
Factors improving the outcome of patients re-irradiated with intensity-modulated radiotherapy (IMRT) for relapse or new head and neck cancer developed in irradiated areas
Introduction
Despite major improvements in the treatment of head and neck (H&N) cancers over the past few decades (1), patients with advanced squamous cell carcinomas (SCC) consistently recur in 10% to 50% of the cases (1-4). Complete surgical resection remains the standard of care, but for the patients unsuitable for complete surgery, therapeutic options or adjuvant treatments include radiotherapy, chemo-radiotherapy, chemotherapy alone and best supportive cares. Exclusive chemotherapy is typically performed with a palliative intent, and the median overall survival (OS) ranges from 5 to 9 months, while the 2-year OS rate is approximately 10% (5-7).
Re-irradiation (re-RT), alone or with concurrent chemotherapy (re-RT-CT), can be used as curative alternative with a 2-year OS rate ranging between 20% and 40% (8-10). However, the use of re-RT is traditionally restricted by fear of the late toxicity, limiting the number of re-irradiated patients and indicating that this treatment should be performed in patients with a low-risk of complications (11). Because the breakthrough of intensity-modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT) allowed a more conformal and targeted dose distribution, higher doses can be delivered with limited complication rates (10,12). To select patients who may benefit from re-RT, some studies identified prognostic factors related to the patient, the disease and the treatment management (3,13,14).
The aim of the present study was to evaluate the outcome of patients treated with curative-intent re-RT with IMRT for recurrent H&N cancers or new H&N cancers developed in previously irradiated areas, and to analyze the prognostic factors based on the literature.
Methods
Patients
We retrospectively reviewed the re-irradiated patients between 2007 and December 2015. Recurrences were considered as all diseases with the same pathological diagnosis than the primary previously diagnosed and treated H&N cancers, occurring in the same area or in corresponding elective lymph nodes, and diagnosed during the 5 years following the completion of the primary treatment course. Others cases were considered second primary cancers. The inclusions criteria were: (I) the first course of radiation was delivered after October 2003; (II) whatever the tumor site; (III) whatever the pathologic subtypes, except lymphomas; (IV) whatever the clinical stages; (V) the primary irradiation should be delivered by external beam radiotherapy; (VI) no total or partial brachytherapy for one or both treatments; (VII) a significant overlapping between the two irradiated volumes; (VIII) the dose of re-RT was at the level of a curative-intend treatment; (IX) exclusive or adjuvant re-RT /re-RT-CT were allowed, and (X) no minimal interval between the two courses of radiotherapy.
Treatments at recurrence or for new cancer in irradiated area
Surgery, chemotherapy
After maximal safe surgical resection without or with re-construction, postoperative re-RT was indicated for patients with node extracapsular extension, perineural invasion and close or positive surgical margins.
Radiation therapy
For the re-RT simulation, patients were immobilized in the treatment position by a thermoplastic mask, including the shoulders. A 2.5-mm slice CT, with an intravenous contrast injection, was performed. For improving delineation, this CT was matched with pre-/post-operative MRI and/or PET when available. The target volume comprised the gross tumor volume (GTV) for exclusive re-RT, or the tumor bed for the post-operative re-RT, expanded with a 0–10-mm isotropic margin to determine the high-risk clinical target volume (CTV). The CTV was corrected to the anatomical barriers free of tumor invasion. Pathological lymph nodes were included in the high-risk CTV. Prophylactic elective lymph nodes and adjacent structures to the high-risk CTV were included in intermediate and low-risk CTVs. The CTVs were expanded by 1 to 10 mm to create the respective planning target volumes (PTVs). Re-RT was performed by IMRT, using 6 MV beams from linear accelerators. Dose levels and integrated boost technic was at the radiation oncologist’s discretion.
Follow-up
During treatment, a physical examination was performed weekly to assess acute toxicities. After completion of radiotherapy, a radiation oncologist and a H&N surgeon clinically evaluated patients approximately every 3 months during the first 2 years, and every 6 months thereafter. A first baseline CT or MRI was performed 2–6 months after treatment and when clinically required. Acute and late toxicities were reported and scored using the Common Terminology Criteria for Adverse Events, version 4 (CTCAE v.4).
Statistics
Primary endpoints were the median actuarial OS, loco-regional progression-free survival (LRPFS), and progression-free survival (PFS). Survival was calculated from the last day of re-RT. The survival rates were calculated with the Kaplan-Meier method. A Cox proportional hazards model was used to determine the prognostic factors of OS, LRPFS, and PFS. Multivariate regression analysis was performed with stepwise variable selection. Kaplan-Meyer survival curves were compared by using a log-rank test. Comparisons between quantitative and qualitative variables were performed using Student’s, Spearman or Pearson correlation tests.
The study obtained institutional review board (n°011113), and participants gave informed consent for the use of their data.
Patient and treatments characteristics
Between 2007 and December 2015, 50 patients (sex ratio F/M: 1/6) who met the criteria were re-irradiated using IMRT.
First irradiation time
The characteristics of the primary disease are shown in Table 1. The median age at primary disease was 56.5 years (range, 39.0–80.0 years). Initial treatment included surgery for 37 patients (74%) and neoadjuvant, adjuvant, or concomitant chemotherapy for 26 patients (52%). The median dose of the first radiotherapy course was 66 Gy (range, 42.6–70 Gy) and was delivered with IMRT for 38% of the patients.
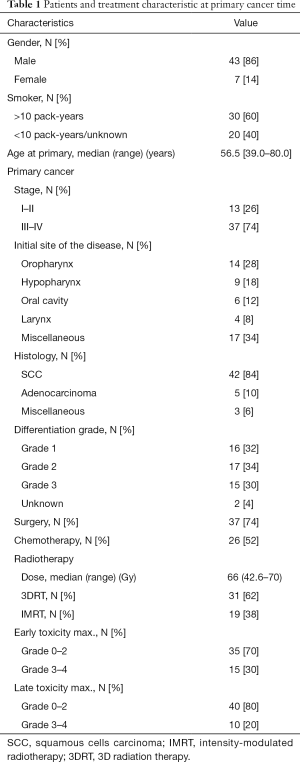
Full table
Second irradiation time
The characteristics of the re-irradiated recurrence or second primary are shown in Table 2. There were 40 recurrences (80%) and 10 new primaries (20%). Forty patients (80%) had a performance status (PS) of 0–1 at the time of re-treatment. Eighteen patients (36%) had only regional lymph node recurrence and 33 patients (66%) were stage III or IV. Surgical resection was performed in 40 patients (80%).
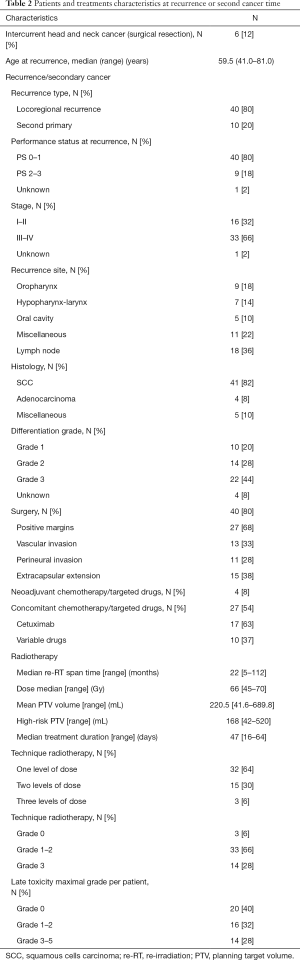
Full table
Four patients received a variable combination of neo-adjuvant chemotherapy or cetuximab. Concomitantly to re-RT, 17 patients received weekly cetuximab and ten patients received a variable combination of chemotherapy.
The median interval between the two courses of irradiation was 22 months (range, 5–112 months), 29 months for patients initially treated with 3-dimensional radiation therapy (3DRT) and 16 months for patients who received IMRT (P<0.001). The median re-RT dose was 66 Gy (range, 45–70 Gy), with a standard fractionation. The mean PTV was 220.5 mL (range, 41.6–689.8 mL).
Results
Median follow-up for surviving patients was 13.6 months (range, 0.8–68.5 months).
OS
The median OS, and 1- and 2-year OS rates were 15.7 months and 62.4% and 33.9%, respectively (Figure 1A). On univariate analysis, significant OS differences were shown for PS (P=0.011), re-RT interval (P=0.012), high-risk volume (P=0.025), re-RT duration time (P=0.033), and the technique of first course radiotherapy (P=0.036). On multivariate analysis, PS 0–1 (HR, 0.518; 95% CI: 0.292–0.917; P=0.024) and 3DRT use during the first irradiation course technique (HR, 0.415; 95% CI: 0.183–0.938; P=0.035) were favorable independent significant prognostic factors of OS.
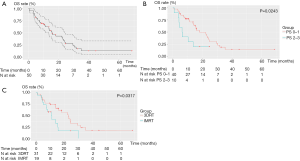
LRPFS
Twenty-seven patients developed local failure (54%). Among these patients, four individuals also had regional recurrence. Only one patient had an isolated regional failure. The median LRPFS and 1- and 2-year LRPFS rates were 8.3 months and 46.6% and 35.9%, respectively (Figure 1B). On univariate analysis, statistically significant differences were found for re-RT interval (P=0.004), re-RT dose (P=0.005), duration of re-RT (P=0.006), age (P=0.015) and, dose-level schedules (P=0.033). On multivariate analysis, a surgical resection before re-RT (HR, 0.107; 95% CI: 0.027–0.428; P=0.002), higher age (HR, 0.894; 95% CI: 0.833–0.960; P=0.002), PS 0–1 (HR, 0.316; 95% CI: 0.140–0.715; P=0.006) and long re-RT interval (HR, 0.970; 95% CI: 0.945–0.996; P=0.024) were favorable independent significant prognostic factors of LRPFS.
PFS
The median PFS and 1- and 2-year PFS rates were 7.0 months and 45.0% and 30.4%, respectively (Figure 1C). On univariate analysis, statistically significant differences were found for the re-RT interval (P=0.002), re-RT duration time (P=0.011) and, age (P=0.019).
On multivariate analysis, a surgical resection before re-RT (HR, 0.129; 95% CI: 0.036–0.466; P=0.002), a PS 0–1 (HR, 0.399; 95% CI: 0.208–0.764; P=0.006), and a long re-RT interval (HR, 0.958; 95% CI: 0.927–0.989; P=0.009) were favorable independent significant prognostic factors.
Toxicity
Fourteen patients (28%) experienced early grade 3 toxicities, dysphagia (n=11), mucositis (n=4), and one case of grade 3 dermatitis. No acute grade 4 or 5 early toxicity was reported (Table 2).
Late grade 3 toxicities appeared for 14 patients (28%), mainly dysphagia (n=7), xerostomia (n=3), soft tissues necrosis (n=3), trismus (n=2), osteoradionecrosis, hearing loss, fistula and dyspnea (one case for each complication). There were one grade 4 and two cases of grade 5 toxicities, following a carotid rupture and a laryngeal edema (Table 2). There was no correlation between the toxicity and interval between the both irradiations.
Discussion
In the present retrospective study, that included 50 patients, we reported a median OS of 15.7 months with a 2-year OS rate of 33.9%, consistent with published data under comparable conditions. Indeed, the median OS ranged from 9.6 to 27.6 months (3,13,15-17), with 2-year OS rates ranging from 32.0% to 67.4% (3,8,13,15-17). In the current study, the 2-year LRPFS and PFS rates were 35.9% and 30.4%, respectively. These figures were slightly below to those previously published, ranging from 37.5% to 65.8% for LRPFS (18) and from 38% to 59% for PFS (19-21). These discrepancies cannot be explained by differences with the published data in terms of (I) age of the patients, which ranged from 43 to 65 years (8,22); (II) rates of rT3 and rT4, which ranged from 52.9% to 84% (3,16,18,22-24); (III) rates of rN2 or rN3, which ranged from 3.7% to 44% (3,16,18,23,24); (IV) rates of the patients who underwent surgical resection, which ranged between 0 and 64% (15,16,18,21-25); (V) of median prescribed doses reported, which ranged from 47.5 to 70.7 Gy (8,13,15-18,20,22-24,26); (VI) volume definitions and margins from the GTV or post-operative bed to the PTV (17,18,20), and (VII) of patients receiving concomitant chemotherapy, which ranged from 20.2% to 100% in previous studies (3,15,16,20-24).
Some factors were no longer specified in most of publications, including the number of relapse and second primary cancers as well as the dose levels schedules and lymph nodes included in the irradiation fields. These factors influencing these results could explain the observed differences. Most of the patients of the current series were treated with TomoTherapy®, classically known to be a robust irradiation technique. The ability of this device to explain the difference of the results should be considered unlikely.
Finally, the most obvious difference between the present cohort and the published series was the mean interval between the two courses of irradiation, which was shorter than previously reported (25 to 49.5 months) (3,13,15,16,18,20-24). This interval is known to be prognostic for loco-regional control and OS (3,26,27) and could at least contribute to the observed difference.
Concerning prognostic factors, we found in multivariate analysis showed that a PS of 0–1 is prognostic for OS, LRPFS and PFS. The impact of a good global status on OS and local control was previously demonstrated in several studies (10,13,14,28). Riaz et al. re-irradiated 257 patients using mostly IMRT at a median dose of 60 Gy with standard fractionation and found an improvement of OS and a difference of local control when the Karnofsky Performance Status (KPS) was >80% (HR, 0.41; P<0.001 and HR, 0.36; P<0.0001, respectively) (13). Salama et al., in a meta-analysis of 115 patients re-irradiated with concomitant chemotherapy, found that a PS of 0–1 was associated with a 3-year OS and PFS rates of 28% and 38%, respectively, whereas these rates were 16% and 21%, respectively for patients with a PS of 2–3 (P=0.04) (14).
We showed that 3DRT at the first treatment was a favorable prognostic factor for OS. Three reasons could explain this observation: first, initially, only 38% of the patients were treated with IMRT; initially, the efficacy of IMRT was perhaps not optimal because of the learning curve and finally, patients who were treated initially with 3DRT had a longer interval between both irradiations than those treated with IMRT. Even if IMRT had demonstrated better target volume coverage than 3DRT (29), a small error in contouring will lead to under-dosing of some parts of the target volume, likely due to of relapse (30).
Surgical resection was associated with an improvement of LRPFS and PFS in multivariate analysis in the present series, which is consistent with the meta-analysis of Salama et al. (14) in which the 3-year LRPFS rate was 51% for patients who underwent surgery compared to 19% for the non-surgical patients (P=0.00005). Recently, this factor was confirmed by several authors (31) in larger series (32) and was introduced in a recursive partitioning analysis (33).
A short interval between the two courses of irradiation was an independent unfavorable prognostic factor for the LRPFS and the PFS. Several published series have highlighted the impact of this factor on the OS rate and loco-regional control rate (3,13,26). In the series of Duprez et al. re-irradiated 84 patients with IMRT, and showed that the disease-free survival rates and the OS rates were significantly higher when the interval was longer than 2 years (3). Hoebers et al., in a series of 58 patients, found an improvement of LCPFS for patients who developed a relapse more than 3 years after the first irradiation (HR, 0.43; P=0.036) (26). The reason for this improvement is not clear, but could be linked to the recovering of the damaged tissue over time after the first irradiation or an improved radiosensitivity of the tumor due to less hypoxia with time.
In the present series some previously indicated prognostic factors were not confirmed. Indeed, the prescribed dose was not significantly associated with LRPFS, although this factor was often indicated previously (13,15,17,34,35). The explanation for this finding is obviously the level of the doses since the median dose was 66 Gy and only four patients received less than 50 Gy, although the previously published dose thresholds were 50 Gy (13,17) or 60 Gy (15,34). A second reason is the potential confusion between the actual delivered dose and the prescribed dose, which limits the relevance of this parameter. Thirdly, the number of levels of dose could be a surrogate for the dose in the present series, and in this case, this factor was showed as a prognostic factor of LRPFS in univariate analysis. To our knowledge, this study is the first to indicate this factor, likely because the use of this technique remains rare in cases of re-RT.
Additionally, we did not show that high-risk GTV was a prognostic factor for any survival. By contrast, Tian et al., in a series of 60 patients with re-irradiated nasopharyngeal cancer, showed a significant improvement of OS when the GTV was smaller than 20 mL (22). Three factors could explain our conclusion: first, the current median high-risk volume was higher at 150 mL, second, the current primitive tumor distribution was more variable, and finally, Tian et al showed a relationship between the tumor volume, complications and, finally cause of death (22), a relationship that we did not observe.
In contrast to previously published data (23), in which young age was typically associated with improved LRPFS, we found that an older age was correlated with LRPFS improvement. The reasons for this difference can be assumed, in the present series, to be a trend toward a correlation between a large interval to re-RT and a higher age (P=0.0695). We showed a highly significance correlation between the interval and LRPFS, and there was a low rate of HPV-positive patients in the present series. In the previously published series, the number of HPV-positive cases was potentially higher. However, the published data were not sufficiently specified to demonstrate this assumption.
Conclusions
The present series showed that IMRT improved OS. Selection of patients is fundamental to obtain the best results. An interval between the both irradiation times longer than 24 months is required. Surgical patients with a good PS were obviously those who could expect the best results. Notably, additional prospective trials are urgently needed to improve the indications and results of patients with local H&N relapses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by Paul Strauss Institutional Review Board (No. n°011113), and participants gave informed consent for the use of their data.
References
- Blanchard P, Baujat B, Holostenco V, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother Oncol 2011;100:33-40. [Crossref] [PubMed]
- Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2012;84:1198-205. [Crossref] [PubMed]
- Duprez F, Madani I, Bonte K, et al. Intensity-modulated radiotherapy for recurrent and second primary head and neck cancer in previously irradiated territory. Radiother Oncol 2009;93:563-9. [Crossref] [PubMed]
- Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys 2012;82:291-8. [Crossref] [PubMed]
- Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial. J Clin Oncol 2013;31:1405-14. [Crossref] [PubMed]
- Bourhis J, Rivera F, Mesia R, et al. Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 2006;24:2866-72. [Crossref] [PubMed]
- Cacicedo J, Navarro A, Alongi F, et al. The role of re-irradiation of secondary and recurrent head and neck carcinomas. Is it a potentially curative treatment? A practical approach. Cancer Treat Rev 2014;40:178-89. [Crossref] [PubMed]
- Chen AM, Farwell DG, Luu Q, et al. Prospective trial of high-dose reirradiation using daily image guidance with intensity-modulated radiotherapy for recurrent and second primary head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011;80:669-76. [Crossref] [PubMed]
- Comet B, Kramar A, Faivre-Pierret M, et al. Salvage stereotactic reirradiation with or without cetuximab for locally recurrent head-and-neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys 2012;84:203-9. [Crossref] [PubMed]
- Cvek J, Knybel L, Skacelikova E, et al. Hyperfractionated stereotactic reirradiation for recurrent head and neck cancer. Strahlenther Onkol 2016;192:40-6. [Crossref] [PubMed]
- Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol 2008;26:5518-23. [Crossref] [PubMed]
- Lartigau EF, Tresch E, Thariat J, et al. Multi institutional phase II study of concomitant stereotactic reirradiation and cetuximab for recurrent head and neck cancer. Radiother Oncol 2013;109:281-5. [Crossref] [PubMed]
- Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol 2014;111:382-7. [Crossref] [PubMed]
- Salama JK, Vokes EE, Chmura SJ, et al. Long-term outcome of concurrent chemotherapy and reirradiation for recurrent and second primary head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2006;64:382-91. [Crossref] [PubMed]
- Curtis KK, Ross HJ, Garrett AL, et al. Outcomes of patients with loco-regionally recurrent or new primary squamous cell carcinomas of the head and neck treated with curative intent reirradiation at Mayo Clinic. Radiat Oncol 2016;11:55. [Crossref] [PubMed]
- Duprez F, Berwouts D, Madani I, et al. High-dose reirradiation with intensity-modulated radiotherapy for recurrent head-and-neck cancer: disease control, survival and toxicity. Radiother Oncol 2014;111:388-92. [Crossref] [PubMed]
- Roeder F, Zwicker F, Saleh-Ebrahimi L, et al. Intensity modulated or fractionated stereotactic reirradiation in patients with recurrent nasopharyngeal cancer. Radiat Oncol 2011;6:22. [Crossref] [PubMed]
- Jeong S, Yoo EJ, Kim JY, et al. Re-irradiation of unresectable recurrent head and neck cancer: using Helical Tomotherapy as image-guided intensity-modulated radiotherapy. Radiat Oncol J 2013;31:206-15. [Crossref] [PubMed]
- Biagioli MC, Harvey M, Roman E, et al. Intensity-modulated radiotherapy with concurrent chemotherapy for previously irradiated, recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 2007;69:1067-73. [Crossref] [PubMed]
- Sher DJ, Haddad RI, Norris CM Jr, et al. Efficacy and toxicity of reirradiation using intensity-modulated radiotherapy for recurrent or second primary head and neck cancer. Cancer 2010;116:4761-8. [Crossref] [PubMed]
- Sulman EP, Schwartz DL, Le TT, et al. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys 2009;73:399-409. [Crossref] [PubMed]
- Tian YM, Guan Y, Xiao WW, et al. Long-term survival and late complications in intensity-modulated radiotherapy of locally recurrent T1 to T2 nasopharyngeal carcinoma. Head Neck 2016;38:225-31. [Crossref] [PubMed]
- Han F, Zhao C, Huang SM, et al. Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:569-76. [Crossref] [PubMed]
- Zwicker F, Roeder F, Hauswald H, et al. Reirradiation with intensity-modulated radiotherapy in recurrent head and neck cancer. Head Neck 2011;33:1695-702. [Crossref] [PubMed]
- Chen L, Nguyen TB, Jones E, et al. Magnetic resonance-based treatment planning for prostate intensity-modulated radiotherapy: creation of digitally reconstructed radiographs. Int J Radiat Oncol Biol Phys 2007;68:903-11. [Crossref] [PubMed]
- Hoebers F, Heemsbergen W, Moor S, et al. Reirradiation for head-and-neck cancer: delicate balance between effectiveness and toxicity. Int J Radiat Oncol Biol Phys 2011;81:e111-8. [Crossref] [PubMed]
- Kress MA, Sen N, Unger KR, et al. Safety and efficacy of hypofractionated stereotactic body reirradiation in head and neck cancer: Long-term follow-up of a large series. Head Neck 2015;37:1403-9. [Crossref] [PubMed]
- Schaefer U, Micke O, Schueller P, et al. Recurrent head and neck cancer: retreatment of previously irradiated areas with combined chemotherapy and radiation therapy-results of a prospective study. Radiology 2000;216:371-6. [Crossref] [PubMed]
- Huang D, Xia P, Akazawa P, et al. Comparison of treatment plans using intensity-modulated radiotherapy and three-dimensional conformal radiotherapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys 2003;56:158-68. [Crossref] [PubMed]
- Giraud P, Jaulerry C, Brunin F, et al. Upper aerodigestive tract cancers: clinical benefits of conformal radiotherapy and intensity modulation. Cancer Radiother 2002;6 Suppl 1:37s-48s. [Crossref] [PubMed]
- Ahlawat P, Rawat S, Kakria A, et al. Reirradiation with IMRT for recurrent head and neck cancer: A single-institutional report on disease control, survival, and toxicity. Rep Pract Oncol Radiother 2017;22:331-9. [Crossref] [PubMed]
- Chang JH, Wu CC, Yuan KS, et al. Locoregionally recurrent head and neck squamous cell carcinoma: incidence, survival, prognostic factors, and treatment outcomes. Oncotarget 2017;8:55600-12. [PubMed]
- Ward MC, Riaz N, Caudell JJ, et al. Refining Patient Selection for Reirradiation of Head and Neck Squamous Carcinoma in the IMRT Era: A Multi-Institution Cohort Study by the MIRI Collaborative. Int J Radiat Oncol Biol Phys 2018;100:586-94. [Crossref] [PubMed]
- Platteaux N, Dirix P, Vanstraelen B, et al. Outcome after re-irradiation of head and neck cancer patients. Strahlenther Onkol 2011;187:23-31. [Crossref] [PubMed]
- Watkins JM, Shirai KS, Wahlquist AE, et al. Toxicity and survival outcomes of hyperfractionated split-course reirradiation and daily concurrent chemotherapy in locoregionally recurrent, previously irradiated head and neck cancers. Head Neck 2009;31:493-502. [Crossref] [PubMed]


