregulation of PD-L1 expression. Sipuleucel-T and Ipilimumab can enhance the T cell infiltration in the tumor microenvironment and therefore may increase the efficacy of PD-1/PD-L1 inhibitors. In 2016 Annual Meeting of the European Society of Medical Oncology, results were presented from an ongoing phase 2 trial of pembrolizumab in CRPC patients who progressed on enzalutamide therapy (NCT02312557). In this study patients were given pembrolizumab 200 mg IV every 3 weekly for four doses along with enzalutamide therapy. Out of 20 patients 4 had reduction on PSA more than 50%. This response rate of 20% is promising and if it is combined with other forms of therapy results may become better. Table 4 summarizes the studies going on monotherapy and combination therapy of PD-1/PD-L1 inhibitors.
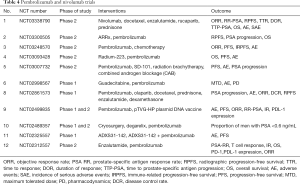
Full table
PARP inhibition in prostate cancer
PARP [poly (ADP Ribose) polymerase] is an enzyme which initiate single stranded DNA repair. In patient with underlying DNA repair problem, if we inhibit PARP there will be no DNA repair and cell will die. This is the basic principle of action of PARP inhibitors. One study showed that genomic testing from bone and soft tissue metastasis of CRPC has DNA repair alterations. There are aberrations in androgen receptor (AR), TP53, PTEN, BRCA1/2 and ATM genes. It also showed that 89% of these are clinically actionable DNA mutation and frequency of these aberrations increase with progression of disease (48). Olaparib is an oral PARP inhibition, which is approved for BRCA1/2 mutant ovarian cancer (49). As prostate cancer patients also have this mutation, active PARP inhibition can be a promising treatment. In a phase 2 study where mCRPC patients not responding to standard therapy are treated with Olaparib and mutational status was assessed by genomic sequencing. It showed that 33% patients have high response to Olaparib therapy and it was associated with defect in DNA repair gene. BRCA and ATM mutations were found in 10% and 12% of patients respectively, out of 49 patients. All patients with BRCA mutations and 80% of ATM mutations had clinical response to Olaparib. It was a small study but it prompted a new therapy for CRPC (50).
PARP inhibition leading to absence of DNA repair and cell death, will increase the antigenicity as there will be increased cytosolic double stranded DNA (51). And this enhanced antigenicity will may increase the efficacy of immune check point inhibitors. Therefore, studies are going on regarding combination therapy of olaparib with durvalumab (PD-L1 inhibitor) in prostate cancer (NCT02484404). At 2017 Annual meeting of American Society of Clinical Oncology, preliminary results were presented, which showed >50 reduction in PSA in 43% patients who got treatment for 2 months (52). Further studies are going on regarding individual and combination therapies of Olaparib, which have been summarized in Table 5. Two new PARP inhibitors Rucaparib (NCT02975934) and Niraparib (NCT02854436) are also under studies for therapy for CRPC.
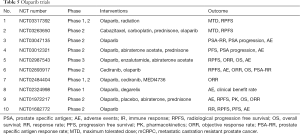
Full table
Combination therapies
Vaccines with immune checkpoint inhibitors
Immune check point inhibitors have significantly failed in improving clinical outcome in prostate cancer patients. There was no improvement in OS with ipilimumab and nivolumab, but the progression free interval improved. It depicts that, there was response, but not up to that extent to become statistically significant. The other likely reason for failure of immune checkpoint inhibitors is not enough inflammation and T cell infiltration in microenvironment of the tumor to cause immune activation and destruction of cancer cells. Vaccine on the other hand didn’t improve PFS but OS improved and with Sipuleucel-T, there is increase in T cell infiltration and inflammation in microenvironment of tumor. Therefore, there should be synergistic effect in combination of vaccines and immune checkpoint inhibitors.
A clinical trial of combination of ipilimumab and therapeutic cancer vaccine Prostvac has shown preliminary evidence of improvement in clinical and immunologic outcome. The median OS was 34.4 months (53) which as compared to Prostvac alone in contemporary study was 26.3 months (54). There was reduction in PSA in 54% of patients and PSA decline more than 50% was seen in 25% of patients. This study suggests potential synergy between vaccines and Immune check point inhibitors. Further studies are going on combination therapy of vaccines and immune checkpoint inhibitors which have been shown in Tables 1-5.
Combination of immune checkpoint inhibitors
Anti CTLA-4 and anti PD-1 both are immune checkpoint inhibitors, but act on different receptors and affect the immune response in different ways, therefore, they may have synergistic effect when combined together. Ipilimumab and nivolumab combination therapy have been tested in Melanoma, which showed improved outcomes as compared to independent therapies of both. But there were grade 3 to 4, adverse reaction in 55% of population (55,56). Currently phase 1 and phase 2 studies are going on regarding combination therapy in CRPC, which have been described in Tables 3 and 4. But increased efficacy of the combination has to weighed against increased side effect profile.
Combination of immunotherapy with standard therapy (chemotherapy and radiotherapy)
Standard therapy reduces the tumor burden by killing the cancer cells. This lysis and degradation of cancer cells release potent cancer antigens which if picked by immune system can lead to activation of T cells and further lysis of cancer cells. But this doesn’t happen as cancer cells modify immune system accordingly and suppress immune activation. If immunotherapy is used with standard therapy, suppression of immunity by cancer cells will be reduced and there will be enhanced activation of immune system which leads to proliferation of T cells and lysis of tumor. Androgen deprivation therapy attenuate immune response, enhance thymic output of naïve T cell, promotes T cell trafficking to prostate and improves tolerance and all of this provides a prime rationale for synergism with immunotherapy (57-60). In preclinical studies it has been shown that chemotherapy (docetaxel) enhances the major histocompatibility complex 1 (MHC-1) and tumor antigen expression and even low dose of radiotherapy does the same (59,61-64). Currently various initial phase trials are going on regarding this combination therapy which have been summarized in Tables 1-5.
Future directions
Vaccine immunotherapy in early stages of cancer
Vaccine immunotherapy is not a direct cancer cell lytic therapy, basically it enhances the immune system to fight against cancer cells and which is a slow process and has to go through multiple steps. And as cancer progresses it has suppressive effect on immune system, and with advanced stages of cancer reactivation of immune system will not be an easy task. Clinical data involving several tumor types have shown that vaccine immunotherapy works best in early stages of disease with limited tumor burden (65,66). It has been shown that there is threefold increase in inflammatory cells in tumor microenvironment when they are treated with Sipuleucel-T prior to radical prostatectomy (7).
Biomarkers
Failure of individual immune check point inhibitors raised the question whether expression of PD-1/PD-L1/CTLA-4 is biomarker for efficacy of immune checkpoint inhibitors. Immune biomarkers are required to increase the efficacy and to reduce the side effect profile. Further studies need to be conducted to look for more specific biomarkers.
Prostate cancer antigens
Immunotherapy vaccines target the antigens which are present normally on prostate like PAP, PSA, prostate specific cell antigen. Prostate cancer undergoes significant somatic mutations and several mutated proteins can serve as a neoantigens (67). If we can find and target these new mutated proteins, efficacy and specificity of vaccine will significantly improve.
Novel targets for immune checkpoint
Finding new immune checkpoints which creates tumor resistance will be the key to design new therapies for cancers. Recently it was discovered that ipilimumab therapy in prostate cancer had led to high level of T cell infiltration along with that there is increased expression of PD-L1 and VISTA (V domain Ig suppressor of T Cell activation), another immune checkpoint (68). Therefore, addition of anti-VISTA therapy to previous immune check point inhibitors can open new frontiers in immunotherapy for prostate cancer. Some other potential targets for immunotherapy of solid tumors are under initial stages of study. Some of them are LAG-3, TIGIT, TIM-3, B7-H3, B7-H4, CD-27, OX40, IDO, CD39, CD73, STAT3, CD137, PSMA, FAP/IL-2.
Conclusions
Although prostate cancer didn’t show satisfactory results with immunotherapy like other tumors, but a considerable amount of progress has been made in developing effective immunotherapy strategy. Future research options regarding prostate cancer are wide open. Some of them are developing biomarkers, starting immunotherapy in early stages, combinational therapies and developing new targets for immunotherapy. As novel therapies are advancing and rational combination therapies are emerging, there is chance we will soon have effective therapy for advanced prostate cancer.
Acknowledgements
Special thanks to Dr. Manisha Dhananjaya.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing incidence of metastatic prostate cancer in the United States (2004-2013) Prostate Cancer Prostatic Dis 2016;19:395-7. [Crossref] [PubMed]
- Harzstark AL, Small EJ. Immunotherapy for prostate cancer using antigen loaded antigen-presenting cells: APC8015 (Provenge). Expert Opin Biol Ther 2007;7:1275-80. [Crossref] [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer N Engl J Med 2010;363:411-22. [Crossref] [PubMed]
- Beer TM, Bernstein GT, Corman JM, et al. Randomized trial of autologous cellular immunotherapy with sipuleucel-T in androgen-dependent prostate cancer. Clin Cancer Res 2011;17:4558-67. [Crossref] [PubMed]
- DiPaola RS, Chen YH, Bubley GJ, et al. A national multicenter phase 2 study of prostate-specific antigen (PSA) pox virus vaccine with sequential androgen ablation therapy in patients with PSA progression: ECOG 9802. Eur Urol 2015;68:365-71. [Crossref] [PubMed]
- Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res 2011;17:907-17. [Crossref] [PubMed]
- Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014.106. [PubMed]
- Holko P, Kawalec P. Economic evaluation of sipuleucel-T immunotherapy in castration-resistant prostate cancer. Expert Rev Anticancer Ther 2014;14:63-73. [Crossref] [PubMed]
- Small EJ, Lance RS, Gardner TA, et al. A Randomized phase II trial of sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. Clin Cancer Res 2015;21:3862-9. [Crossref] [PubMed]
- Antonarakis ES, Kibel AS, Adams GW, et al. Antigen-specific immune responses through 24 months in the STAND trial: a randomized phase 2 study evaluating optimal sequencing of sipuleucel-T (sip-T) and androgen deprivation therapy (ADT) in biochemically-recurrent prostate cancer (BRPC). J Clin Oncol 2015;33:abstr 171.
- Hu R, George DJ, Zhang T. What is the role of sipuleucel-T in the treatment of patients with advanced prostate cancer? An update on the evidence. Ther Adv Urol 2016;8:272-8. [Crossref] [PubMed]
- Graff JN, Chamberlain ED. Sipuleucel-T in the treatment of prostate cancer: an evidence-based review of its place in therapy. Core Evid 2014;10:1-10. [Crossref] [PubMed]
- Silvestri I, Cattarino S, Giantulli S, et al. A perspective of immunotherapy for prostate cancer. Cancers (Basel) 2016.8. [PubMed]
- Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol 2007;178:1515-20. [Crossref] [PubMed]
- Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28:1099-105. [Crossref] [PubMed]
- Hodge JW, Chakraborty M, Kudo-Saito C, et al. Multiple costimulatory. J Immunol 2005;174:5994-6004. [Crossref] [PubMed]
- Gulley JL, Madan RA, Tsang KY, et al. Immune impact induced by PROSTVAC (PSA TRICOM),a therapeutic vaccine for prostate cancer. Cancer Immunol Res 2014;2:133-41. [Crossref] [PubMed]
- Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J 2010;16:304-10. [Crossref] [PubMed]
- Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor--secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res 2007;13:3883-91. [Crossref] [PubMed]
- Higano C, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer vs docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC). Genitourinary Cancer Symposium: Proc Am Soc Clin Oncol, 2009:Abstract # LBA150.
- Small E, Demkow T, Gerritsen W, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel vs docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC). Genitourinary Cancer Symposium: Proc Am Soc Clin Oncol 2009:Abstract #7.
- Podrazil M, Horvath R, Becht E, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015;6:18192-205. [Crossref] [PubMed]
- Tomasz MB, Nicholas V, Jiřina B, et al. Autologous dendritic cell immunotherapy (DCVAC/PCa) added to docetaxel chemotherapy in a Phase III trial (viable) in men with advanced (mCRPC) prostate cancer. J Immunother Cancer 2015;3:164. [Crossref]
- Rojas-Martínez A, Manzanera AG, Sukin SW, et al. Intra prostatic distribution and long-term follow-up after AdV-tk immunotherapy as neoadjuvant to surgery in patients with prostate cancer. Cancer Gene Ther 2013;20:642-9. [Crossref] [PubMed]
- Alme AK, Karir BS, Faltas BM, et al. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: an overview. Urol Oncol 2016;34:171-81. [Crossref] [PubMed]
- Goswami S, Aparicio A, Subudhi SK. Immune checkpoint therapies in prostate cancer. Cancer J 2016;22:117-20. [Crossref] [PubMed]
- Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994;1:405-13. [Crossref] [PubMed]
- Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013;1:32-42. [Crossref] [PubMed]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002;3:611-8. [Crossref] [PubMed]
- Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 2007;13:1810-5. [Crossref] [PubMed]
- Fong L, Rini B, Kavanaugh B, et al. CTLA-4 blockade-based immunotherapy for prostate cancer. J Clin Oncol 2004;22:abstr 2590.
- Kwon ED, Drake CG, Scher HI, et al. CA184-043 Investigators. Ipilimumab versus placebo after radiotherapy in patients with metastatic castrationresistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700-12. [Crossref] [PubMed]
- Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017;35:40-7. [Crossref] [PubMed]
- Okazaki T, Maeda A, Nishimura H, et al. PD-1 immunoreceptor inhibits B cell receptormediated signaling by recruiting src homology 2 domaincontaining tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA 2001;98:13866-71. [Crossref] [PubMed]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity Nat Rev Immunol 2004;4:336-47. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints In cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009;10:1185-92. [Crossref] [PubMed]
- Zhang X, Schwartz JC, Guo X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 2004;20:337-47. [Crossref] [PubMed]
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205-14. [Crossref] [PubMed]
- Raedler LA, Writer M. Opdivo (nivolumab): second PD-1 inhibitor receives FDA approval for unresectable or metastatic melanoma. Am Health Drug Benefits 2015;8:180-3. [PubMed]
- Kazandjian D, Suzman DL, Blumenthal G, et al. FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 2016;21:634-42. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti- PD-1 therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent antiprogrammed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Martin AM, Nirschl TR, Nirschl CJ, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis 2015;18:325-32. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer Cell 2015;161:1215-28. [erratum appears in Cell. 2015;162(2):454]. [Crossref] [PubMed]
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123-34. [Crossref] [PubMed]
- Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697-708. [Crossref] [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [Crossref] [PubMed]
- Karzai F, Madan RA, Owens H, et al. A phase II study of the anti- programmed death ligand-1antibody durvalumab (D;MEDI4736) in combination with PARP inhibitor, olaparib(O), in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017;35:abstr 162.
- Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a pox viral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010;59:663-74. [Crossref] [PubMed]
- McNeel DG, Eickhoff J, Jeraj R, et al. DNA vaccine with pembrolizumab to elicit anti tumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017;35:abstr 168.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab hormonotherapy in untreated melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Heng TS, Goldberg GL, Gray DH, et al. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol 2005;175:2982-93. [Crossref] [PubMed]
- Ardiani A, Farsaci B, Rogers CJ, et al. Combination therapy with a secondgeneration androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res 2013;19:6205-18. [Crossref] [PubMed]
- Drake CG, Doody AD, Mihalyo MA, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer cell 2005;7:239-49. [Crossref] [PubMed]
- Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 2001;98:14565-70. [Crossref] [PubMed]
- Chakraborty M, Wansley EK, Carrasquillo JA, et al. The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res 2008;14:4241-9. [Crossref] [PubMed]
- Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res 2008;14:3536-44. [Crossref] [PubMed]
- Hodge JW, Garnett CT, Farsaci B, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer 2013;133:624-36. [Crossref] [PubMed]
- Malamas AS, Gameiro SR, Knudson KM, et al. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulinmediated immunogenic modulation. Oncotarget 2016;7:86937-47. [Crossref] [PubMed]
- Madan RA, Gulley JL. Prospects for the future of prostate cancer vaccines. Expert Rev Vaccines 2016;15:271-4. [PubMed]
- Madan RA, Bilusic M, Heery C, et al. Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol 2012;39:296-304. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017;23:551-5. [Crossref] [PubMed]


