
CD30+ T-cell lymphoproliferative disorders
Introduction
The cutaneous T-cell lymphomas (CTCL) are a diverse group of non-Hodgkin’s lymphomas characterized by accumulation of clonal T-cells within the skin. CD30+ primary cutaneous lymphoproliferative disorders represent the second most common type of CTCL and include the diseases lymphomatoid papulosis (LyP), primary cutaneous anaplastic large-cell lymphoma (PC-ALCL) and borderline cases (1). These diseases are characterized by immunophenotypic expression of the CD30 surface antigen, a cytokine receptor within the tumor necrosis factor family (2). Both LyP and PC-ALCL carry an excellent prognosis and share similar histologic and immunophenotypic features, so both clinical presentation and disease course remain important for correct diagnosis and avoidance of excessive or unnecessary therapy (3).
LyP
LyP is characterized by self-healing recurrent papulonodular skin eruptions that typically evolve and regress over the course of a few weeks (4). The lesions may ulcerate or become hemorrhagic or necrotic. The peak incidence occurs in the fifth decade, and there is a male predominance with a male-to-female ratio of 2–3:1 (5,6). LyP is associated with an increased lifetime risk for development of systemic lymphoma or other lymphoproliferative disorders, which can be seen in approximately 20% of cases (6-8). Mycosis fungoides (MF) is the most commonly associated lymphoma seen with LyP. Of note, LyP can co-exist in the same patient with other CTCL, such as MF.
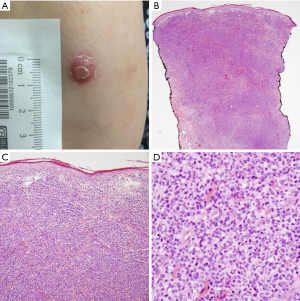
Six major histopathologic subtypes of LyP have been described, Types A-E and LyP with 6p25.3 rearrangement (Table 1) (9). Type A is the most common, comprising approximately 75% of all cases of LyP (3). It is characterized by large CD30+ lymphocytes (resembling Reed-Sternberg cells) that are surrounded by an inflammatory background of neutrophils, eosinophils and histiocytes. Type B is rare (<5% of cases) and consists of small CD30+ or CD30− atypical lymphocytes in a predominately epidermotropic pattern, which closely resemble the malignant cell of mycoses fungoides. Type C demonstrates sheets of large CD30+ cells with only minimal inflammatory infiltrate (Figure 1). Type D involves epidermotropic CD8+ CD30+ T-lymphocytes that resemble primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, although these lesions behave like LyP. Type E involves small to medium-sized CD8+ CD30+ pleomorphic lymphocytes that are angiodestructive and frequently ulcerate (10). Finally, LyP with 6p25.3 rearrangement is characterized by the weakly CD30+ small to medium-sized T-cells with cerebriform nuclei and strongly CD30+ larger blast cells in the dermis (11). Histologically, this latter subtype appears quite aggressive, but lesions spontaneously regress, which is characteristic of LyP. Patients with LyP with 6p25.3 rearrangement are often older than other subtypes. It should be noted that patients can present with more than one histologic subtype (12).

Full table
Clonal T-cell receptors can be detected in approximately 84% of cutaneous lesions and 42% of blood among patients with LyP (13). Unlike systemic ALCL, the t(2;5) translocation is not typically seen in LyP (14). As mentioned above, a small subset of LyP is characterized by chromosomal rearrangements of the DUSP22-IRF4 locus on 6p25.3. This mutation is not specific to LyP and has been found in both systemic ALCL and PC-ALCL and is associated with a good prognosis in the former entity (15).
Although the lesions of LyP may recur, they are largely characterized by spontaneous regression without medical intervention. The precise etiology of this regression is largely unknown, but increased apoptosis is thought to play a role. The pro-apoptotic protein, Bax, has been shown to be increased in the skin lesions from LyP and PC-ALCL, but not in the skin lesions from systemic lymphomas with secondary cutaneous involvement, which do not undergo spontaneous regression and often display an aggressive clinical course (16). The apoptotic index of these CD30+ lymphoproliferative diseases is also higher than their systemic counterparts.
LyP tends to have a chronic course, and an expectant observational approach may be appropriate for many patients. In patients with asymptomatic limited disease, observation is generally recommended (17). Moreover, systemic treatment has not been shown to alter the course of the disease or decrease the risk of development of systemic lymphoma, so systemic treatments must be carefully weighed against the possibility of toxicities (1). Topical steroids may be useful for amelioration of local symptoms, such as pruritus. Topical nitrogen mustards, such as mechlorethamine, can also be utilized (17). However, in patients with extensive skin involvement or lesions on cosmetically sensitive areas, such as the face or hands, disease-directed systemic therapy may be beneficial.
Methotrexate is generally recommended as an appropriate initial therapy for patients who may require systemic treatment. In a series among 45 patients with LyP, treatment with methotrexate provided long-term control in 87% of patients (18). Doses varied between 10 and 60 mg per week. Clinical improvement was generally noted at doses of 15–20 mg per week. Ten patients discontinued methotrexate but still had over 24 months free of disease recurrence. The most commonly observed toxicities in included fatigue (47%), increased serum hepatic transaminase levels (27%), and nausea (22%).
Psoralen and ultraviolet A (PUVA) therapy can be considered in patients refractory to methotrexate or those for which methotrexate is contraindicated, although prospective data is lacking. In a review of 19 patients with LyP treated with PUVA, 68% experienced partial response (PR), and 26% experienced complete response (CR) (1). All patients relapsed shortly after cessation of treatment. Multi-agent systemic chemotherapy is generally avoided due to high relapse rate seen with LyP.
Brentuximab vedotin (BV) is a monoclonal antibody directed against CD30 that is conjugated with the microtubule toxin, monomethyl auristatin E. In a phase II study among patients with CD30+ primary cutaneous lymphoproliferative disorders, nine patients with histologically-confirmed LyP were included (19). The overall response rate (ORR) was 100%, with five patients achieving a CR and four patients demonstrating a PR. The most commonly observed toxicities were peripheral neuropathy (67%), fatigue (35%), and rash (24%). Currently, BV is not approved by the Food and Drug Administration (FDA) for LyP.
PC-ALCL
PC-ALCL is clinically characterized by a solitary tumor or grouped nodules, generally with rapid growth and possible ulceration (3). The extremities and head/neck areas are commonly involved with approximately 20% of patients demonstrating multifocal disease. The vast majority of patients present with skin-only disease, but approximately 10% present with extracutaneous disease (20). In contrast to LyP, spontaneous regression of the skin lesions is seen in only 25–40% of cases (3,21). Following any regression, disease recurrence is common. PC-ALCL typically occurs in adults in their sixth decade of life, although cases in children have been reported, with a male predominance (again with a male to female ratio of 2–3:1) (3). PC-ALCL is also a common type of CTCL seen in individuals with human immunodeficiency virus (HIV) infection (22). No prior history or clinical features of MF should be described, as a diagnosis of MF with large cell transformation would be more likely. Distinction from systemic ALCL is also necessary for proper diagnosis, prognosis and treatment.
Pathologically, PC-ALCL is defined by a nodular infiltrate consisting of sheets of large T-cells with an anaplastic, pleomorphic or immunoblastic morphology (9). By definition, at last 75% of the malignant cells should express the CD30 antigen. Epidermotropism may be present. In a majority of cases, neutrophils and eosinophils are rare, although there is a neutrophil-rich variant, which may clinically present with pyogenic features (23). The immunophenotype of the malignant cells is often consistent with an activated CD4+ T-cell, although CD4-/CD8+, CD4+/CD8+ and null-cell phenotypes have been observed (9).
TCR clonality has been observed in nearly 90% of all cases of PC-ALCL (24). The t(2;5) gene rearrangement involving the ALK gene is an exceedingly rare event, compared to nearly 60% of all systemic ALCL, which can help differentiate the two entities in ambiguous cases (14,25). In one series, rearrangements of the IRF4-DUSP22 locus on 6p25.3 were noted in 26% of PC-ALCL cases (26). In large multicenter study, fluorescence in situ hybridization (FISH) testing for IRF4 translocations has a sensitivity and positive predictive value for PC-ALCL of 99% and 90%, respectively (27).
Initial treatment for localized disease, including solitary or grouped lesions, often consists of surgery or localized radiation to the involved areas. In one retrospective study, 95% of patients with solitary skin lesions achieved a CR following surgical excision or radiation, although the recurrence rate following these therapies is approximately 40% (20). If recurrent disease remains localized, repetitive excision or radiation therapy could again be considered, although the morbidity of repetitive local therapy should be considered.
For individuals with widespread lesions, systemic therapy may be indicated. Oral weekly methotrexate is again a commonly-employed therapeutic option. In one study, the ORR to methotrexate was 77% (18). Oral bexarotene is another potential option, although data regarding its effectiveness for PC-ALCL appears to be limited to case reports (28). Multi-agent cytotoxic chemotherapy, such as the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) has been utilized before with response rates approaching 90% (1,20). Unfortunately, relapse rates are high, and combination therapy has not been proven to be beneficial compared to single-agent treatment.
BV has been shown to be effective for PC-ALCL and is currently approved by the FDA for relapsed disease. The ALCANZA trial was a phase 3 study that randomized patients with previously-treated CD30+ CTCL, including 31 patients with PC-ALCL, to receive BV or physician’s choice (either methotrexate or bexarotene) (29). The ORR was 75% in patients treated with BV versus 33% for those in the physician’s choice arm. Among patients with PC-ALCL in the BV arm, 33% achieved a CR. Peripheral neuropathy was a notable toxicity in the BV arm, which occurred in 67% of patients.
In patients with nodal or visceral involvement, single-agent therapy is again often preferred (1). BV has been shown to be active in this setting (29). Other agents that have shown activity in CTCL, including PC-ALCL, are gemcitabine, romidepsin and pralatrexate, among others (30-32). Aside from BV, as described above, these agents have not been directly compared against each other. Radiation therapy can be considered for those patients with nodal disease in select cases or for consolidation (17).
In general, the prognosis of PC-ALCL is good, with a 5-year overall survival (OS) of 75–90% (3,20,33). Patients with extensive limb disease, which is defined as multiple skin tumors within one limb or contiguous body regions, have a poorer prognosis with a 5-year OS of 50% (33). Other factors for poor OS include older age and progression to extracutaneous disease.
Borderline cases
Borderline cases refer to cutaneous lesions for which a distinction between LyP and PC-ALCL is not possible based on clinicopathologic presentation. For example, a patient may present with a recurrent, self-healing skin eruption (classically associated with LyP), but with histopathologic features more characteristic for PC-ALCL. The clinical course over time may be necessary to truly draw a distinction between borderline lesions.
Primary mucosal CD30+ T-cell lymphoproliferations
CD30+ T-cell lymphoproliferative disorders have been found at mucosal sites within the head and neck, particularly the oral mucosa. Primary mucosal CD30+ T-cell lymphoproliferative disorders (PM-LPD) are now considered a distinct clinicopathologic entity with a median age at diagnosis of approximately 54 years with a slight male predominance (34). Concurrent cutaneous lesions can occur (35). The majority of patients present with a nodule or ulcer within the oral cavity.
Histologically, the PM-LPD appears similar to PC-ALCL (34,36). The malignant cells are uniformly CD30+, often with irregular, eccentric nuclei. An inflammatory infiltrate of neutrophils and/or eosinophils is often present. In a series of 14 patients, nine received systemic therapy with CHOP, two received external radiation therapy, and three received no adjuvant therapy following resection (34). After a median follow-up of 38 months, all patients were alive and without disease recurrence.
Breast-implant associated anaplastic large cell lymphoma (BIA-ALCL)
Primary breast lymphomas are rare disorders and are mostly of B-cell origin (37,38). BIA-ALCL is a rare T-cell lymphoma that arises around breast implants and was first described in 1997, which presented as mass involving the capsule surrounding the saline-filled implant (39). Since then, numerous cases have been described and represent approximately 6% of all primary breast lymphomas (37,40). The median age at time of presentation appears to be approximately 50 years, with a mean time of diagnosis nearly 10 years after initial placement of the implant (41,42). Patients often present with breast enlargement, pain, lymphadenopathy, or a palpable mass or periprosthetic fluid collection.
The pathophysiology of BIA-ALCL is largely unknown, but chronic inflammation is hypothesized to play a role in its pathogenesis. Inflammation has been shown to play a role in development of ectopic lymphoid tissue, especially in the setting of certain chronic infections, such as Helicobacter pylori and hepatitis C virus, which can also lead to development of lymphoma (43,44). Animal models have demonstrated that textured breast implants have been associated with significantly more biofilm and lymphocyte infiltration within the surrounding fibrous capsule compared to smooth implants (45). Nearly all cases of BIA-ALCL have been associated with textured implants (41,46). The majority of patients have disease confirmed within the fibrous capsule, although some patients will present with a distinct tumor mass (47).
Diagnosis is confirmed by biopsy of the tumor mass or aspiration of the periprosthetic effusion. Pathology specimens demonstrate large pleomorphic lymphocytes with abundant cytoplasm. Immunohistochemically, the malignant cells stain diffusely positive for CD30 and have a T-cell immunophenotype. TCR gene rearrangement studies generally indicate monoclonality (40,47). ALK is not typically expressed. Activating mutations in STAT3 and JAK1 have been reported (48). In a review series of 36 cases of BIA-ALCL, none demonstrated overexpression of ALK or rearrangements of DUSP22 or TP53, which are all reported in systemic ALCL (49). In addition, 100% of evaluable cases demonstrated pSTAT3 expression by immunohistochemistry with activating mutations in the JAK-STAT pathway confirmed in several of these cases. This pathologic consistency among cases of BIA-ALCL is in contrast to the heterogeneity seen in systemic ALCL.
Once a diagnosis of BIA-ALCL is established, complete staging is recommended with PET/CT, mainly to determine whether disease is limited to the breast and capsule, rather than more disseminated disease. Staging may also useful to help distinguish BIA-ALCL from systemic (nodal) ALCL with breast involvement. The TNM staging system is generally used (17).
Regardless of stage, initial treatment involves surgical removal of the capsule and implant with consideration of removal of the contralateral breast implant, as nearly 5% of patient have been found to have contralateral disease (46). Excision of suspicious lymph nodes is recommended, but definite axillary node removal is not always necessary (17). Patients who achieve a complete resection from surgery or have disease confined to the capsule have improved OS compared to those who do not (46,50).
For patients with disease that extends beyond the capsule (stage II or higher), adjuvant systemic therapy is recommended (17). As seen in systemic ALCL, anthracycline-based chemotherapy, such as CHOP or CHOEP (CHOP with addition of etoposide), remains the cornerstone for therapy. BV and radiation therapy may be useful in certain circumstances, but data are lacking (51). Overall, prognosis remains good with prompt diagnosis and aggressive surgical management.
Conclusions
CD30+ T-cell LPD represent a spectrum of heterogenous disorders, in which both clinical and pathological phenotype equally inform the diagnosis. The standardization of diagnosis and treatment has been impeded by the relative rarity of these disorders and the lack of prospective data. Treatment paradigms generally follow the same principles as for other CTCL, although the uniform expression of the cytokine receptor CD30 offers a target for CD30-directed therapies. Genetic and molecular profiling has helped shed some light on pathobiology, but further work is needed to gain more insights to help optimize diagnosis and therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood 2011;118:4024-35. [Crossref] [PubMed]
- Durkop H, Latza U, Hummel M, et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell 1992;68:421-7. [Crossref] [PubMed]
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood 2000;95:3653-61. [PubMed]
- Macaulay WL. Lymphomatoid papulosis. A continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol 1968;97:23-30. [Crossref] [PubMed]
- Assaf C, Gellrich S, Steinhoff M, et al. Cutaneous lymphomas in Germany: an analysis of the Central Cutaneous Lymphoma Registry of the German Society of Dermatology (DDG). J Dtsch Dermatol Ges 2007;5:662-8. [Crossref] [PubMed]
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol 2009;34:576-81. [Crossref] [PubMed]
- Wang HH, Myers T, Lach LJ, et al. Increased risk of lymphoid and nonlymphoid malignancies in patients with lymphomatoid papulosis. Cancer 1999;86:1240-5. [Crossref] [PubMed]
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: Treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol 2016;74:59-67. [Crossref] [PubMed]
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [Crossref] [PubMed]
- Kempf W, Kazakov DV, Scharer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol 2013;37:1-13. [Crossref] [PubMed]
- Karai LJ, Kadin ME, Hsi ED, et al. Chromosomal rearrangements of 6p25.3 define a new subtype of lymphomatoid papulosis. Am J Surg Pathol 2013;37:1173-81. [Crossref] [PubMed]
- El Shabrawi-Caelen L, Kerl H, Cerroni L. Lymphomatoid papulosis: reappraisal of clinicopathologic presentation and classification into subtypes A, B, and C. Arch Dermatol 2004;140:441-7. [Crossref] [PubMed]
- Humme D, Lukowsky A, Steinhoff M, et al. Dominance of nonmalignant T-cell clones and distortion of the TCR repertoire in the peripheral blood of patients with cutaneous CD30+ lymphoproliferative disorders. J Invest Dermatol 2009;129:89-98. [Crossref] [PubMed]
- DeCoteau JF, Butmarc JR, Kinney MC, et al. The t(2;5) chromosomal translocation is not a common feature of primary cutaneous CD30+ lymphoproliferative disorders: comparison with anaplastic large-cell lymphoma of nodal origin. Blood 1996;87:3437-41. [PubMed]
- Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014;124:1473-80. [Crossref] [PubMed]
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol 2005;14:380-5. [Crossref] [PubMed]
- National Comprehensive Cancer Network. T-cell Lymphomas (Version 4.2018). Available online: https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf, accessed date: July 8, 2018.
- Vonderheid EC, Sajjadian A, Kadin ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J Am Acad Dermatol 1996;34:470-81. [Crossref] [PubMed]
- Duvic M, Tetzlaff MT, Gangar P, et al. Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis. J Clin Oncol 2015;33:3759-65. [Crossref] [PubMed]
- Liu HL, Hoppe RT, Kohler S, et al. CD30+ cutaneous lymphoproliferative disorders: the Stanford experience in lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma. J Am Acad Dermatol 2003;49:1049-58. [Crossref] [PubMed]
- Kadin ME, Carpenter C. Systemic and primary cutaneous anaplastic large cell lymphomas. Semin Hematol 2003;40:244-56. [Crossref] [PubMed]
- Kerschmann RL, Berger TG, Weiss LM, et al. Cutaneous presentations of lymphoma in human immunodeficiency virus disease. Predominance of T cell lineage. Arch Dermatol 1995;131:1281-8. [Crossref] [PubMed]
- Burg G, Kempf W, Kazakov DV, et al. Pyogenic lymphoma of the skin: a peculiar variant of primary cutaneous neutrophil-rich CD30+ anaplastic large-cell lymphoma. Clinicopathological study of four cases and review of the literature. Br J Dermatol 2003;148:580-6. [Crossref] [PubMed]
- Macgrogan G, Vergier B, Dubus P, et al. CD30-positive cutaneous large cell lymphomas. A comparative study of clinicopathologic and molecular features of 16 cases. Am J Clin Pathol 1996;105:440-50. [Crossref] [PubMed]
- Herbst H, Sander C, Tronnier M, et al. Absence of anaplastic lymphoma kinase (ALK) and Epstein-Barr virus gene products in primary cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis. Br J Dermatol 1997;137:680-6. [Crossref] [PubMed]
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol 2010;130:816-25. [Crossref] [PubMed]
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol 2011;24:596-605. [Crossref] [PubMed]
- French LE, Shapiro M, Junkins-Hopkins JM, et al. Regression of multifocal, skin-restricted, CD30-positive large T-cell lymphoma with interferon alfa and bexarotene therapy. J Am Acad Dermatol 2001;45:914-8. [Crossref] [PubMed]
- Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet 2017;390:555-66. [Crossref] [PubMed]
- Duvic M, Talpur R, Wen S, et al. Phase II evaluation of gemcitabine monotherapy for cutaneous T-cell lymphoma. Clin Lymphoma Myeloma 2006;7:51-8. [Crossref] [PubMed]
- Horwitz SM, Kim YH, Foss F, et al. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood 2012;119:4115-22. [Crossref] [PubMed]
- Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol 2010;28:4485-91. [Crossref] [PubMed]
- Woo DK, Jones CR, Vanoli-Storz MN, et al. Prognostic factors in primary cutaneous anaplastic large cell lymphoma: characterization of clinical subset with worse outcome. Arch Dermatol 2009;145:667-74. [Crossref] [PubMed]
- Wang W, Cai Y, Sheng W, et al. The spectrum of primary mucosal CD30-positive T-cell lymphoproliferative disorders of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117:96-104. [Crossref] [PubMed]
- Alobeid B, Pan LX, Milligan L, et al. Eosinophil-rich CD30+ lymphoproliferative disorder of the oral mucosa. A form of "traumatic eosinophilic granuloma". Am J Clin Pathol 2004;121:43-50. [Crossref] [PubMed]
- Sciallis AP, Law ME, Inwards DJ, et al. Mucosal CD30-positive T-cell lymphoproliferations of the head and neck show a clinicopathologic spectrum similar to cutaneous CD30-positive T-cell lymphoproliferative disorders. Mod Pathol 2012;25:983-92. [Crossref] [PubMed]
- Talwalkar SS, Miranda RN, Valbuena JR, et al. Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol 2008;32:1299-309. [Crossref] [PubMed]
- Validire P, Capovilla M, Asselain B, et al. Primary breast non-Hodgkin's lymphoma: a large single center study of initial characteristics, natural history, and prognostic factors. Am J Hematol 2009;84:133-9. [Crossref] [PubMed]
- Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 1997;100:554-5. [Crossref] [PubMed]
- Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 2015;135:695-705. [Crossref] [PubMed]
- Doren EL, Miranda RN, Selber JC, et al. U.S. Epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2017;139:1042-50. [Crossref] [PubMed]
- Popplewell L, Thomas SH, Huang Q, et al. Primary anaplastic large-cell lymphoma associated with breast implants. Leuk Lymphoma 2011;52:1481-7. [Crossref] [PubMed]
- Kong J, Deng X, Wang Z, et al. Hepatitis C virus F protein: A double-edged sword in the potential contribution of chronic inflammation to carcinogenesis. Mol Med Rep 2009;2:461-9. [PubMed]
- Matsumoto Y, Marusawa H, Kinoshita K, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med 2007;13:470-6. [Crossref] [PubMed]
- Hu H, Jacombs A, Vickery K, et al. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg 2015;135:319-29. [Crossref] [PubMed]
- Clemens MW, Medeiros LJ, Butler CE, et al. Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant-Associated Anaplastic Large-Cell Lymphoma. J Clin Oncol 2016;34:160-8. [Crossref] [PubMed]
- Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 2014;32:114-20. [Crossref] [PubMed]
- Blombery P, Thompson ER, Jones K, et al. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica 2016;101:e387-90. [Crossref] [PubMed]
- Oishi N, Brody GS, Ketterling RP, et al. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood 2018;132:544-7. [Crossref] [PubMed]
- Ferrufino-Schmidt MC, Medeiros LJ, Liu H, et al. Clinicopathologic Features and Prognostic Impact of Lymph Node Involvement in Patients With Breast Implant-associated Anaplastic Large Cell Lymphoma. Am J Surg Pathol 2018;42:293-305. [Crossref] [PubMed]
- Johnson L, O'Donoghue JM, McLean N, et al. Breast implant associated anaplastic large cell lymphoma: The UK experience. Recommendations on its management and implications for informed consent. Eur J Surg Oncol 2017;43:1393-401. [Crossref] [PubMed]


