Endocrine therapy for ER-positive/HER2-negative metastatic breast cancer
Introduction
According to GLOBOCAN database, breast cancer is the most frequent cancer among women in the world, the most common cause of cancer-related mortality in women in less developed countries and the second most frequent cause of cancer death in more developed regions (198,000 deaths) (1). It is noteworthy that incidence rates are increasing in most of the countries, but mortality rates are decreasing only in high-income countries (HICs) (2).
Estrogen receptor positive (ER+) tumors are the most common form of breast cancer and are responsible for most of the deaths from the disease (3). Blocking the estrogen receptor (ER) pathway might be considered the first molecularly targeted therapeutic strategy for cancer and continues to be a mainstay of treatment for all stages of ER+ breast tumors (3).
Breast cancers are known to undergo genomic evolution during the course of the disease, with the acquisition of new molecular and phenotypic alterations that are associated with resistance to therapeutic strategies leading to disease progression. Hence, a proportion of breast cancer patients with early-stage disease experience recurrence despite curative intent locoregional therapy and (neo)adjuvant systemic treatments such as chemotherapy and endocrine therapy (ET). In the metastatic setting, currently available systemic treatments will lead to initial benefits in the majority of patients, with disease stabilization or tumor shrinkage. Nonetheless, due to a variety of mechanisms of resistance to treatment, breast cancer cells persist and disease progression invariably occurs. Therefore, advanced breast cancer remains an incurable, lethal, systemic disease (4).
Recent advances in the ability to understand the molecular biology of interactions between the ER pathway and important growth factor, metabolic and cell division pathways have brought the possibility of improving therapeutic results by modulating endocrine signaling and interfering with a variety of mechanisms of resistance to ET. Regrettably, limitations in the design of clinical studies published in this field have made it challenging to develop predictive biomarkers and most of the contemporary treatment strategies, although associated with important clinical benefits, have been directed to an unselected population of patients. Additionally, the optimal sequencing strategies of available therapeutic agents remain unknown.
This article aims to summarize the most important clinical studies and review current optimal therapeutic strategies. Moreover, topics about breast cancer care in low- and middle-income countries (LMIC) will be addressed.
The current ET armamentarium
Since Beatson’s historical observation that locally advanced breast tumors can regress after oophorectomy (5), estrogen deprivation has become an essential treatment for HR+ breast cancer. ET therapy may target the ER directly with the use of selective ER modulators (SERMs) or selective ER degraders (SERDs), or it can work by blocking estrogen synthesis, by the use of aromatase inhibitors (AIs) in postmenopausal patients or ovarian function suppression (OFS) in premenopausal patients, decreasing the activation of the ER pathway signaling and avoiding tumor cell replication. The current armamentarium of ET is summarized in Table 1.

Full table
Tamoxifen
Tamoxifen was approved by the Food and Drug Administration (FDA) in 1977 for the treatment of women with advanced breast cancer and several years afterward for adjuvant treatment of primary breast cancer. For decades, tamoxifen has been the most used agent for the endocrine treatment across all stages of ER+ breast cancer. Tamoxifen is included in the World Health Organization (WHO) list of essential drugs for the treatment of breast cancer. It is estimated that more than 500,000 women are alive today as a result of tamoxifen therapy, and millions more have benefited from palliation and extended disease-free survival (DFS). Tamoxifen is used for the treatment of ER+ invasive breast cancer in the neoadjuvant, adjuvant, and metastatic settings (6,7). Tamoxifen is also used to prevent recurrences from surgically treated ER+ ductal carcinoma in situ (DCIS) and for breast cancer prevention in high-risk patients (8). In the metastatic setting, a meta-analysis that evaluated more than 5,000 patients treated with tamoxifen reported an overall response rate (ORR) of 34% review and a clinical benefit rate (CBR) of 53% (9).
AIs
The AIs suppress plasma estrogen levels in postmenopausal women by inhibiting or inactivating aromatase, the enzyme responsible for the synthesis of estrogens from androgenic substrates. Unlike tamoxifen, AIs have no partial agonist activity. In postmenopausal women in whom estrogen synthesis occurs mainly in peripheral tissues, third-generation AIs (anastrozole, letrozole and exemestane) have demonstrated efficacy while decreasing circulating estrogen levels (10,11). Following several decades of tamoxifen therapy dominance in first line setting for treatment of ER+ metastatic breast cancer (MBC), AIs became the preferred mode of therapy in this setting approximately two decades ago. The clinical trials that compared first-line therapy with AIs and tamoxifen are well summarized elsewhere and are not given in details here (3). Additionally, recent trials established AIs as one of the preferred treatment strategies in the neoadjuvant and adjuvant settings for selected groups of patients with ER+ early-stage breast cancer (12). Anastrozole and letrozole are non-steroidal AIs with reversible binding to aromatase, whereas exemestane is a steroidal AI that irreversibly binds to the enzyme (13). While there is no clinical evidence demonstrating that either AI is better than the other, what is evident is that AIs are superior to tamoxifen, and this was demonstrated in a pivotal meta-analysis of 8,504 patients with HR+ MBC, documenting superior survival with AIs versus tamoxifen (HR, 0.89; 95% CI: 0.80–0.99) (14).
SERDs
SERDs, exemplified by fulvestrant, are a class of endocrine agents that act by binding to the ER and induce rapid degradation thereby making the the receptor unavailable or unresponsive to estrogen. These drugs attenuate the ability of ER to activate gene transcription and also increase ER turnover and degradation leading to profound inhibition of estrogen signaling. A key characteristic of SERDs that distinguishes their mechanisms of action from that of SERMs, is that fulvestrant consistently reduces estrogen and progesterone receptor levels in the tumor and in other tissues as well, without having agonist effects. The use of a sub-optimal dose of fulvestrant has demonstrated it was as effective as anastrozole in tamoxifen failures (15). Recently published randomized clinical trials reported that treatment with higher doses of fulvestrant improves disease control and improved progression-free survival in comparison with anastrozole in patients with HR+ MBC that have not been previously exposed to ET (16). Fulvestrant has been proven useful in the treatment of advanced breast cancer and as of the year 2016, has been approved as monotherapy in first line setting for treatment of ER+ MBC per results of the FALCON trial (16,17). Fulvestrant is not approved for early-stage or neoadjuvant treatment of breast cancer. However, the ALTERNATE trial which is intended to evaluate the use of fulvestrant in the neoadjuvant treatment of ER+ early stage breast cancer is currently open and accruing (18).
Fulvestrant was initially approved in the year 2002 at the dose of 250 mg intramuscular injection (IM) every 28 days (19). However, follow up studies showed a strong dose-dependent biological effect of fulvestrant, which resulted in change in the recommended dose to 500 mg IM every 28 days with loading on days 1, 14 and 28. Strong dose-dependent biologic effect was initially observed in NEWEST neoadjuvant study where 500 mg dosing of fulvestrant was shown to more effectively reduce the tumor Ki67 compared to the 250 mg dosing (20). The phase III trial CONFIRM randomized postmenopausal patients with ER+ MBC with progression after tamoxifen or AIs to receive fulvestrant 250 or 500 mg (days 0, 14, 28 and every 28 days thereafter). Both doses had similar ORR, CBR and toxicity profile. The higher dose was associated with a statistically significant longer PFS. Overall survival (OS) was later reported showing a significant 4.1 (P=0.02) months difference in favoring the 500 mg dose of fulvestrant (21,22). Following this study, the approved dose of fulvestrant was updated to 500 mg IM dosing monthly with the loading dose (23). A significant improvement in PFS versus anastrozole was first seen in a phase II study (24), favoring fulvestrant versus anastrozole, and was later confirmed in the recently published phase III FALCON trial (16). The results included improvement of median PFS to 16.6 months (95% CI: 13.83–20.99) in fulvestrant arm versus 13.8 months (11.99–16.59) in the AI arm. Furthermore, results of a subsequent analysis of the data in this trial demonstrated that the improvement in PFS with fulvestrant versus anastrozole was preferentially observed in patients with non-visceral metastatic disease, and further studies are required to and understand this finding. It is important to note that patients in this study were not exposed to adjuvant ET, a patient population for which there is a paucity of data, especially in developed countries where the majority of breast cancers are diagnosed through screening, therefore limiting the generalizability of the findings. Perhaps the most important conclusion from this series of fulvestrant studies is that SERD-type drugs should be further studied, particularly in the adjuvant setting where improved endocrine therapy would have the greatest impact.
Estrogen and progesterone receptor agonists
Several alternative hormone therapies have been utilized with variable success over the last decades and remain as options to be considered with the goal of delaying chemotherapy for advanced disease as long as possible. Megestrol acetate and estradiol represent cheaper options that need to be taken into consideration. Many women may be on long-term treatment on drugs not always fully covered by insurance and in LMIC, novel therapies are unavailable due to their high price.
Progestins (megestrol acetate and medroxyprogesterone) have a reported ORR of 25% but are associated with side effects such as weight gain, fluid retention and an increase in the risk of thromboembolic events (25-28). Medium and high dose estradiol or diethylstilbestrol (DES) have been paradoxically used in the treatment HR+ MBC with similar response rates compared to tamoxifen but a higher toxicity profile (29-31). Estradiol may be administered at 6 mg daily, which was shown to be as effective and much less toxic than the previously recommended 30 mg daily dose (29). Bisphosphonates must be co-administered to avoid hypercalcemia and medroxyprogesterone acetate should be given for the last 5 days of the month to avoid dysfunctional uterine bleeding. Ideal patients for estradiol therapy are those that have experienced long term disease control with an AI before disease progression.
Combination of endocrine agents
A logical approach to improve the effectiveness of ET is to combine different classes of agent. Trials comparing the combination of fulvestrant with anastrozole versus anastrozole monotherapy have reported conflicting results. The SWOG 0226 trial reported advantages in terms of OS and PFS in favoring the combination arm (32). However, the FACT study did not show any clinical advantage with the combination (33). Subgroup analysis of these trials suggested that the benefits were probably limited to patients who have not previously been exposed to adjuvant tamoxifen and differences in the eligibility criteria of the studies probably explain the conflicting results. Additionally, in patients with HR+ MBC that had progressed after first-line therapy with anastrozole or letrozole, the SoFEA trial showed that the combination of fulvestrant with anastrozole is not more effective than the monotherapy with fulvestrant or exemestane. Once again, a subgroup analysis suggested that those patients with both ER and PR expression, a surrogate for a more hormone-sensitive phenotype, might achieve superior benefit (34). Based on this information, it can be hypothesized that patients with ET naive advanced breast cancer and those with highly endocrine-sensitive tumors could derive the largest benefit from combination-ET. Nonetheless, we believe that we should wait for further evidence before considering combination-ET in routine clinical practice. Additionally, no data on the use of 500 mg fulvestrant monthly dosing in combination with an AI has been published to date.
ET combinations with targeted therapies
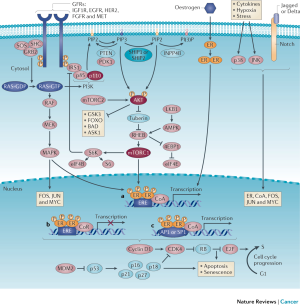
Over the last decade, targeted therapies that modulate mechanisms of ET resistance have been incorporated into clinical practice. Two general patterns of ET resistance are recognized clinically: intrinsic resistance, referring to cases where ER+ cancers never adequately respond to ET and acquired resistance, which occurs after an initial response to endocrine manipulation (35). These definitions are not precise and the underlying mechanisms between intrinsic and acquired resistance are likely to overlap. Several cell-autonomous and non-cell-autonomous alterations in ER+ breast cancer and a variety of components of the interaction of breast cancer cells with the tumor microenvironment could lead to mechanisms of resistance to ET. These mechanisms are described in Figure 1 and include deregulation of the ER pathway, the cell-cycle machinery, growth factor receptor signaling, secondary messengers, apoptosis and senescence, epithelial-to-mesenchymal transition (EMT), cancer stem cell (CSC) persistence, and signaling within the tumor microenvironment. Comprehensive reviews regarding mechanisms of ET resistance have been published elsewhere and are beyond the scope of this article (4,36,37). We do not discuss the subgroup of ER+ HER2 positive patients in detail here but do point out there are a number of phase 2 and phase 3 trials that indicate that the combination of HER2 targeting with ET produced prolonged responses in some patients (38-40).
mTOR inhibitors
Deregulation of the PI3K/AKT/mTOR pathway function has been described in diverse tumor types, especially breast cancer, and has been correlated with cancer pathogenesis, resistance to therapies and tumor progression (41). Extensive pre-clinical data indicate significant cross-talk between the PI3K/Akt/mTOR and the ER signaling pathways (42,43). The mTOR inhibitor everolimus has been approved by the FDA for the treatment of HR+ MBC in combination with exemestane after failure to a NSAI based on PFS benefits seen in the BOLERO2 trial (44). The BOLERO-2 study was the first phase III clinical trial to demonstrate PFS benefit with the inhibition of one of the activated adaptive pathways mediating resistance to ET. This trial randomized postmenopausal patients with ER+ MBC with prior exposure to anastrozole or letrozole for therapy with everolimus in combination with exemestane versus exemestane alone. With a median follow-up (FU) of 18 months, patients in the everolimus arm had a significantly reduced risk of disease progression with a PFS of 7.8 versus 3.2 months in the placebo arm (HR, 0.45; 95% CI: 0.38–0.54, P<0.0001) (44). The benefit was consistent regardless of factors such as prior ET exposure and presence of visceral metastasis. Despite clinical benefits and statistically significant extension of PFS, this combination did not confer any OS benefit (45). Besides, half of the patients in everolimus arm had grade 3/4 toxicity, being most related stomatitis, hepatic impairment, anemia and hyperglycemia. Nonetheless, a post hoc analysis confirmed that the use of everolimus did not impact adversely on health-related quality of life (HR-QOL) (46). Fulvestrant 500 mg and everolimus at a dose of 10 mg daily have been tested in patients with ER+ MBC post progression on AI in a phase II trial. This trial showed a significant improvement in PFS (median PFS was 10.4 months in the experimental arm versus 5.1 months in the ET alone arm; HR, 0.61, 95% CI: 0.40–0.92; stratified log-rank P=0.02) with the combination of mTOR inhibition and fulvestrant. However, grade 3 toxicities were more commonly observed in the combination arm compared with fulvestrant alone (53% vs. 23%). Additionally, the recently presented MANTA trial also highlighted the benefit of combining fulvestrant with everolimus. Patients who progressed after previous AI were randomized to treatments with fulvestrant, fulvestrant plus the PI3K vistusertib, or fulvestrant plus everolimus. The PFS for fulvestrant plus everolimus was superior to fulvestrant plus vistusertib or fulvestrant monotherapy (12.3 vs. 7.6 months, HR, 0.63, 95% CI: 0.45–0.9, P=0.1) (47). Thus, everolimus will remain a useful approach to endocrine therapy resistant breast cancer. The use of steroid mouthwash and judicious dose reductions will increase the tolerability if patients are monitored carefully.
CDK4/6 inhibitors
The most significant advance in the management of luminal MBC over the last few years has undoubtedly been the introduction of a new class of agent, the CDK4/6 inhibitors, in combination with an endocrine drug (48). Three agents (palbociclib, ribociclib and abemaciclib) have been approved and incorporated into clinical practice in combination with ET both in first and second line settings.
Palbociclib was the first CDK4/6 inhibitor approved by the FDA in 2015 and is the compound with the most mature data evaluating its efficacy and safety in a variety of clinical settings. In vitro studies demonstrated that palbociclib was associated with tumor growth inhibition specifically in luminal cells (49). The following results of a phase I trial, in which palbociclib was combined with letrozole, revealed 41% of partial response and 30% of stable disease, after a schedule change due to grade 3/4 rates of neutropenia. The first strong evidence of clinical activity was observed in the PALOMA 1 study, a randomized two-arm phase II trial of 165 patients, comparing palbociclib + letrozole versus placebo + letrozole, which demonstrated 20.2 versus 10.2 months of median progression-free survival, favoring the experimental arm (50). Although most frequent toxicity was neutropenia (54%), neutropenic fever was not reported as a frequent complication. The PALOMA-2 phase 3 trial, a 2:1 randomized two-arm phase III trial of letrozole with palbociclib versus letrozole alone was published in 2016. Here 666 patients were included with no previous treatment for metastatic disease. However, at least 48% received (neo)adjuvant chemotherapy and 56% adjuvant hormonal therapy. The trial was positive for the primary endpoint and the median PFS was 24.8 versus 14.5 months, favoring the experimental arm (HR, 0.58; 95% CI: 0.46–0.72, P<0.000001) (51).
The phase III PALOMA-3 trial was designed to compare palbociclib in combination with fulvestrant versus fulvestrant monotherapy in patients with ER-positive/HER-2 negative MBC that relapsed or progressed on prior ET (52). With 521 patients, the PALOMA-3 study showed increased median PFS of 9.2 months (95% CI: 7.5–not estimable) for the palbociclib/fulvestrant arm versus 3.8 months (95% CI: 3.5–5.5 months) for the placebo/fulvestrant arm (HR, 0.42; P<0.001). OS data are not yet mature for either of these studies. A preplanned subgroup analysis of Asian patients included in the PALOMA-3 study showed similar PFS benefits with palbociclib, with a higher rate of grade 3/4 neutropenia (92%) and febrile neutropenia (4.1%) (53). The median time to deterioration of the global HR-QOL score was not reached but favored the palbociclib group over the ET monotherapy arm (HR, 0.641, 95% CI: 0.45–0.91) (54). With PALOMA-3 results, the FDA approved palbociclib in combination with fulvestrant for the treatment of women with ER-positive/HER2-negative MBC after progression of disease with previous ET in 2016 (55).
Ribociclib is another CDK4/6 inhibitor with similar pharmacologic properties and therapeutic effects as palbociclib. In the first-line setting, the MONALEESA II trial randomized the treatment of 668 patients to receive letrozole plus placebo or ribociclib 600 mg per day, three weeks on and one week off. The study met its primary endpoint as the duration of PFS was significantly longer in the ribociclib group (HR, 0.56; 95% CI: 0.43–0.72). After 18 months, the PFS rate was 63% (95% CI: 54.6–70.3%) in the ribociclib group and 42% (95% CI: 34.8–49.5%) in the placebo group. The improved efficacy outcomes were maintained with longer follow-up and at the time of second interim analysis the median PFS was 25.3 months in the ribociclib group and 16 months in the placebo group (HR, 0.56, 95% CI: 0.45–0.7). The side effects profile of ribociclib is very similar to palbociclib with afebrile neutropenia and fatigue being the most common toxicities. A QT interval prolongation was described in a few patients in the MONALEESA II trial. The events were reversible and managed with dose adjustments or interruptions. Recommendations for ribociclib treatment include ECG monitoring. The recently published MONALEESA 7 trial evaluated exclusively the premenopausal population in the first-line setting. Patients were randomized to receive ribociclib or placebo plus goserelin (for ovarian suppression) and an AI or tamoxifen. The median PFS was 23.8 versus 13.0 months (HR, 0.55, 95% CI: 0.44–0.69; P<0.0000001) favoring the ribociclib arm (56). In the second-line setting, the MONALEESA-3 trial has completed enrolment but results are still awaited.
Abemaciclib is the third CDK4/6 inhibitor approved for the treatment of ER+ HER2-negative MBC. Two phase III trials assessing the combination of abemaciclib with ET for ER-positive MBC patients were designed (57,58). MONARCH 3 is a phase III study designed to compare the combination of abemaciclib at the dose of 150 mg twice daily combined with the AIs anastrozole or letrozole versus placebo plus an AI as first-line treatment (58). Recently presented results showed that the median PFS was not yet reached in the abemaciclib arm versus 14.7 months with the NSAI alone (HR, 0.543; 95% CI: 0.409–0.723; P=0.000021). In those with measurable disease, ORR was 59.2% with the CDK4/6 inhibitor and 43.8% in the control arm (P=0.004). The phase III MONARCH 2 trial compared the combination of abemaciclib with fulvestrant in women with ER+ HER2-negative MBC with disease progression while receiving neoadjuvant or adjuvant ET, within less than 12 months from the end of adjuvant ET, or while receiving first-line treatment for advanced disease (57). With 669 patients, this study showed that abemaciclib plus fulvestrant increased PFS when compared to placebo plus fulvestrant (median, 16.4 vs. 9.3 months; HR, 0.55, P<0.001). The most frequent AEs of abemaciclib versus placebo at any grade were: diarrhea (86.4% versus 24.7%), neutropenia (46% versus 4%), nausea (45.1% versus 22.9%) and fatigue (39.9% versus 26.9%). Diarrhea typically occurred in the first cycle and was effectively controlled with antidiarrheal medications and dose reduction. The phase II trial MONARCH 1 was designed to assess safety and efficacy of abemaciclib monotherapy in patients with refractory ER-positive MBC (59). Women with ER-positive/Her2-negative MBC with disease progression after prior ET and 1 or 2 chemotherapy regimens were eligible. Abemaciclib 200 mg was administered orally on a continuous schedule twice daily. The study included 132 patients, 90% with visceral disease, the objective response rate was 19.7%, with CBR of 42.4% and a median PFS of 6 months.
Comprehensive reviews about clinical management of potential toxicities and drug interactions related to CDK4/6 inhibitors in MBC have been published elsewhere (60) and are also an excellent resource for further consideration.
Other agents
The scope of this article is to discuss targeted therapies that have been approved for the treatment of breast cancer. Nevertheless, it is important to mention other promising agents including a variety of compounds currently being tested in phase III trials such as phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitors (61) and histone deacetylase (HDAC) inhibitors (62,63) as well as drugs targeting the Src family (64).
Current treatment algorithm for HR + MBC
Historically, ET has been an active therapeutic strategy associated with both limited toxicity and significant efficacy. Several clinical principles guide our treatment recommendations: ET in the absence of visceral crisis or involvement, optimal sequencing of ET treatments, and the treatment after the development of ET resistance (48). For patients with HR-positive MBC, there is now an unprecedented number of endocrine-based treatment alternatives that can enhance long-term outcomes and that can be used before the need of chemotherapy. Regrettably, even after many decades of clinical studies, there is still a lack of definitive recommendations about the optimal strategy for sequencing endocrine agents in patients with advanced breast cancer (65).
Moreover, despite many efforts in translational studies, currently, no tested biomarker has succeeded in distinguishing between those patients more likely to benefit from the use of targeted therapies, and those who may be adequately managed with endocrine monotherapy. Subgroup analyses from the previously mentioned phase III trials have so far also failed to help us identify which group of patients could avoid combination treatment, as the benefit in terms of decreased risk of progression favored the combination arms for all the pre-defined subgroups without statistically significant differences based on a variety of factors such as age, menopausal status, race, presence of visceral metastases, prior ET sensitivity, prior chemotherapy, and disease-free interval (DFI) (37). However, recent data derived from the MONARCH-3 study suggested the benefit from the addition of abemaciclib was not seen in patients with a longer DFI (>36 months) from the end of adjuvant ET (57). Additionally, a lesser benefit from the combination was observed in the subgroup of patients with bone-only disease. This information could indicate that endocrine monotherapy could be used in the subgroup of patients with clinical characteristics typical of a strongly endocrine-responsive disease, and that the use of targeted therapies such as CDK4/6 inhibitors could potentially be saved for subsequent lines of treatment. However, as this hypothesis comes from a subgroup analysis with a relatively low number of patients in a single study, these data require further confirmation in a larger study (66). On the other hand, the updated analysis of the PALOMA-2 trial showed that the benefit of adding the CDk4/6 inhibitor occurred irrespective of patterns of disease recurrence and DFI.
Several factors need to be considered when selecting the optimal ET agent for the treatment of ER+ MBC. These factors include patient’s characteristics (i.e., menopausal status, comorbidities, adherence) as well as tumor and disease characteristics like the site of metastasis, tumor burden, need for rapid symptom control, DFI and response to previous ET. Nonetheless, an assortment of additional factors such as cost, access to innovative drugs, availability of clinical research as well as financial and social hardships can make this decision even more challenging. Individual breast tumors have different patterns of response and resistance to each ET strategy and the ability to select which endocrine agent an individual patient’s cancer is most sensitive to is a realistic, as well as a clinically worthwhile goal (3).
OS reports are highly awaited as well as future trials addressing sequencing strategies (44). Furthermore, biomarkers for patient selection as well as cost-effectiveness analysis are awaited. The appropriate moment and patient selection in clinical practice for prescribing these drugs is an ongoing debate. In our opinion, this advanced decision must be individually discussed with patients (51). Until there are clear overall survival benefits from one strategy versus another, treatments must be focused on optimizing symptom control and reducing toxicity.
First-line setting
Patients who are ET naive and present with de novo MBC are candidates for (true) first-line ET. Therapeutic options in the first-line setting include endocrine monotherapy or the combination of endocrine agents and CDK4/6 inhibitors. The most important phase III trials are summarized in Table 2.

Full table
Upfront use of CDK4/6 was analyzed in phase III trials that compared the combination of three available agents (palbociclib, ribociclib and abemaciclib) with an AI versus AI plus placebo. In summary, the three studies demonstrated significant statistical and clinical benefits in terms of improved PFS and time to chemotherapy with a tolerable toxicity profile. It is important to emphasize that, so far, no OS benefit has been demonstrated. Nonetheless, first-line treatment with CDK4/6 inhibitors in combination with letrozole or anastrozole is being increasingly adopted as the preferential strategy, especially in the USA and Western Europe (67).
The first-line trials of CDK4/6 inhibitors enrolled patients with ‘de novo’ MBC and patients that experienced recurrence after a long DFI following the completion of adjuvant ET, therefore, representing a population with potentially highly endocrine sensitive tumors. Endocrine-naive patients presenting with MBC are increasingly rare in regions of the globe where breast cancer screening programs allow detection of cancers at an early stage and treatment with adjuvant ET is commonly prescribed in the vast majority of patients. Nevertheless, for this subgroup of patients, the use of first-line fulvestrant is a valid approach based on the FALCON trial, especially in patients without visceral metastasis (16).
Despite the evidence of superior outcomes with other alternatives, still we consider that the use of an AI or tamoxifen is a valid option as first-line ET for selected patients, particularly in tumors with characteristics associated with endocrine sensitivity and/or in resource limited settings such as LMIC. PFS achieved with AI therapy as first-line treatment for MBC have shown progressive improvement. The median PFS increased from 6–9 months in the earlier trials to 13–16 months in the current era, representing an absolute gain of approximately seven months, without the addition of any other drugs (68). These findings, probably attributed to better patient selection, have significant implications in the design of future clinical trials including issues related to sample size and FU periods estimations as well as in the translation of the results into clinical practice.
Second-line setting
Patients with disease resistance to first-line treatment and patients who progress during adjuvant ET or within the initial 12 months after the completion of adjuvant ET are considered candidates for second-line ET. As previously described, several factors should be considered while selecting the optimal second-line strategy. Subsequent use of endocrine agents should always take into considerations what were the previous lines of treatment as well as the type and duration of response to previous ET. Two major patterns of endocrine resistance are recognized clinically; “intrinsic resistance” whereby ER+ cancers never respond adequately to ET and “acquired resistance” which occurs following an initial response (69). An arbitrary cutoff of 2 years for relapse (in the early stage setting) or 6 months for progression (in the metastatic setting) has been used to define intrinsic versus acquired resistance in clinical trials. However, these distinctions are somewhat arbitrary and the underlying mechanisms between intrinsic and acquired resistance are likely to overlap. In addition, resistance to ET may be agent-selective. For example, after failure of AI therapy, tumors can respond to other ET approaches such as another AI, ER modulators (tamoxifen) or down-regulators (fulvestrant) and even estradiol (29).
Important trials of second-line ET are reviewed in Table 3. In AI-refractory disease, the use of combination of endocrine agents with targeted therapies aiming to modulate mechanisms of endocrine resistance have been incorporated into clinical practice initially with the mTOR inhibitor everolimus and more recently with the use of CDK4/6 inhibitors in combination with fulvestrant.

Full table
The CDK4/6 inhibitors palbociclib and abemaciclib have also shown significant clinical benefits and prolongation of median PFS when used in combination with fulvestrant in the PALOMA-3 (52) and MONARCH-2 (57) phase III trials, respectively. These studies included patients who relapsed during first-line treatment with AI for MBC as well as patients that recurred during adjuvant AI or experienced a short DFI after completion of adjuvant ET. Also, in a similar population with NSAI refractory tumors, the BOLERO-2 trial showed better outcomes with treatment with everolimus in combination with exemestane, in comparison with exemestane monotherapy.
As discussed previously, the contemporary unavailability of predictive biological markers leaves us with clinical factors such as type of previous ET exposure and timing of disease progression as the only elements to help us define the optimal therapeutic strategy. In patients with long PFS (as a surrogate of endocrine sensitivity) the sequential use of endocrine agents can be considered a valid option. Despite limited data, drug treatment withdrawal in selected patients with progressive advanced ER+ breast cancer may eventually be utilized (70). Tamoxifen, megestrol acetate, estradiol and androgens also remain potential treatment options in those patients with long-lasting ER-sensitive disease as well as in limited resources settings, where drug availability is limited.
Premenopausal patients
In premenopausal patients with HR+ advanced breast cancer, for decades the standard ET has been tamoxifen or ovarian function suppression. Studies that compared tamoxifen with surgical castration have shown similar efficacy (71,72). In a meta-analysis the combination of a GnRH agonist with tamoxifen resulted in a significant increase in median PFS and OS in comparison with either agent alone (73). The use of AI monotherapy in premenopausal patients is not recommended since these women have functioning ovaries that respond to AI induced surge on gonadotrophins. Therefore, AIs can only be administered in combination with OFS, that can be achieved either by the use of a GnRH analog or by surgical castration. After progression on tamoxifen and with the indication of further ET, the NCCN guideline and a limited amount of clinical data (74-76) suggest that premenopausal and perimenopausal patients with ER+ MBC should be treated with OFS, and treated in the same manner as postmenopausal patients. As mentioned above, the recently presented MONALEESA-7 trial was designed to evaluate the role of the CDK4/6 inhibitor ribociclib specifically in the premenopausal population and demonstrated similar benefits regarding PFS and response rates as seen in the trials with postmenopausal patients (56).
ER+ MBC in LMIC
It is projected that the new cases of cancer will increase from about 14 million in 2012 to 22 million in 2030, with the bulk of new cases in Africa, Asia and Latin America (77). LMIC in these regions are facing an epidemiologic transition towards a pattern of morbidity and mortality similar to HICs, with a rising incidence of certain forms of cancer such as colon, lung and breast cancer. At the same time, they still have a high incidence of infection-related cancers such as cervical and liver cancers. We can anticipate a huge humanitarian and economic impact on these countries that needs to be urgently addressed. Considering only access to radiotherapy services, from 2002 to 2012 there was an increase in cases requiring radiotherapy of 69% in LMIC and of 31% in HICs. Meanwhile, during the period from 2004 to 2013, the increase in radiotherapy machines was 31% in LMICs and 61% in HICS (78). The global expenditure on cancer medicines rose to more than $100 billion in 2015 and is projected to reach $150 billion, mostly in HICs. The World Health Organization (WHO) developed two important projects to address the needs of LMICs: a list of basic and priority medical devices required for cancer treatment and a list of essential medicines (79). The list of essential medicines for MBC includes drugs such as trastuzumab, paclitaxel, docetaxel, vinorelbine (IV) and gemcitabine. But the accessibility to these drugs is threatened by insufficient availability and affordability. In many LMICs, even the medicines in the WHO essential list are available as an out-of- pocket expense and with unreliable supply.
In regard specifically to breast cancer in LMICs, there are some initiatives that have the potential to change this landscape. Most of the reviews on breast cancer in LMICs are based on strategies developed in HICs and most the national cancer control programs are global solutions implemented without considering local conditions (80). It is crucial that research identifies local challenges and proposes realistic, accessible and affordable interventions. The collaboration with institutions in HICs may provide the expertise and grants necessary to build a research infrastructure and enhance the understanding of breast cancer. Another possible initiative is to design resource-stratified guidelines for prevention, screening and treatment of breast cancer, as available for cervical cancer (81). However, any adequate program to control breast cancer must involve health education, prevention, screening, early detection and treatment. A collaborative effort between the governments, industry and social and advocacy group with the creation of a global fund to support LMICs could be a more efficient solution (82).
Studies have shown that physicians involved in oncology treatment in LMICs are aware of existing cancer treatment guidelines. Nevertheless, implementation of these guidelines hinders their usage because of a variety of factors such as lack of adequate facilities, absence of access to medications, and overly complex guidelines (83). Despite the significant advancements in medical oncology over the last decade, there is a paucity of information about global access to oncology medicines particularly in LMIC, where 70% of cancer mortality occurs (84). The majority of LMIC does not have access to modern oncology treatments like monoclonal antibodies, SERDs and protein kinase inhibitors. Even in the region of the Latin America where ET agents are well presented in national essential medicine lists, publications have described disparities in relation to access to AIs in the public sector of upper middle-income countries (85,86). Regional disparities between the public and private health systems make these issues even more challenging.
Therefore, the treatment strategies of advanced ER+ breast cancer should be tailored according to the regional and socio-economic contexts. Once CDK4/6 inhibitors and SERDs are unavailable in the majority of LMIC, AI monotherapy should be considered as the preferred therapeutic option in the first line setting for postmenopausal patients with ER+ MBC. Guidelines recommending chemotherapy in visceral crisis or in patients with known or suspected endocrine resistance should be followed. For premenopausal patients with limited access to GnRH agonists, surgical castration can be considered. In the second-line setting, given the unavailability of CDk4/6 and mTOR inhibitors in the majority of LMIC countries, therapeutic options include less-expensive alternatives such as tamoxifen and, particularly for patients with sensitivity to initial hormone manipulation, estrogen and progesterone receptor agonists.
Future perspectives
The significant heterogeneity of ER+ breast cancer is not adequately demonstrated with the use of traditional clinicopathological features such as histological subtype, grade, and ER expression status.
As previously discussed, estrogen-independent tumor growth often exists de novo at MBC diagnosis or develop during the course of breast cancer treatment. Even though it is clear that significant advances occurred in the last years bringing meaningful benefits to patients, there are still important unanswered questions and one of the research priorities in this field should be the development of clinically-relevant predictive biomarkers to allow optimal sequencing of ET strategies and to guide a more personalized patient care.
Genomic instability is considered an enabling feature of cancer that promotes the acquisition of other hallmarks of cancer and furthermore creates genetic heterogeneity from cell to cell (87). The development of massively parallel genome sequencing techniques, combined with advances in computation methodologies, has led to the identification of a variety of processes that leave a mutational footprint in the cancer genome (88). In the past decade, research started to incorporate these novel bioinformatic tools to gain further insight into breast cancer heterogeneity. However, most studies have sampled a given patient’s disease once, which provides only a snapshot of sub-clonal heterogeneity in treatment-naïve human cancers (89). However recent research using new approaches to analyze the genomic landscape of breast cancer, such as liquid biopsies, allowed an evolutionary analysis of cancer genomes in different periods of time and under different treatment-selective pressures.
A series of recent publications that reported the relatively frequent presence of mutations in the ligand-binding domain of the ESR1 gene in patients with hormone-resistant ER+ advanced breast cancer introduced new concepts for the understanding and modulation of endocrine resistance (90). Research analyzing circulating tumor DNA, which better captures tumoral heterogeneity, reported that ESR1 mutations are found in approximately one-third of patients with MBC that progressed on treatment with an AI (91,92). The use of liquid biopsies for assessing ESR1 mutations is a promising tool for the continued investigation of the clinical implications of these molecular alterations as a predictive biomarker, stratification factor and as a therapeutic target.
A variety of novel therapeutic agents such as PI3K inhibitors, HDAC inhibitors, and others in development will probably increase our therapeutic armamentarium in this patient population. Additionally, efforts to develop new endocrine agents with advantages in terms of antiestrogenic activity potency are ongoing and have led to the discovery and characterization of a new generation of SERDs (93,94) and a new class of SERM/SERD hybrids (95), some of which are now undergoing clinical evaluation.
Acknowledgements
Dr. Ellis received a Cancer Prevention Institute of Texas Established Investigator Award, A McNair Medical Institute Scholarship, and support from the Breast Cancer Research Foundation, The Susan G. Komen Foundation and The US National Cancer Institute.
Footnote
Conflicts of Interest: T Reinert reports research funding from AstraZeneca and speaker honoraria from AstraZeneca, Pfizer and Novartis. MJ Ellis reports ad hoc consulting fees from AstraZeneca, Pfizer, Abbvie, Xeno Pharmaceuticals, Puma and Novartis. J Bines reports consulting fees from Pfizer and travel expenses from AstraZeneca. The other authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. Available online: http://globocan.iarc.fr. 2012.
- Jemal A, Vineis P, Bray F, et al. The Cancer Atlas 2nd ed. Atlanta, GA: American Cancer Society, 2014.
- Reinert T, Barrios C. Optimal management of hormone receptor positive metastatic breast cancer in 2016. Ther Adv Med Oncol 2015;7:304-20. [Crossref] [PubMed]
- Ma CX, Reinert T, Chmielewska I, et al. Mechanisms of aromatase inhibitors resistance. Nat Rev Cancer 2015;15:261-75. [Crossref] [PubMed]
- Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma. Sugestion for a new method with illustrative cases. Lancet 1896;2:104-7. [Crossref]
- Network NCC. National Comprehensive Cancer Network Breast Cancer (Version 2.2015), Available online: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. September 12, 2015.
- Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:324-54. [Crossref] [PubMed]
- Breast Cancer Risk Reduction. National Comprehensive Cancer Network. NCCN Guidelines Version 1.2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf. Accessed August 31, 2017.
- Litherland S, Jackson IM. Antiestrogens in the management of hormone-dependent cancer. Cancer Treat Rev 1988;15:183-94. [Crossref] [PubMed]
- Lonning PE, Eikesdal HP. Aromatase inhibition: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer 2013;20:r183-201. [Crossref] [PubMed]
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med 2003;348:2431-42. [Crossref] [PubMed]
- (EBCTCG)† EBCTCG. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015;386:1341-52. [Crossref] [PubMed]
- Buzdar AU, Robertson JF, Eiermann W, et al. An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane. Cancer 2002;95:2006-16. [Crossref] [PubMed]
- Mauri D, Pavlidis N, Polyzos NP, et al. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst 2006;98:1285-91. [Crossref] [PubMed]
- Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: a prospective combined analysis of two multicenter trials. Cancer 2003;98:229-38. [Crossref] [PubMed]
- Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 2016;388:2997-3005. [Crossref]
- Faslodex receives US FDA approval as monotherapy for expanded use in breast cancer. Available online: https://www.astrazeneca.com/media-centre/press-releases/2017/faslodex-receives-us-fda-approval-as-monotherapy-for-expanded-use-in-breast-cancer.html. Accessed 10/24/2017.
- Suman VJ, Ellis MJ, Ma CX. The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol 2015;4:34. [PubMed]
- Bross PF, Cohen MH, Williams GA, et al. FDA drug approval summaries: fulvestrant. Oncologist 2002;7:477-80. [Crossref] [PubMed]
- Kuter I, Gee JM, Hegg R, et al. Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: results from NEWEST, a randomized Phase II study. Breast Cancer Res Treat 2012;133:237-46. [Crossref] [PubMed]
- Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol 2010;28:4594-600. [Crossref] [PubMed]
- Di Leo A, Jerusalem G, Petruzelka L, et al. Final overall survival: fulvestrant 500 mg vs. 250 mg in the randomized CONFIRM trial. J Natl Cancer Inst 2014;106. [Crossref] [PubMed]
- . Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021344s014lbl.pdfFaslodex (Fulvestrant Injection).
- Ellis MJ, Llombart-Cussac A, Feltl D, et al. Fulvestrant 500 mg Versus Anastrozole 1 mg for the First-Line Treatment of Advanced Breast Cancer: Overall Survival Analysis From the Phase II FIRST Study. J Clin Oncol 2015;33:3781-7. [Crossref] [PubMed]
- Abrams J, Aisner J, Cirrincione C, et al. Dose-response trial of megestrol acetate in advanced breast cancer: cancer and leukemia group B phase III study 8741. J Clin Oncol 1999;17:64. [Crossref] [PubMed]
- Bines J, Dietmsann R, Obadia RM, et al. Activity of megestrol acetate in postmenopausal women with advanced breast cancer after nonsteroidal aromatase inhibitor failure: a phase II trial†. Ann Oncol 2014;25:831-36. [Crossref] [PubMed]
- Coombes RC, Dearnley D, Humphreys J, et al. Danazol treatment of advanced breast cancer. Cancer Treat Rep 1980;64:1073. [PubMed]
- Manni A, Arafat BM, Pearson OH. Androgen-induced remissions after antiestrogen and hypophysectomy in stage IV breast cancer. Cancer 1981;48:2507. [Crossref] [PubMed]
- Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs. high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA 2009;302:774-80. [Crossref] [PubMed]
- Ingle JN, Ahmann DL, Green SJ, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med 1981;304:16. [Crossref] [PubMed]
- Iwase H, Yamamoto Y, Yamamoto-Ibusuki M, et al. Ethinylestradiol is beneficial for postmenopausal patients with heavily pre-treated metastatic breast cancer after prior aromatase inhibitor treatment: a prospective study. Br J Cancer 2013;109:1537. [Crossref] [PubMed]
- Mehta RS, Barlow WE, Albain KS, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 2012;367:435-44. [Crossref] [PubMed]
- Bergh J, Johnson PE, Lidbrink EK, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 2012;30:1919-25. [Crossref] [PubMed]
- Johnston SRD, Kilburn LS, Ellis P, et al. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 2013;14:989-98. [Crossref] [PubMed]
- Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) dagger. Ann Oncol 2014;25:1871-88. [Crossref] [PubMed]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233-47. [Crossref] [PubMed]
- Cruz M, Reinert T, Cristofanilli M. Emerging innovative therapeutic approaches leveraging cyclin-dependent kinase inhibitors to treat advanced breast cancer. Clin Pharmacol Ther 2018;103:1009-19. [Crossref] [PubMed]
- Rimawi M, Ferrero J, Haba-Rodriguez J, et al. Primary analysis of PERTAIN: A randomized, two-arm, multicenter phase II trial assessing the efficacy and safety of pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in first-line patients with HER-2 positive and hormone receptor-positive metastatic breast cancer. Proc SABCS 2016.
- Johnston SRD, Hegg R, Im S, et al. Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: ALTERNATIVE. J Clin Oncol 2018;36:741-8. [Crossref] [PubMed]
- Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 2009;27:5529-37. [Crossref]
- Martin LA, Andre F, Campone M, et al. mTOR inhibitors in advanced breast cancer: ready for prime time? Cancer Treat Rev 2013;39:742-52. [Crossref] [PubMed]
- deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res 2004;10:8059-67. [Crossref] [PubMed]
- Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem 2001;276:9817-24. [Crossref] [PubMed]
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9. [Crossref] [PubMed]
- Piccart M, Hortobagyi G, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol 2014;25:2357-62. [Crossref] [PubMed]
- Augereau P, Patsouris A, Bourbouloux E, et al. Hormonoresistance in advanced breast cancer: a new revolution in endocrine therapy. Ther Adv Med Oncol 2017;9:335-46. [Crossref] [PubMed]
- Schmid P, Zaiss M, Harper-Wynne C, et al. MANTA - a randomized phase II study of fulvestrant in combination with the dual mTOR inhibitor AZD2014 or everolimus or fulvestrant alone in estrogen receptor-positive advanced or metastatic breast cancer. Cancer Res 2018;78:Abstract GS2-07.
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:3111. [Crossref] [PubMed]
- Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref]
- Turner NC, Ro J, Andre F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 2015;373:209-19. [Crossref] [PubMed]
- Iwata H, Im SA, Masuda N, et al. PALOMA-3: Phase III Trial of Fulvestrant With or Without Palbociclib in Premenopausal and Postmenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer That Progressed on Prior Endocrine Therapy-Safety and Efficacy in Asian Patients. J Glob Oncol 2017;3:289-303. [Crossref] [PubMed]
- Ettl J, Harbeck N. The safety and efficacy of palbociclib in the treatment of metastatic breast cancer. Expert Rev Anticancer Ther 2017;17:661-8. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375:1738-48. [Crossref]
- Tripathy D, Sohn J, Im SA, et al. First-line ribociclib vs. placebo with goserelin and tamoxifen or a non-steroidal aromatase inhibitor in premenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer: Results from the randomized phase III MONALEESA-7 trial. SABCS Proceedings 2017.
- Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
- Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 2017;35:3638-46. [Crossref] [PubMed]
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin Cancer Res 2017;23:5218-24. [Crossref] [PubMed]
- Spring LM, Zangardi ML, Moy B, et al. Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations. Oncologist 2017;22:1039-48. [Crossref] [PubMed]
- Van Tine BA, Crowder RJ, Ellis MJ. ER. Cancer Discov 2011;1:287-8. [Crossref] [PubMed]
- Damaskos C, Grampis N, Valsami S, et al. Histone Deacetylase Inhibitors: An Attractive Therapeutic Strategy Against Breast Cancer. Anticancer Res 2017;37:35-46. [Crossref] [PubMed]
- Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized Phase II, Double-Blind, Placebo-Controlled Study of Exemestane With or Without Entinostat in Postmenopausal Women With Locally Recurrent or Metastatic Estrogen Receptor-Positive Breast Cancer Progressing on Treatment With a Nonsteroidal Aromatase Inhibitor. J Clin Oncol 2013;31:2128-35. [Crossref] [PubMed]
- Larsen SL, Duun-Henriksen AK, Duun-Henriksen AK, et al. SRC drives growth of antiestrogen resistant breast cancer cell lines and is a marker for reduced benefit of tamoxifen treatment. PLos One 2015;10. [Crossref] [PubMed]
- Barrios C, Forbes JF, Jonat W, et al. The sequential use of endocrine treatment for advanced breast cancer: where are we? Ann Oncol 2012;23:1378-86. [Crossref] [PubMed]
- Malorni L, Biganzoli L. Is There Still a Role for First-Line Single Agent Endocrine Therapy in HR+ and HER2– Advanced Breast Cancer? Breast Care 2017;12:288-9. [Crossref] [PubMed]
- Wolff AC. CDK4 and CDK6 Inhibition in Breast Cancer - A New Standard. N Engl J Med 2016;375:1993-4. [Crossref]
- Reinert T, Debiasi M, Bines J, et al. Trends in progression-free survival (PFS) and time to progression (TTP) over time within first-line aromatase inhibitors trials in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat 2018;168:457-65. [Crossref] [PubMed]
- Ellis M. Overcoming Endocrine Therapy Resistance by Signal Transduction Inhibition. Oncologist 2004;9:20-6. [Crossref] [PubMed]
- Chavarri-Guerra Y, Szymonifka J, Szymonifka J, et al. Drug withdrawal in women with progressive metastatic breast cancer while on aromatase inhibitor therapy. Br J Cancer 2014;111:2046-50. [Crossref] [PubMed]
- Buchanan RB, Blamey RW, Durrant KR, et al. A randomized comparison of tamoxifen with surgical oophorectomy in premenopausal patients with advanced breast cancer. J Clin Oncol 1986;4:1326-30. [Crossref] [PubMed]
- Boccardo F, Rubagotti A, Perrotta A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol 1994;5:337-42. [Crossref] [PubMed]
- Klijn JG, Blamey RW, Boccardo F, et al. Combined tamoxifen and luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist alone in premenopausal advanced breast cancer: a meta-analysis of four randomized trials. J Clin Oncol 2001;19:343-53. [PubMed]
- Klijn JG, Beex LV, Mauriac L, et al. Combined treatment with buserelin and tamoxifen in premenopausal metastatic breast cancer: a randomized study. J Natl Cancer Inst 2000;92:903-11. [Crossref] [PubMed]
- Park IH, Ro J, Lee KS, et al. Phase II parallel group study showing comparable efficacy between premenopausal metastatic breast cancer patients treated with letrozole plus goserelin and postmenopausal patients treated with letrozole alone as first-line hormone therapy. J Clin Oncol 2010;28:2705-11. [Crossref] [PubMed]
- Carlson RW, Schurman CM, Schurman CM, et al. Phase II Trial of Anastrozole Plus Goserelin in the Treatment of Hormone Receptor–Positive, Metastatic Carcinoma of the Breast in Premenopausal Women. J Clin Oncol 2010;28:3917-21. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Yap ML, Zubizarreta E, Bray F, et al. Global acces to radiotherapy services: have we made progress during the past decade? J Glob Oncol 2016;2:207-15. [Crossref] [PubMed]
- The selection and use of essential medicines. Report of the WHO Expert Committee.
- Prasad V, Kumar H, Mailandlody S. Ethics of clinical trials in low-resource settings: lessons from recent trials in cancer medicine. J Glob Oncol 2015;2:1-3. [Crossref] [PubMed]
- Chuang LT, Temin S, Camacho R, et al. Management and Care of Women With Invasive Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline. J Glob Oncol 2016;2:311-40. [Crossref] [PubMed]
- Lopes GJ, Souza JA, Barrios C. Acess to cancer medications in low- and middle- income countries. Nat Ver Clin Oncol 2013;10:314-22. [Crossref]
- Ismalla N, Salako O, Mutiu J, et al. Oncology Guidelines Usage in a Low- and Middle-Income Country. J Global Oncol 2018;4:1-6. [Crossref]
- Bazargani YT, de Boer A, Schellens JH, et al. Selection of oncology medicines in low- and middle-income countries. Ann Oncol 2014;25:270-76. [Crossref] [PubMed]
- Lee BL, Liedke PER, Barrios CH, et al. Breast cancer in Brazil: present status and future goals. Lancet Oncol 2012;13:e95-102. [Crossref] [PubMed]
- Liedke PER, Finkelstein DM, Szymonifka J, et al. Outcomes of breast cancer in Brazil related to health care coverage: a retrospective cohort study. Cancer Epidemiol Biomarkers Prev 2014;23:126-33. [Crossref] [PubMed]
- Hanahan D, Wieinberg R. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Helleday T, Eshtad S, Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet 2014;15:585-98. [Crossref] [PubMed]
- Venkatesan S, Swanton C, Taylor B, et al. Treatment-induced mutagenesis and selective pressures sculpt cancer evolution. Cold Spring Harb Perspect Med 2017;7. [Crossref] [PubMed]
- Reinert T, Saad ED, Barrios CH, et al. Clinical Implications of ESR1 Mutations in Hormone Receptor-Positive Advanced Breast Cancer. Front Oncol 2017;7:26. [Crossref] [PubMed]
- Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 2016;7:11579. [Crossref] [PubMed]
- Fribbens C, O'Leary B, Kilburn L, et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. J Clin Oncol 2016;34:2961-8. [Crossref] [PubMed]
- Weir HM, Bradbury RH, Lawson M, et al. AZD9496: An Oral Estrogen Receptor Inhibitor That Blocks the Growth of ER-Positive and ESR1- Mutant Breast Tumors in Preclinical Models. Cancer Res 2016;76:3307-18. [Crossref] [PubMed]
- Joseph JD, Darimont B, Zhou W, et al. The selective estrogen receptor downregulator GDC-0810 is efficacious in diverse models of ER+ breast cancer. eLife 2016;5. [Crossref]
- Wardell SE, Nelson ER, Chao CA, et al. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res 2013;19:2420-31. [Crossref] [PubMed]


